Tissue Engineering of the Corneal Endothelium: A Review of Carrier Materials
Abstract
:1. Introduction
1.1. The Cornea
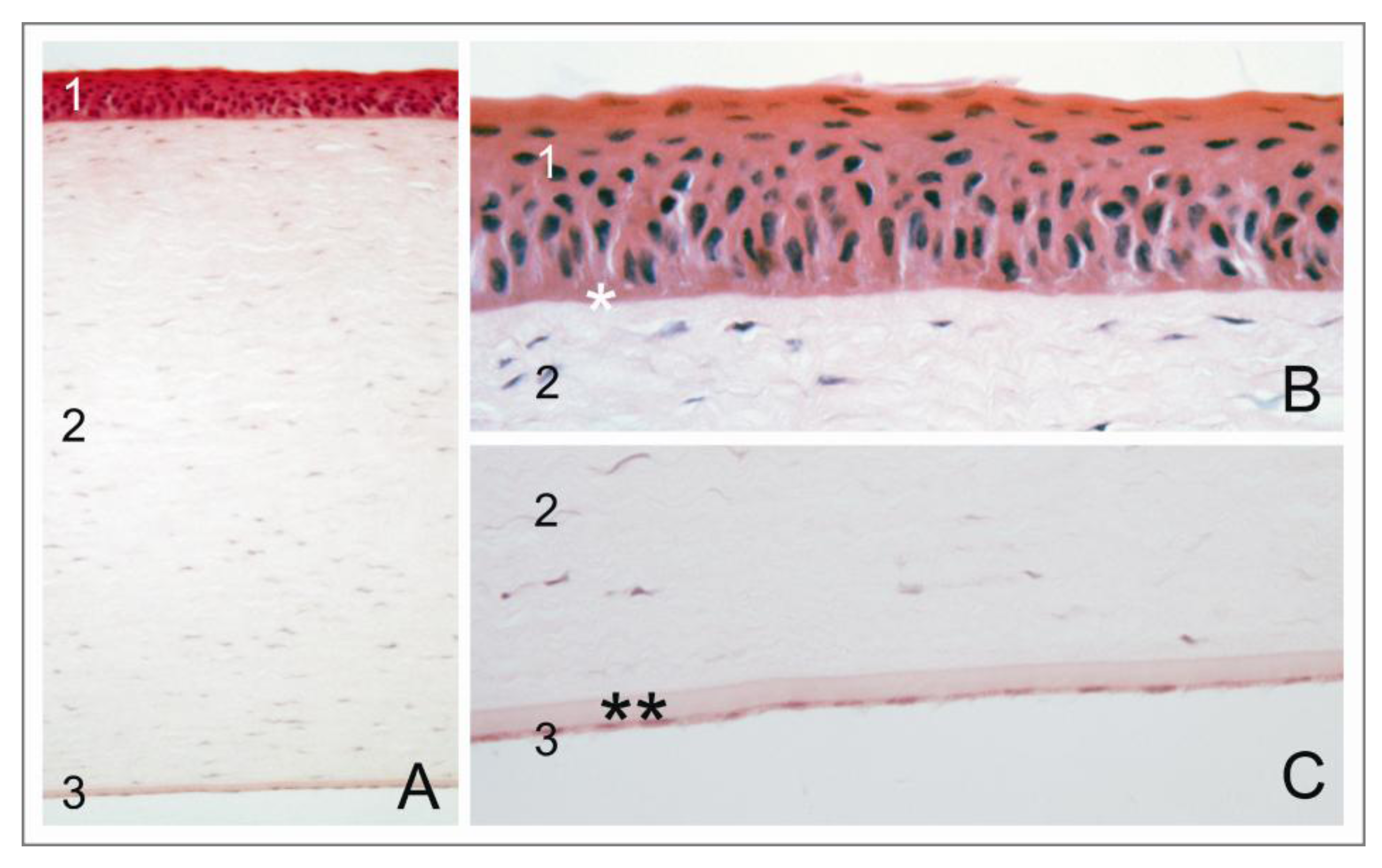
1.2. The Corneal Endothelium
1.3. Corneal Endotheliopathies and Therapy by Donor Cornea Transplantation
1.4. Keratoprostheses
2. Tissue Engineering of the Corneal Endothelium
2.1. General Considerations
2.2. Naturally Grown Membranes
2.3. Biological Polymers
2.4. Composites Made from Biological and Synthetic Polymers
2.5. Synthetic Materials
2.6. Special Focus: Thermo-Responsive Polymers as Cell Culture Carriers for Corneal Endothelial Cells
2.6.1. General Requirements and Working Principle

2.6.2. Thermo-Responsive Polymer Materials
2.6.3. Preparation of Thermo-Responsive Cell Culture Carriers
| Process based on | Polymer/copolymer/blend | Monomer unit(s) |
|---|---|---|
| without additional energy input | grafting to | grafting from |
| with additional energy input | simultaneous cross-linking and immobilization by
| simultaneous polymerization and immobilization by
|
2.6.4. Characterization of Thermo-Responsive Polymer Coatings
2.6.5. Application of Thermo-Responsive Polymers for Corneal Endothelial Tissue Engineering


3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Brubaker, R.F. The flow of aqueous humor in the human eye. Trans. Am. Ophthalmol. Soc. 1982, 80, 391–474. [Google Scholar]
- Nishida, T.; Saika, S. Cornea and Sclera: Anatomy and Physiology. In Cornea—Fundamentals, Diagnosis and Management; Krachmer, J.H., Mannis, M.J., Holland, E.J., Eds.; Mosby Elsevier: Linn, MO, USA, 2011; pp. 3–24. [Google Scholar]
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract. Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef]
- Doughty, M.J.; Zaman, M.L. Human corneal thickness and its impact on intraocular pressure measures: A review and meta-analysis approach. Surv. Ophthalmol. 2000, 44, 367–408. [Google Scholar] [CrossRef]
- Funderburgh, J.L. The Corneal Stroma. In Encyclopedia of the Eye; Dartt, D.A., Besharse, J.C., Dana, R., Eds.; Elsevier Ltd.: Boston, MA, USA, 2010; pp. 515–521. [Google Scholar]
- Hanna, C.; Bicknell, D.S.; O’Brien, J.E. Cell turnover in the adult human eye. AMA Arch. Ophthalmol. 1961, 156, 695–698. [Google Scholar]
- Sun, T.-T.; Lavker, R.M. Corneal epithelial stem cells: Past, present, and future. J. Investig. Dermatol. Symp. Proc. 2004, 9, 202–207. [Google Scholar] [CrossRef]
- Oliveira-Soto, L.; Efron, N. Morphology of corneal nerves using confocal microscopy. Cornea 2001, 20, 374–384. [Google Scholar] [CrossRef]
- Patel, D.V; McGhee, C.N.J. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: A review. Br. J. Ophthalmol. 2009, 93, 853–860. [Google Scholar] [CrossRef]
- Edelhauser, H.F. The balance between corneal transparency and edema. Invest. Ophthalmol. Vis. Sci. 2006, 47, 1755–1767. [Google Scholar] [CrossRef]
- Von der Mark, K.; Park, J. Engineering biocompatible implant surfaces Part II: Cellular recognition of biomaterial surfaces: Lessons from cell-matrix interactions. Prog. Mater. Sci. 2013, 58, 327–381. [Google Scholar] [CrossRef]
- Komai, Y.; Ushiki, T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest. Ophthalmol. Vis. Sci. 1991, 32, 2244–2258. [Google Scholar]
- Kabosova, A.; Azar, D.T.; Bannikov, G.A.; Campbell, K.P.; Durbeej, M.; Ghohestani, R.F.; Jones, J.C.R.; Kenney, M.C.; Koch, M.; Ninomiya, Y.; et al. Compositional differences between infant and adult human corneal basement membranes. Invest. Ophthalmol. Vis. Sci. 2007, 48, 4989–4999. [Google Scholar] [CrossRef]
- Tuft, S.J.; Coster, D.J. The corneal endothelium. Eye 1990, 4, 389–424. [Google Scholar] [CrossRef]
- McGowan, S.L.; Edelhauser, H.F.; Pfister, R.R.; Whikehart, D.R. Stem cell markers in the human posterior limbus and corneal endothelium of unwounded and wounded corneas. Mol. Vis. 2007, 13, 1984–2000. [Google Scholar]
- Bourne, W.M.; Nelson, L.R.; Hodge, D.O. Central corneal endothelial cell changes over a ten-year period. Invest. Ophthalmol. Vis. Sci. 1997, 38, 779–782. [Google Scholar]
- Kaufman, H.E.; Capella, J.A.; Robbins, J.E. The human corneal endothelium. Am. J. Ophthalmol. 1966, 61, 835–841. [Google Scholar]
- Hoppenreijs, V.P.T.; Pels, E.; Gijs, F.J.M.; Treffers, W.F. Corneal endothelium and growth factors. Surv. Ophthalmol. 1996, 41, 155–164. [Google Scholar] [CrossRef]
- Lu, Q.; Fuchsluger, T.A.; Jurkunas, U.V. Regulation of corneal endothelial cell proliferation. In Encyclopedia of the Eye; Dartt, D.A., Besharse, J.C., Dana, R., Eds.; Elsevier: Boston, MA, USA, 2010; pp. 15–20. [Google Scholar]
- Senoo, T.; Joyce, N.C. Cell cycle kinetics in corneal endothelium from old and young donors. Invest. Ophthalmol. Vis. Sci. 2000, 41, 660–667. [Google Scholar]
- Zhu, C.; Joyce, N.C. Proliferative response of corneal endothelial cells from young and older donors. Invest. Ophthalmol. Vis. Sci. 2004, 45, 1743–1751. [Google Scholar] [CrossRef]
- Joyce, N.C. Cell cycle status in human corneal endothelium. Exp. Eye Res. 2005, 81, 629–638. [Google Scholar] [CrossRef]
- Paull, A.C.; Whikehart, D.R. Expression of the p53 family of proteins in central and peripheral human corneal endothelial cells. Mol. Vis. 2005, 11, 328–334. [Google Scholar]
- Kelley, M.J.; Rose, A.Y.; Keller, K.E.; Hessle, H.; Samples, J.R.; Acott, T.S. Stem cells in the trabecular meshwork: Present and future promises. Exp. Eye Res. 2009, 88, 747–751. [Google Scholar] [CrossRef]
- Dikstein, B.Y.S.; Maurice, D.M. The metabolic basis to the fluid pump in the cornea. J. Physiol. 1972, 221, 29–41. [Google Scholar]
- Edelhauser, H.F. The resiliency of the corneal endothelium to refractive and intraocular surgery. Cornea 2000, 19, 263–273. [Google Scholar] [CrossRef]
- Fischbarg, J.; Maurice, D.M. An update on corneal hydration control. Exp. Eye Res. 2004, 78, 537–541. [Google Scholar] [CrossRef]
- Srinivas, S.P. Dynamic regulation of barrier integrity of the corneal endothelium. Optom. Vis. Sci. 2010, 87, E239–E254. [Google Scholar]
- Hogan, M.J.; Alvarado, J.A.; Weddell, J. Histology of the Human Eye; W.B. Saunders Company: Philadelphia, PA, USA, 1971; p. 687. [Google Scholar]
- Maurice, D.M. The location of the fluid pump in the cornea. J. Physiol. 1972, 221, 43–54. [Google Scholar]
- Guggenheim, J.A.; Hodson, S.A. Localization of Na+/K+-ATPase in the bovine corneal endothelium. Biochim. Biophys. Acta 1994, 1189, 127–134. [Google Scholar] [CrossRef]
- Maurice, D.M. The permeability to sodium ions of the living rabbit’s cornea. J. Physiol. 1951, 12, 367–391. [Google Scholar]
- Fischbarg, J.; Aires, B.; Council, N. Fluid transport across leaky epithelia: Central role of the tight junction and supporting role of aquaporins. Physiol. Rev. 2010, 90, 1271–1290. [Google Scholar] [CrossRef]
- Fischbarg, J.; Diecke, F.P.J.; Iserovich, P.; Rubashkin, A. The role of the tight junction in paracellular fluid transport across corneal endothelium. Electro-osmosis as a driving force. J. Membr. Biol. 2006, 210, 117–130. [Google Scholar] [CrossRef]
- Diecke, F.P.J.; Ma, L.; Iserovich, P.; Fischbarg, J. Corneal endothelium transports fluid in the absence of net solute transport. Biochim. Biophys. Acta 2007, 1768, 2043–2048. [Google Scholar] [CrossRef]
- Fischbarg, J.; Diecke, F.P.J. A mathematical model of electrolyte and fluid transport across corneal endothelium. J. Membr. Biol. 2005, 203, 41–56. [Google Scholar] [CrossRef]
- Engelmann, K.; Bednarz, J.; Valtink, M. Prospects for endothelial transplantation. Exp. Eye Res. 2004, 78, 573–578. [Google Scholar] [CrossRef]
- Krachmer, J.H. Posterior polymorphous corneal dystrophy: A disease characterized by epithelial-like endothelial cells which influence management and prognosis. Trans. Am. Ophthalmol. Soc. 1985, 83, 413–475. [Google Scholar]
- McCartney, A.C.; Kirkness, C.M. Comparison between posterior polymorphous dystrophy and congenital hereditary endothelial dystrophy of the cornea. Eye 1988, 2, 63–70. [Google Scholar] [CrossRef]
- Adamis, A.P.; Filatov, V.; Tripathi, B.J.; Tripathi, R.C. Fuchs’ endothelial dystrophy of the cornea. Surv. Ophthalmol. 1993, 38, 149–168. [Google Scholar] [CrossRef]
- Elhalis, H.; Azizi, B.; Jurkunas, U.V. Fuchs endothelial corneal dystrophy. Ocul. Surf. 2011, 8, 173–184. [Google Scholar] [CrossRef]
- Proulx, S.; Brunette, I. Methods being developed for preparation, delivery and transplantation of a tissue-engineered corneal endothelium. Exp. Eye Res. 2012, 95, 68–75. [Google Scholar] [CrossRef]
- Tan, D.T.H.; Dart, J.K.G.; Holland, E.J.; Kinoshita, S. Corneal transplantation. Lancet 2012, 379, 1749–1761. [Google Scholar] [CrossRef]
- Reinhard, T.; Böhringer, D.; Enczmann, J.; Wernet, P.; Sundmacher, R. HLA-Matching bei perforierender Keratoplastik. Deutsch. Ärzteblatt 2003, 100, A1198–A1210. (in German). [Google Scholar]
- Maier, P.; Reinhard, T. Keratoplasty: Laminate or penetrate? Part 1: Penetrating keratoplasty. Ophthalmologe 2009, 106, 563–569. [Google Scholar] [CrossRef]
- Böhringer, D.; Böhringer, S.; Poxleitner, K.; Birnbaum, F.; Schwartzkopff, J.; Maier, P.; Sundmacher, R.; Reinhard, T. Long-term graft survival in penetrating keratoplasty: The biexponential model of chronic endothelial cell loss revisited. Cornea 2010, 29, 1113–1117. [Google Scholar] [CrossRef]
- Lass, J.H.; Sugar, A.; Benetz, B.A.; Beck, R.W.; Dontchev, M.; Gal, R.L.; Kollman, C.; Gross, R.; Heck, E.; Holland, E.J.; et al. Endothelial cell density to predict endothelial graft failure after penetrating keratoplasty. AMA Arch. Ophthalmol. 2010, 128, 63–69. [Google Scholar] [CrossRef]
- Melles, G.R.J. Posterior lamellar keratoplasty DLEK to DSEK to DMEK. Cornea 2006, 25, 879–818. [Google Scholar] [CrossRef]
- Cursiefen, C.; Kruse, F.E. Posteriore lamelläre Keratoplastik (DSAEK). Ophthalmologe 2009, 106, 939–953. (in Geraman). [Google Scholar] [CrossRef]
- Cursiefen, C.; Kruse, F.E. DMEK: Posteriore lamelläre Keratoplastiktechnik Zusammenfassung. Ophthalmologe 2010, 107, 370–376. (in Geraman). [Google Scholar] [CrossRef]
- Dapena, I.; Ham, L.; Melles, G.R.J. Endothelial keratoplasty: DSEK/DSAEK or DMEK-the thinner the better? Curr. Opin. Ophthalmol. 2009, 20, 299–307. [Google Scholar] [CrossRef]
- Chuo, J.Y.; Yeung, S.N.; Rocha, G. Modern corneal and refractive procedures. Expert Rev. Ophthalmol. 2011, 6, 247–266. [Google Scholar] [CrossRef]
- Kook, D.; Derhartunian, V.; Bug, R.; Kohnen, T. Top-hat shaped corneal trephination for penetrating keratoplasty using the femtosecond laser: A histomorphological study. Cornea 2009, 28, 795–800. [Google Scholar] [CrossRef]
- Engelmann, K.; Valtink, M.; Lindemann, D.; Nitschke, M. Die Transplantation des kornealen Endothels—Möglichkeiten und Grenzen. Klin. Monbl. Augenheilkd. 2011, 228, 712–723. (in Geraman). [Google Scholar] [CrossRef]
- Albon, J.; Tullo, A.B.; Aktar, S.; Boulton, M.E. Apoptosis in the endothelium of human corneas for transplantation. Invest. Ophthalmol. Vis. Sci. 2000, 41, 2887–2893. [Google Scholar]
- Gimeno, F.L.; Lang, M.; Mehta, J.S.; Tan, D.T. Descemet’ s stripping automated endothelial keratoplasty: Past, present and future. Expert Rev. Ophthalmol. 2010, 5, 303–311. [Google Scholar] [CrossRef]
- Price, M.O.; Price, F.W. Endothelial cell loss after descemet stripping with endothelial keratoplasty influencing factors and 2-year trend. Ophthalmology 2008, 115, 857–865. [Google Scholar] [CrossRef]
- Terry, M.A.; Chen, E.S.; Shamie, N.; Hoar, K.L.; Friend, D.J. Endothelial cell loss after Descemet’s stripping endothelial keratoplasty in a large prospective series. Ophthalmology 2008, 115, 488–496. [Google Scholar]
- Price, M.O.; Gorovoy, M.; Benetz, B.A; Price, F.W.; Menegay, H.J.; Debanne, S.M.; Lass, J.H. Descemet’s stripping automated endothelial keratoplasty outcomes compared with penetrating keratoplasty from the Cornea Donor Study. Ophthalmology 2010, 117, 438–444. [Google Scholar] [CrossRef]
- Daneshgar, F.; Ziagharib, H. Review of posterior lamellar keratoplasty techniques. J. Transplant. Technol. Res. 2011, S2, 1–8. [Google Scholar]
- Khor, W.-B.; Mehta, J.S.; Tan, D.T.-H. Descemet stripping automated endothelial keratoplasty with a graft insertion device: Surgical technique and early clinical results. Am. J. Ophthalmol. 2011, 151, 223–232. [Google Scholar] [CrossRef]
- Bednarz, J.; Doubilei, V.; Wollnik, P.C.; Engelmann, K. Effect of three different media on serum free culture of donor corneas and isolated human corneal endothelial cells. Br. J. Ophthalmol. 2001, 85, 1416–1420. [Google Scholar] [CrossRef]
- Hempel, B.; Bednarz, J.; Engelmann, K. Use of a serum-free medium for long-term storage of human corneas. Influence on endothelial cell density and corneal metabolism. Graef. Arch. Clin. Exp. 2001, 239, 801–805. [Google Scholar] [CrossRef]
- Jäckel, T.; Knels, L.; Valtink, M.; Funk, R.H.W.; Engelmann, K. Serum-free corneal organ culture medium (SFM) but not conventional minimal essential organ culture medium (MEM) protects human corneal endothelial cells from apoptotic and necrotic cell death. Br. J. Ophthalmol. 2011, 95, 123–130. [Google Scholar] [CrossRef]
- European Parliament and the Council, DIRECTIVE 2004/23/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 31 March 2004 on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells. Europe, 2004; pp. L 102/48–L 102/58. Available online: http://europa.eu/legislation_summaries/public_health/threats_to_health/c11573_en.htm#act (accessed on 14 October 2013).
- Pels, E.; Rijneveld, W.J. Organ culture preservation for corneal tissue. In Eye Banking; Bredehorn-Mayr, T., Duncker, G.I.W., Armitage, W.J., Eds.; Developmental Ophthalmology: Basel, Switzerland, 2009; Volume 43, pp. 31–46. [Google Scholar]
- Engelmann, K.; Friedl, P. Optimization of culture conditions for human corneal endothelial cells. In Vitro Cell Dev. Biol. Anim. 1989, 25, 1065–1072. [Google Scholar] [CrossRef]
- Engelmann, K.; Friedl, P. Growth of human corneal endothelial cells in a serum-reduced medium. Cornea 1995, 14, 62–70. [Google Scholar]
- Møller-Pedersen, T.; Hartmann, U.; Møller, H.J.; Ehlers, N.; Engelmann, K. Evaluation of potential organ culture media for eye banking using human donor corneas. Br. J. Ophthalmol. 2001, 85, 1075–9107. [Google Scholar] [CrossRef]
- Møller-Pedersen, T.; Hartmann, U.; Ehlers, N.; Engelmann, K. Evaluation of potential organ culture media for eye banking using a human corneal endothelial cell growth assay. Graef. Arch. Clin. Exp. 2001, 239, 778–782. [Google Scholar] [CrossRef]
- Gomaa, A.; Comyn, O.; Liu, C. Keratoprostheses in clinical practice—A review. Clin. Exp. Ophthalmol. 2010, 38, 211–224. [Google Scholar] [CrossRef]
- Ruberti, J.W.; Roy, A.S.; Roberts, C.J. Corneal biomechanics and biomaterials. Annu. Rev. Biomed. Eng. 2011, 13, 269–295. [Google Scholar] [CrossRef]
- Dohlman, C.H.; Harissi-Dagher, M.; Khan, B.F.; Sippel, K.; Aquavella, J.V; Graney, J.M. Introduction to the use of the Boston keratoprosthesis. Expert Rev. Ophthalmol. 2006, 1, 41–48. [Google Scholar] [CrossRef]
- Myung, D.; Duhamel, P.-E.; Cochran, J.; Noolandi, J.; Ta, C.; Frank, C. Development of hydrogel-based keratoprostheses: A materials perspective. Biotechnol. Prog. 2009, 24, 735–741. [Google Scholar]
- Hicks, C.R.; Werner, L.; Vijayasekaran, S.; Mamalis, N.; Apple, D.J. Histology of AlphaCor skirts: Evaluation of biointegration. Cornea 2005, 24, 933–940. [Google Scholar] [CrossRef]
- Duan, X.; Sheardown, H. Incorporation of cell-adhesion peptides into collagen scaffolds promotes corneal epithelial stratification. J. Biomater. Sci. Polym. Ed. 2007, 18, 701–711. [Google Scholar] [CrossRef]
- Klenkler, B.J.; Griffith, M.; Becerril, C.; West-Mays, J.A.; Sheardown, H. EGF-grafted PDMS surfaces in artificial cornea applications. Biomaterials 2005, 26, 7286–7296. [Google Scholar] [CrossRef]
- Choi, J.S.; Williams, J.K.; Greven, M.; Walter, K.A.; Laber, P.W.; Khang, G.; Soker, S. Bioengineering endothelialized neo-corneas using donor-derived corneal endothelial cells and decellularized corneal stroma. Biomaterials 2010, 31, 6738–6745. [Google Scholar] [CrossRef]
- Ruberti, J.W.; Zieske, J.D.; Trinkaus-Randall, V. Corneal-tissue replacement. In Principles of Tissue Engineering; Lanza, R.P., Langer, R.S., Vacanti, J.P., Eds.; Elsevier Inc.: Oxford, UK, 2007; pp. 1025–1047. [Google Scholar]
- Chen, K.H.; Azar, D.; Joyce, N.C. Transplantation of adult human corneal endothelium ex vivo: A morphologic study. Cornea 2001, 20, 731–737. [Google Scholar] [CrossRef]
- Mimura, T.; Amano, S.; Usui, T.; Araie, M.; Ono, K.; Akihiro, H.; Yokoo, S.; Yamagami, S. Transplantation of corneas reconstructed with cultured adult human corneal endothelial cells in nude rats. Exp. Eye Res. 2004, 79, 231–237. [Google Scholar] [CrossRef]
- Engelmann, K.; Drexler, D.; Böhnke, M. Transplantation of adult human or porcine corneal endothelial cells onto human recipients in vitro. Part I: Cell culturing and transplantation procedure. Cornea 1999, 18, 199–206. [Google Scholar] [CrossRef]
- Engelmann, K.; Bednarz, J.; Böhnke, M. Endothelzelltransplantation und Wachstumsverhalten des humanen kornealen Endothels. Ophthalmologe 1999, 96, 555–562. (in German). [Google Scholar] [CrossRef]
- Engelmann, K.; Böhnke, M.; Friedl, P. Isolation and long-term cultivation of human corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 1988, 29, 1656–1662. [Google Scholar]
- Aboalchamat, B.; Engelmann, K.; Böhnke, M.; Eggli, P.; Bednarz, J. Morphological and functional analysis of immortalized human corneal endothelial cells after transplantation. Exp. Eye Res. 1999, 69, 547–553. [Google Scholar] [CrossRef]
- Van Horn, D.L.; Sendele, D.D.; Seideman, S.; Buco, P.J. Regenerative capacity of the corneal endothelium in rabbit and cat. Invest. Ophthalmol. Vis. Sci. 1977, 16, 597–613. [Google Scholar]
- Patel, S.V; Bachman, L.A.; Hann, C.R.; Bahler, C.K.; Fautsch, M.P. Human corneal endothelial cell transplantation in a human ex vivo model. Invest. Ophthalmol. Vis. Sci. 2009, 50, 2123–2131. [Google Scholar] [CrossRef]
- Reichl, S.; Bednarz, J.; Müller-Goymann, C.C. Human corneal equivalent as cell culture model for in vitro drug permeation studies. Br. J. Ophthalmol. 2004, 88, 560–565. [Google Scholar] [CrossRef]
- Reichl, S.; Döhring, S.; Bednarz, J.; Müller-Goymann, C.C. Human cornea construct HCC—An alternative for in vitro permeation studies? A comparison with human donor corneas. Eur. J. Pharm. Biopharm. 2005, 60, 305–308. [Google Scholar] [CrossRef]
- Gomes, J.A.P.; Romano, A.; Santos, M.S.; Dua, H.S. Amniotic membrane use in ophthalmology. Curr. Opin. Ophthalmol. 2005, 16, 233–240. [Google Scholar] [CrossRef]
- Ishino, Y.; Sano, Y.; Nakamura, T.; Connon, C.J.; Rigby, H.; Fullwood, N.J.; Kinoshita, S. Amniotic membrane as a carrier for cultivated human corneal endothelial cell transplantation. Invest. Ophthalmol. Vis. Sci. 2004, 45, 800–806. [Google Scholar] [CrossRef]
- Wencan, W.; Mao, Y.; Wentao, Y.; Fan, L.; Jia, Q.; Qinmei, W.; Xiangtian, Z. Using basement membrane of human amniotic membrane as a cell carrier for cultivated cat corneal endothelial cell transplantation. Curr. Eye Res. 2007, 32, 199–215. [Google Scholar] [CrossRef]
- Kim, H.-J.; Ryu, Y.-H.; Ahn, J.-I.; Park, J.-K.; Kim, J.-C. Characterization of immortalized human corneal endothelial cell line using HPV 16 E6/E7 on lyophilized human amniotic membrane. Korean J. Ophthalmol. 2006, 20, 47–54. [Google Scholar] [CrossRef]
- Lange, T.M.; Wood, T.O.; McLaughlin, B.J. Corneal endothelial cell transplantation using Descemet’s membrane as a carrier. J. Cataract. Refract. Surg. 1993, 19, 232–235. [Google Scholar] [CrossRef]
- Yoeruek, E.; Saygili, O.; Spitzer, M.S.; Tatar, O.; Bartz-Schmidt, K.U.; Szurman, P. Human anterior lens capsule as carrier matrix for cultivated human corneal endothelial cells. Cornea 2009, 28, 416–420. [Google Scholar] [CrossRef]
- Ju, C.; Gao, L.; Wu, X.; Pang, K. A human corneal endothelium equivalent constructed with acellular porcine corneal matrix. Indian J. Med. Res. 2012, 135, 887–894. [Google Scholar]
- Hashimoto, Y.; Funamoto, S.; Sasaki, S.; Honda, T.; Hattori, S.; Nam, K.; Kimura, T.; Mochizuki, M.; Fujisato, T.; Kobayashi, H.; et al. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials 2010, 31, 3941–3948. [Google Scholar] [CrossRef]
- Bayyoud, T.; Thaler, S.; Hofmann, J.; Maurus, C.; Stephan, M.; Szurman, P.; Yoeruek, E. Decellularized bovine corneal posterior lamellae as carrier matrix for cultivated human corneal endothelial cells. Curr. Eye Res. 2012, 37, 179–186. [Google Scholar] [CrossRef]
- McCulley, J.P.; Maurice, D.M.; Schwartz, B.D. Comeal endothelial transplantation. Ophthalmology 1980, 87, 194–201. [Google Scholar]
- Schwartz, B.D.; McCulley, J.P. Morphology of transplanted corneal endothelium derived from tissue culture. Invest. Ophthalmol. Vis. Sci. 1981, 20, 467–480. [Google Scholar]
- Maurice, D.M.; James, P.M.; Schwartz, B.D. The use of cultured corneal endothelium in keratoplasty. Vis. Res. 1981, 21, 173–174. [Google Scholar] [CrossRef]
- Watanabe, R.; Hayashi, R.; Kimura, Y.; Tanaka, Y.; Kageyama, T.; Hara, S.; Tabata, Y.; Nishida, K. A novel gelatin hydrogel carrier sheet for corneal endothelial transplantation. Tissue Eng. Part A 2011, 17, 1–8. [Google Scholar]
- Koizumi, N.; Sakamoto, Y.; Okumura, N.; Okahara, N.; Tsuchiya, H.; Torii, R.; Cooper, L.J.; Ban, Y.; Tanioka, H.; Kinoshita, S. Cultivated corneal endothelial cell sheet transplantation in a primate model. Invest. Ophthalmol. Vis. Sci. 2007, 48, 4519–4526. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Yokoo, S.; Usui, T.; Tanaka, K.; Hattori, S.; Irie, S.; Miyata, K.; Araie, M.; Amano, S. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Invest. Ophthalmol. Vis. Sci. 2004, 45, 2992–2997. [Google Scholar] [CrossRef]
- Harkin, D.G.; George, K.A.; Madden, P.W.; Schwab, I.R.; Hutmacher, D.W.; Chirila, T.V. Silk fibroin in ocular tissue reconstruction. Biomaterials 2011, 32, 2445–2458. [Google Scholar] [CrossRef]
- Valtink, M.; Gruschwitz, R.; Funk, R.H.W.; Engelmann, K. Two clonal cell lines of immortalized human corneal endothelial cells show either differentiated or precursor cell characteristics. Cells Tissues Organs 2008, 187, 286–294. [Google Scholar] [CrossRef]
- Madden, P.W.; Lai, J.N.X.; George, K.A.; Giovenco, T.; Harkin, D.G.; Chirila, T.V. Human corneal endothelial cell growth on a silk fibroin membrane. Biomaterials 2011, 32, 4076–4084. [Google Scholar] [CrossRef]
- Griffith, M. Functional human corneal equivalents constructed from cell lines. Science 1999, 286, 2169–2172. [Google Scholar] [CrossRef]
- Liu, Y.; Gan, L.; Carlsson, D.J.; Fagerholm, P.; Lagali, N.; Watsky, M.A; Munger, R.; Hodge, W.G.; Priest, D.; Griffith, M. A simple, cross-linked collagen tissue substitute for corneal implantation. Invest. Ophthalmol. Vis. Sci. 2006, 47, 1869–1875. [Google Scholar] [CrossRef]
- Liu, W.; Merrett, K.; Griffith, M.; Fagerholm, P.; Dravida, S.; Heyne, B.; Scaiano, J.C.; Watsky, M.A.; Shinozaki, N.; Lagali, N.; et al. Recombinant human collagen for tissue engineered corneal substitutes. Biomaterials 2008, 29, 1147–1158. [Google Scholar] [CrossRef]
- Griffith, M.; Jackson, W.B.; Lagali, N.; Merrett, K.; Li, F.; Fagerholm, P. Artificial corneas: A regenerative medicine approach. Eye 2009, 23, 1985–1989. [Google Scholar] [CrossRef]
- Orwin, E.J.; Hubel, A. In vitro culture characteristics of corneal epithelial, endothelial, and keratocyte cells in a native collagen matrix. Tissue Eng. 2000, 6, 307–320. [Google Scholar] [CrossRef]
- Gao, X.; Liu, W.; Han, B.; Wei, X.; Yang, C. Preparation and properties of a chitosan-based carrier of corneal endothelial cells. J. Mater. Sci. Mater. Med. 2008, 19, 3611–3619. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, W.; Han, B.; Yang, C.; Ma, Q.; Zhao, W.; Rong, M.; Li, H. Fabrication and characters of a corneal endothelial cells scaffold based on chitosan. J. Mater. Sci. Mater. Med. 2011, 22, 175–183. [Google Scholar] [CrossRef]
- Wang, T.-J.; Wang, I.-J.; Chen, S.; Chen, Y.-H.; Young, T.-H. The phenotypic response of bovine corneal endothelial cells on chitosan/polycaprolactone blends. Colloids Surf. B Biointerfaces 2012, 90, 236–243. [Google Scholar] [CrossRef]
- Mohay, J.; Lange, T.M.; Soltau, J.B.; Wood, T.O.; McLaughlin, B.J. Transplantation of corneal endothelial cells. Cornea 1994, 13, 173–182. [Google Scholar] [CrossRef]
- Hadlock, T.; Singh, S.; Vacanti, J.P.; McLaughlin, B.J. Ocular cell monolayers cultured on biodegradable substrates. Tissue Eng. 1999, 5, 187–196. [Google Scholar] [CrossRef]
- Wang, T.-J.; Wang, I.-J.; Chen, Y.-H.; Lu, J.-N.; Young, T.-H. Polyvinylidene fluoride for proliferation and preservation of bovine corneal endothelial cells by enhancing type IV collagen production and deposition. J. Biomed. Mater. Res. A 2012, 100, 252–260. [Google Scholar]
- Matsuda, N.; Shimizu, T.; Yamato, M.; Okano, T. Tissue engineering based on cell sheet technology. Adv. Mater. Weinh. 2007, 19, 3089–3099. [Google Scholar] [CrossRef]
- Ravichandran, R.; Sundarrajan, S.; Venugopal, J.R.; Mukherjee, S.; Ramakrishna, S. Advances in polymeric systems for tissue engineering and biomedical applications. Macromol. Biosci. 2012, 12, 286–311. [Google Scholar] [CrossRef]
- Kobayashi, J.; Okano, T. Fabrication of a thermoresponsive cell culture dish: A key technology for cell sheet tissue engineering. Sci. Technol. Adv. Mater. Weinh. 2010, 11, 1–12. [Google Scholar]
- Canavan, H.E.; Cheng, X.; Graham, D.J.; Ratner, B.D.; Castner, D.G. Cell sheet detachment affects the extracellular matrix: A surface science study comparing thermal liftoff, enzymatic, and mechanical methods. J. Biomed. Mater. Res. A 2005, 75, 1–13. [Google Scholar]
- Canavan, H.E.; Cheng, X.; Graham, D.J.; Ratner, B.D.; Castner, D.G. A plasma-deposited surface for cell sheet engineering: Advantages over mechanical dissociation of cells. Plasma Process. Polym. 2006, 3, 516–523. [Google Scholar] [CrossRef]
- Joseph, N.; Kumar, A.P.R.; Kumary, T. Tunable stimuli-responsive polymers for cell sheet engineering. In Regenerative Medicine and Tissue Engineering—Cells and Biomaterials; Eberli, D., Ed.; InTech: Rijeka, Croatia, 2010; pp. 503–512. [Google Scholar]
- Da Silva, R.M.P.; Mano, J.F.; Reis, R.L. Smart thermoresponsive coatings and surfaces for tissue engineering: Switching cell-material boundaries. Trends Biotechnol. 2007, 25, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef]
- De Las Heras Alarcón, C.; Pennadam, S.; Alexander, C. Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 2005, 34, 276–285. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Okuhara, M.; Sakai, H.; Sakurai, Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials 1995, 16, 297–303. [Google Scholar] [CrossRef]
- Reed, J.A.; Lucero, A.E.; Cooperstein, M.A.; Canavan, H.E. The effects of cell culture parameters on cell release kinetics from thermoresponsive surfaces. J. Appl. Biomater. Biomech. 2008, 6, 81–88. [Google Scholar]
- Cooperstein, M.A.; Canavan, H.E. Biological cell detachment from poly(N-isopropyl acrylamide) and its applications. Langmuir 2010, 26, 7695–7707. [Google Scholar] [CrossRef]
- Yamato, M.; Okuhara, M.; Karikusaa, F.; Kikuchi, A.; Sakurai, Y.; Okano, T. Signal transduction and cytoskeletal reorganization are required for cell detachment from cell culture surfaces grafted with a temperature-responsive polymer. J. Biomed. Mater. Res. 1999, 44, 44–52. [Google Scholar] [CrossRef]
- Yamato, M.; Konno, C.; Kushida, A.; Hirose, M.; Utsumi, M.; Kikuchi, A.; Okano, T. Release of adsorbed fibronectin from temperature-responsive culture surfaces requires cellular activity. Biomaterials 2000, 21, 981–986. [Google Scholar] [CrossRef]
- Barker, T.H. The role of ECM proteins and protein fragments in guiding cell behavior in regenerative medicine. Biomaterials 2011, 32, 4211–4214. [Google Scholar] [CrossRef]
- Collier, J.H.; Segura, T. Evolving the use of peptides as components of biomaterials. Biomaterials 2011, 32, 4198–4204. [Google Scholar] [CrossRef]
- Takahashi, H.; Matsuzaka, N.; Nakayama, M.; Kikuchi, A.; Yamato, M.; Okano, T. Terminally functionalized thermoresponsive polymer brushes for simultaneously promoting cell adhesion and cell sheet harvest. Biomacromolecules 2012, 13, 253–260. [Google Scholar] [CrossRef]
- Gramm, S.; Teichmann, J.; Nitschke, M.; Gohs, U.; Eichhorn, K.-J.; Werner, C. Electron beam immobilization of functionalized poly(vinyl methyl ether) thin films on polymer surfaces—Towards stimuli responsive coatings for biomedical purposes. Express Polym. Lett. 2011, 5, 970–976. [Google Scholar] [CrossRef]
- Ebara, M.; Yamato, M.; Aoyagi, T.; Kikuchi, A.; Sakai, K.; Okano, T. Immobilization of cell-adhesive peptides to temperature-responsive surfaces facilitates both serum-free cell adhesion and noninvasive cell harvest. Tissue Eng. 2004, 10, 1125–1135. [Google Scholar]
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Ultrathin poly(N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir 2004, 20, 5506–5511. [Google Scholar] [CrossRef]
- Fukumori, K.; Akiyama, Y.; Kumashiro, Y.; Kobayashi, J.; Yamato, M.; Sakai, K.; Okano, T. Characterization of ultra-thin temperature-responsive polymer layer and its polymer thickness dependency on cell attachment/detachment properties. Macromol. Biosci. 2010, 10, 1117–1129. [Google Scholar] [CrossRef]
- Cole, M.A.; Voelcker, N.H.; Thissen, H.; Griesser, H.J. Stimuli-responsive interfaces and systems for the control of protein-surface and cell-surface interactions. Biomaterials 2009, 30, 1827–1850. [Google Scholar] [CrossRef]
- Crespy, D.; Rossi, R.M. Temperature-responsive polymers with LCST in the physiological range and their applications in textiles. Polym. Int. 2007, 56, 1461–1468. [Google Scholar] [CrossRef]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Schäfer-Soenen, H.; Moerkerke, R.; Berghmans, H.; Koningsveld, R.; Dusek, K.; Sloc, K. Zero and off-zero critical concentrations in systems containing polydisperse polymers with very high molar masses. 2. The system water-poly(vinyl methyl ether). Macromolecules 1997, 30, 410–416. [Google Scholar] [CrossRef]
- Hegewald, J.; Schmidt, T.; Gohs, U.; Günther, M.; Reichelt, R.; Stiller, B.; Arndt, K.-F. Electron beam irradiation of poly(vinyl methyl ether) films: 1. Synthesis and film topography. Langmuir 2005, 21, 6073–6080. [Google Scholar] [CrossRef]
- Hegewald, J.; Schmidt, T.; Eichhorn, K.-J.; Kretschmer, K.; Kuckling, D.; Arndt, K.-F. Electron beam irradiation of poly(vinyl methyl ether) films. 2. Temperature-dependent swelling behavior. Langmuir 2006, 22, 5152–5159. [Google Scholar] [CrossRef]
- Mikheeva, L.M.; Grinberg, N.V; Mashkevich, A.Y.; Grinberg, V.Y.; Thi, L.; Thanh, M.; Makhaeva, E.E.; Khokhlov, A.R. Microcalorimetric study of thermal cooperative transitions in Poly (N-vinylcaprolactam) hydrogels. Macromolecules 1997, 30, 2693–2699. [Google Scholar] [CrossRef]
- Rzaev, Z.M.O.; Dinçer, S.; Pişkin, E. Functional copolymers of N-isopropylacrylamide for bioengineering applications. Prog. Polym. Sci. 2007, 32, 534–595. [Google Scholar] [CrossRef]
- Takezawa, T.; Mori, Y.; Yoshizato, K. Cell culture on a thermo-responsive polymer surface. Nat. Biotechnol. 1990, 8, 854–856. [Google Scholar] [CrossRef]
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Macromol. Rapid Commun. 1990, 11, 571–576. [Google Scholar] [CrossRef]
- Yamato, M.; Akiyama, Y.; Kobayashi, J.; Yang, J.; Kikuchi, A.; Okano, T. Temperature-responsive cell culture surfaces for regenerative medicine with cell sheet engineering. Prog. Polym. Sci. 2007, 32, 1123–1133. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Shimizu, T.; Sasagawa, T.; Sekine, H.; Sakaguchi, K.; Kikuchi, T.; Sekine, W.; Sekiya, S.; Yamato, M.; Umezu, M.; et al. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat. Protoc. 2012, 7, 850–858. [Google Scholar] [CrossRef]
- Ide, T.; Nishida, K.; Yamato, M.; Sumide, T.; Utsumi, M.; Nozaki, T.; Kikuchi, A.; Okano, T.; Tano, Y. Structural characterization of bioengineered human corneal endothelial cell sheets fabricated on temperature-responsive culture dishes. Biomaterials 2006, 27, 607–614. [Google Scholar] [CrossRef]
- Sumide, T.; Nishida, K.; Yamato, M.; Ide, T.; Hayashida, Y.; Watanabe, K.; Yang, J.; Kohno, C.; Kikuchi, A.; Maeda, N.; et al. Functional human corneal endothelial cell sheets harvested from temperature-responsive culture surfaces. FASEB J. 2006, 20, 392–394. [Google Scholar]
- Hsiue, G.-H.; Lai, J.-Y.; Chen, K.-H.; Hsu, W.-M. A novel strategy for corneal endothelial reconstruction with a bioengineered cell sheet. Transplantation 2006, 81, 473–476. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Chen, K.-H.; Hsu, W.-M.; Hsiue, G.-H.; Lee, Y.-H. Bioengineered human corneal endothelium for transplantation. AMA Arch. Ophthalmol. 2006, 124, 1441–1448. [Google Scholar] [CrossRef]
- Ebara, M.; Yamato, M.; Hirose, M.; Aoyagi, T.; Kikuchi, A.; Sakai, K.; Okano, T. Copolymerization of 2-carboxyisopropylacrylamide with N-isopropylacrylamide accelerates cell detachment from grafted surfaces by reducing temperature. Biomacromolecules 2003, 4, 344–349. [Google Scholar] [CrossRef]
- Gramm, S.; Komber, H.; Schmaljohann, D. Copolymerization kinetics of N -isopropylacrylamide and diethylene glycol monomethylether monomethacrylate determined by online NMR spectroscopy. J. Polym. Sci. Part. A Polym. Chem. 2005, 43, 142–148. [Google Scholar] [CrossRef]
- Nitschke, M.; Gramm, S.; Götze, T.; Valtink, M.; Drichel, J.; Voit, B.; Engelmann, K.; Werner, C. Thermo-responsive poly(NiPAAm-co-DEGMA) substrates for gentle harvest of human corneal endothelial cell sheets. J. Biomed. Mater. Res. Part A 2007, 80A, 1003–1010. [Google Scholar] [CrossRef]
- Teichmann, J.; Valtink, M.; Gramm, S.; Nitschke, M.; Werner, C.; Funk, R.H.W.; Engelmann, K. Human corneal endothelial cell sheets for transplantation: Thermo-responsive cell culture carriers to meet cell-specific requirements. Acta Biomater. 2013, 9, 5031–5039. [Google Scholar] [CrossRef]
- Nagase, K.; Kobayashi, J.; Okano, T. Temperature-responsive intelligent interfaces for biomolecular separation and cell sheet engineering. J. R. Soc. Interface 2009, 6, S293–S309. [Google Scholar] [CrossRef]
- Takei, Y.G.; Aoki, T.; Sanui, K.; Ogata, N.; Sakurai, Y.; Okano, T. Dynamic contact angle measurement of temperature-responsive surface properties for poly(n-isopropylacrylamide) grafted surfaces. Macromolecules 1994, 27, 6163–6166. [Google Scholar] [CrossRef]
- Yakushiji, T.; Sakai, K.; Kikuchi, A.; Aoyagi, T.; Sakurai, Y.; Okano, T. Effects of cross-linked structure on temperature-responsive hydrophobic interaction of Poly(N-isopropylacrylamide) hydrogel-modified surfaces with steroids. Anal. Chem. 1999, 71, 1125–1130. [Google Scholar] [CrossRef]
- Mizutani, A.; Kikuchi, A.; Yamato, M.; Kanazawa, H.; Okano, T. Preparation of thermoresponsive polymer brush surfaces and their interaction with cells. Biomaterials 2008, 29, 2073–2081. [Google Scholar] [CrossRef]
- Morra, M.; Cassinelli, C. Thermal recovery of cells cultured on poly(n-isopropylacrylamide) surface-grafted polystyrene dishes. In Surface Modification by Polymeric Biomaterials; Ratner, B.D., Castner, D.G., Eds.; Plenum Press: New York, NY, USA, 1997; pp. 175–181. [Google Scholar]
- Biederman, H.; Osada, Y. Plasma Polymerization Processes, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 1992; p. 210. [Google Scholar]
- Pan, Y.V.; Wesley, R.A.; Luginbuhl, R.; Denton, D.D.; Ratner, B.D. Plasma polymerized n-isopropylacrylamide: Synthesis and characterization of a smart thermally responsive coating. Biomacromolecules 2001, 2, 32–36. [Google Scholar] [CrossRef]
- Chu, L.-Q.; Zou, X.-N.; Knoll, W.; Förch, R. Thermosensitive surfaces fabricated by plasma polymerization of N,N-diethylacrylamide. Surf. Coat. Technol. 2008, 202, 2047–2051. [Google Scholar] [CrossRef]
- McHerron, D.C.; Wilkes, G.L. Electron beam irradiation of polystyrene-poly(vinyl methyl ether) blends. Polymer 1993, 34, 3976–3985. [Google Scholar] [CrossRef]
- Nitschke, M.; Zschoche, S.; Baier, A; Simon, F.; Werner, C. Low pressure plasma immobilization of thin hydrogel films on polymer surfaces. Surf. Coat. Technol. 2004, 185, 120–125. [Google Scholar] [CrossRef]
- Schmaljohann, D.; Beyerlein, D.; Nitschke, M.; Werner, C. Thermo-reversible swelling of thin hydrogel films immobilized by low-pressure plasma. Langmuir 2004, 20, 10107–10114. [Google Scholar] [CrossRef]
- He, X.-L.; Nie, P.-P.; Chen, B.-Z.; Li, X.-X.; Chen, L.; Guo, G.; Zhang, R. A novel method to fabricate thermoresponsive microstructures with improved cell attachment/detachment properties. J. Biomed. Mater. Res. A 2012, 100, 1946–1953. [Google Scholar]
- Xu, F.-J.; Kang, E.-T.; Neoh, K.-G. pH- and temperature-responsive hydrogels from crosslinked triblock copolymers prepared via consecutive atom transfer radical polymerizations. Biomaterials 2006, 27, 2787–2797. [Google Scholar] [CrossRef]
- Bullett, N.A.; Talib, R.A.; Short, R.D.; McArthur, S.L.; Shard, A.G. Chemical and thermo-responsive characterisation of surfaces formed by plasma polymerisation of N-Isopropyl acrylamide. Surf. Interface Anal. 2006, 38, 1109–1116. [Google Scholar] [CrossRef]
- Tsuda, Y.; Kikuchi, A.; Yamato, M.; Nakao, A.; Sakurai, Y.; Umezu, M.; Okano, T. The use of patterned dual thermoresponsive surfaces for the collective recovery as co-cultured cell sheets. Biomaterials 2005, 26, 1885–1893. [Google Scholar] [CrossRef]
- Yoshikatsu Kushida, A.; Yamato, M.; Kikuchi, A.; Okano, T. Surface characterization of Poly(N-isopropylacrylamide) grafted tissue culture polystyrene by electron beam irradiation, using atomic force microscopy, and X-ray photoelectron spectroscopy. J. Nanosci. Nanotechnol. 2007, 7, 796–802. [Google Scholar] [CrossRef]
- Cheng, X.; Canavan, H.E.; Stein, M.J.; Hull, J.R.; Kweskin, S.J.; Wagner, M.S.; Somorjai, G.A; Castner, D.G.; Ratner, B.D. Surface chemical and mechanical properties of plasma-polymerized N-isopropylacrylamide. Langmuir 2005, 21, 7833–7841. [Google Scholar] [CrossRef]
- Yamato, M.; Konno, C.; Koike, S.; Isoi, Y.; Shimizu, T.; Kikuchi, A.; Makino, K.; Okano, T. Nanofabrication for micropatterned cell arrays by combining electron beam-irradiated polymer grafting and localized laser ablation. J. Biomed. Mater. Res. Part A 2003, 67, 1065–1071. [Google Scholar]
- Cheng, X.; Canavan, H.E.; Graham, D.J.; Castner, D.G.; Ratner, B.D. Temperature dependent activity and structure of adsorbed proteins on plasma polymerized N-isopropyl acrylamide. Biointerphases 2006, 1, 61–72. [Google Scholar] [CrossRef]
- Canavan, H.E.; Cheng, X.; Graham, D.J.; Ratner, B.D.; Castner, D.G. Surface characterization of the extracellular matrix remaining after cell detachment from a thermoresponsive polymer. Langmuir 2005, 21, 1949–1955. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Kikuchi, A.; Yamato, M.; Okano, T. Bio-functionalized thermoresponsive interfaces facilitating cell adhesion and proliferation. Biomaterials 2006, 27, 5069–5078. [Google Scholar] [CrossRef]
- Joseph, N.; Prasad, T.; Raj, V.; Kumar, A.P.R.; Sreenivasan, K.; Kumary, T.V. A cytocompatible poly(n-isopropylacrylamide-co-glycidylmethacrylate) coated surface as new substrate for corneal tissue engineering. J. Bioact. Compat. Polym. 2010, 25, 58–74. [Google Scholar] [CrossRef]
- Ebara, M.; Yamato, M.; Aoyagi, T.; Kikuchi, A.; Sakai, K.; Okano, T. Temperature-responsive cell culture surfaces enable “on-off” affinity control between cell integrins and RGDS ligands. Biomacromolecules 2004, 5, 505–510. [Google Scholar] [CrossRef]
- Wang, X.; Mccord, M.G. Grafting of poly(n-isopropylacrylamide) onto nylon and polystyrene surfaces by atmospheric plasma treatment followed with free radical graft copolymerization. J. Appl. Polym. Sci. 2006, 104, 3614–3621. [Google Scholar] [CrossRef]
- Anil Kumar, P.R.; Sreenivasan, K.; Kumary, T.V. Alternate method for grafting thermoresponsive polymer for transferring in vitro cell sheet structures. J. Appl. Polym. Sci. 2007, 105, 2245–2251. [Google Scholar] [CrossRef]
- Kooij, E.S.; Sui, X.; Hempenius, M.A.; Zandvliet, H.J.W.; Vancso, G.J. Probing the thermal collapse of poly(n-isopropylacrylamide) grafts by quantitative in situ ellipsometry. J. Phys. Chem. A B 2012, 116, 9261–9268. [Google Scholar]
- Schmaljohann, D.; Nitschke, M.; Schulze, R.; Eing, A.; Werner, C.; Eichhorn, K.-J. In situ study of the thermoresponsive behavior of micropatterned hydrogel films by imaging ellipsometry. Langmuir 2005, 21, 2317–2322. [Google Scholar] [CrossRef]
- Cordeiro, A.L.; Zimmermann, R.; Gramm, S.; Nitschke, M.; Janke, A.; Schäfer, N.; Grundke, K.; Werner, C. Temperature dependent physicochemical properties of poly(n-isopropylacrylamide-co-N-(1-phenylethyl)acrylamide) thin films. Soft Matter 2009, 5, 1367–1377. [Google Scholar] [CrossRef]
- Kurkuri, M.D.; Nussio, M.R.; Deslandes, A.; Voelcker, N.H. Thermosensitive copolymer coatings with enhanced wettability switching. Langmuir 2008, 24, 4238–4244. [Google Scholar] [CrossRef]
- Matzelle, T.R.; Ivanov, D.A.; Landwehr, D.; Heinrich, L.A.; Herkt-Bruns, C.; Reichelt, R.; Kruse, N. Micromechanical properties of “Smart” gels: Studies by scanning force and scanning electron microscopy of PNIPAAm. J. Phys. Chem. A B 2002, 106, 2861–2866. [Google Scholar] [CrossRef]
- Matzelle, T.R.; Geuskens, G.; Kruse, N. Elastic properties of poly(n-isopropylacrylamide) and poly(acrylamide) hydrogels studied by scanning force microscopy. Macromolecules 2003, 36, 2926–2931. [Google Scholar] [CrossRef]
- Jhon, Y.K.; Bhat, R.R.; Jeong, C.; Rojas, O.J.; Szleifer, I.; Genzer, J. Salt-induced depression of lower critical solution temperature in a surface-grafted neutral thermoresponsive polymer. Macromol. Rapid Commun. 2006, 27, 697–701. [Google Scholar] [CrossRef]
- Alf, M.E.; Hatton, T.A.; Gleason, K.K. Novel N-isopropylacrylamide based polymer architecture for faster LCST transition kinetics. Polymer 2011, 52, 4429–4434. [Google Scholar] [CrossRef]
- Alf, M.E.; Hatton, T.A.; Gleason, K.K. Insights into thin, thermally responsive polymer layers through quartz crystal microbalance with dissipation. Langmuir 2011, 27, 10691–10698. [Google Scholar] [CrossRef]
- Yang, J.; Yamato, M.; Shimizu, T.; Sekine, H.; Ohashi, K.; Kanzaki, M.; Ohki, T.; Nishida, K.; Okano, T. Reconstruction of functional tissues with cell sheet engineering. Biomaterials 2007, 28, 5033–5043. [Google Scholar] [CrossRef]
- Asakawa, N.; Shimizu, T.; Tsuda, Y.; Sekiya, S.; Sasagawa, T.; Yamato, M.; Fukai, F.; Okano, T. Pre-vascularization of in vitro three-dimensional tissues created by cell sheet engineering. Biomaterials 2010, 31, 3903–3909. [Google Scholar] [CrossRef]
- Sasagawa, T.; Shimizu, T.; Sekiya, S.; Haraguchi, Y.; Yamato, M.; Sawa, Y.; Okano, T. Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials 2010, 31, 1646–1654. [Google Scholar] [CrossRef]
- Tsuda, Y.; Shimizu, T.; Yamato, M.; Kikuchi, A.; Sasagawa, T.; Sekiya, S.; Kobayashi, J.; Chen, G.; Okano, T. Cellular control of tissue architectures using a three-dimensional tissue fabrication technique. Biomaterials 2007, 28, 4939–4946. [Google Scholar] [CrossRef]
- Tsuda, Y.; Kikuchi, A.; Yamato, M.; Chen, G.; Okano, T. Heterotypic cell interactions on a dually patterned surface. Biochem. Biophys. Res. Commun. 2006, 348, 937–944. [Google Scholar] [CrossRef]
- Elloumi Hannachi, I.; Itoga, K.; Kumashiro, Y.; Kobayashi, J.; Yamato, M.; Okano, T. Fabrication of transferable micropatterned-co-cultured cell sheets with microcontact printing. Biomaterials 2009, 30, 5427–5432. [Google Scholar] [CrossRef]
- Jun, I.; Kim, S.J.; Lee, J.-H.; Lee, Y.J.; Shin, Y.M.; Choi, E.; Park, K.M.; Park, J.; Park, K.D.; Shin, H. Transfer printing of cell layers with an anisotropic extracellular matrix assembly using cell-interactive and thermosensitive hydrogels. Adv. Funct. Mater. 2012, 22, 4060–4069. [Google Scholar] [CrossRef]
- Yang, J.; Yamato, M.; Kohno, C.; Nishimoto, A.; Sekine, H.; Fukai, F.; Okano, T. Cell sheet engineering: Recreating tissues without biodegradable scaffolds. Biomaterials 2005, 26, 6415–6422. [Google Scholar] [CrossRef]
- Yamato, M.; Sjöqvist, S. Basic Considerations with cell sheets. In Tissue Engineering in Regenerative Medicine; Bernstein, H.S., Ed.; Humana Press: New York, NY, USA, 2011; pp. 143–160. [Google Scholar]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Hsiue, G.-H. Functional biomedical polymers for corneal regenerative medicine. React. Funct. Polym. 2007, 67, 1284–1291. [Google Scholar] [CrossRef]
- Götze, T.; Valtink, M.; Nitschke, M.; Gramm, S.; Hanke, T.; Engelmann, K.; Werner, C. Cultivation of an immortalized human corneal endothelial cell population and two distinct clonal subpopulations on thermo-responsive carriers. Graef. Arch. Clin. Exp. 2008, 246, 1575–1583. [Google Scholar] [CrossRef]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Maeda, N.; Watanabe, H.; Yamamoto, K.; Nagai, S.; Kikuchi, A.; Tano, Y.; et al. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation 2004, 77, 379–385. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Lu, P.-L.; Chen, K.-H.; Tabata, Y.; Hsiue, G.-H. Effect of charge and molecular weight on the functionality of gelatin carriers for corneal endothelial cell therapy. Biomacromolecules 2006, 7, 1836–1844. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Li, Y.-T. Functional assessment of cross-linked porous gelatin hydrogels for bioengineered cell sheet carriers. Biomacromolecules 2010, 11, 1387–1397. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Ma, D.H.-K.; Lai, M.-H.; Li, Y.-T.; Chang, R.-J.; Chen, L.-M. Characterization of cross-linked porous gelatin carriers and their interaction with corneal endothelium: Biopolymer concentration effect. PloS One 2013, 8, 1–12. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Teichmann, J.; Valtink, M.; Nitschke, M.; Gramm, S.; Funk, R.H.W.; Engelmann, K.; Werner, C. Tissue Engineering of the Corneal Endothelium: A Review of Carrier Materials. J. Funct. Biomater. 2013, 4, 178-208. https://doi.org/10.3390/jfb4040178
Teichmann J, Valtink M, Nitschke M, Gramm S, Funk RHW, Engelmann K, Werner C. Tissue Engineering of the Corneal Endothelium: A Review of Carrier Materials. Journal of Functional Biomaterials. 2013; 4(4):178-208. https://doi.org/10.3390/jfb4040178
Chicago/Turabian StyleTeichmann, Juliane, Monika Valtink, Mirko Nitschke, Stefan Gramm, Richard H.W. Funk, Katrin Engelmann, and Carsten Werner. 2013. "Tissue Engineering of the Corneal Endothelium: A Review of Carrier Materials" Journal of Functional Biomaterials 4, no. 4: 178-208. https://doi.org/10.3390/jfb4040178






