Consequences of Ultra-Violet Irradiation on the Mechanical Properties of Spider Silk
Abstract
:1. Introduction
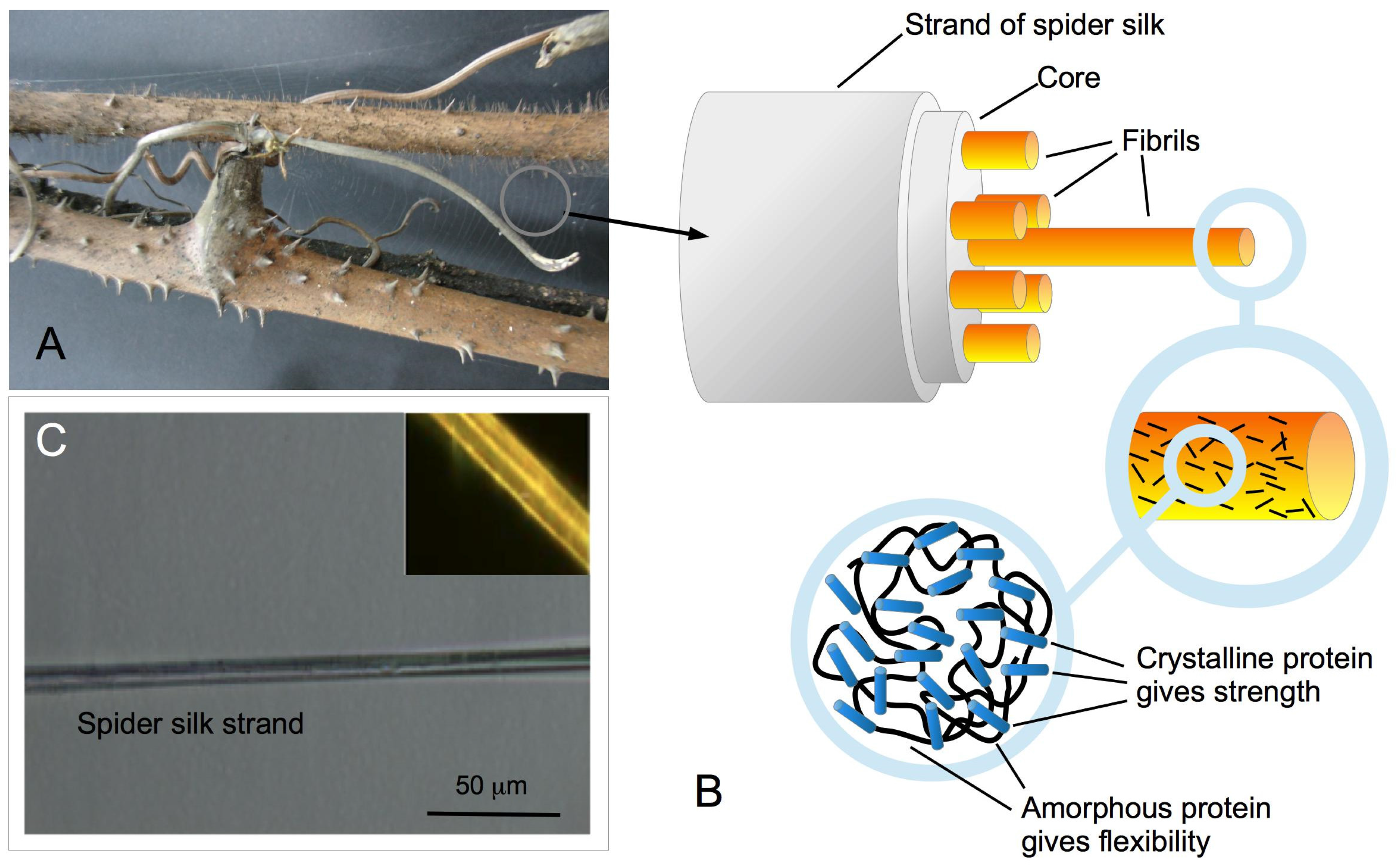
2. Results
2.1. Microscopy


2.2. Micro-Tensile Test


2.3. Biochemical Compositional Analyses

3. Discussion
3.1. Effects on the Mechanical Properties
3.2. Effects of UV on the Biochemical Composition
3.3. Implications on the Structure-Function Relationships

4. Experimental Methods
4.1. Ultra-Violet Irradiation of Spider Silks
| Specimen Number | Control (μm) | UV Treatment | ||
|---|---|---|---|---|
| 10 min (μm) | 20 min (μm) | 30 min (μm) | ||
| 1 | 17.8 | 6.9 | 6.1 | 6.5 |
| 2 | 6.5 | 4.0 | 7.3 | 10.5 |
| 3 | 4.6 | 7.5 | 11.2 | 7.3 |
| 4 | 10.4 | 9.8 | 11.2 | 8.5 |
| 5 | 6.8 | 7.0 | 7.2 | 10.2 |
| 6 | 3.3 | 10.3 | 7.6 | 6.2 |
| 7 | 4.5 | 9.2 | 31.3 | 9.6 |
| 8 | 3.6 | 9.3 | 16.2 | 12.6 |
| 9 | 5.8 | 9.8 | 11.9 | 12.8 |
| 10 | 7.2 | 11.4 | 5.5 | 8.0 |
4.2. Physical Examination and Chemical Characterization
4.3. Micro-Tensile Tests

4.4. Statistical Analysis
5. Conclusions
- The spider silk experiences decrease in fracture strength, extensibility, and fracture toughness with increased irradiation duration. In particular with regards to the rate of degradation, it is found that the strength of the spider silk degrades most rapidly; the extensibility of the spider silk degrades the slowest.
- UVC irradiation affects the surface morphology of the spider silks; small compact masses (attributing to fragments of the peptide chains) are observed to cover the surface, with interconnecting bridges.
- FT-IR spectroscopy reveals a shift in the peak position of a key functional group from a higher to a lower wavenumber (for UV irradiated spider silk), suggesting the occurrence of bond rupture within the peptide chains, which in turn implicates the diminution in the strength, extensibility, and fracture toughness.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gosline, J.M.; Guerette, P.A.; Ortlepp, C.S.; Savage, K.N. The mechanical design of spider silks: From fibroin sequence to mechanical function. J. Exp. Biol. 1999, 202, 3295–3303. [Google Scholar] [PubMed]
- Osaki, S. Spider silk violin strings with a unique packing structure generate a soft and profound timbre. Phys. Rev. Lett. 2012, 108. [Google Scholar] [CrossRef]
- Vollrath, F.; Knight, D.P. Liquid crystalline spinning of spider silk. Nature 2001, 410, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.K.; Kawabata, S.; Inoue, M.; Niwa, M.; Fossey, S.; Song, J.W. Engineering properties of spider silk. MRS Proc. 2001, 702. [Google Scholar] [CrossRef]
- Bourzac, K. Web of intrigue. Nature 2015, 519, S4–S6. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Wendt, H.; Hillmer, A.; Reimers, K.; Kuhbier, J.W.; Scha, F.; Allmeling, C.; Kasper, C.; Vogt, P.M. Artificial skin—Culturing of different skin cell lines for generating an artificial skin substitute on cross-weaved spider silk fibres. PLoS ONE 2011, 6, e21833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennecke, K.; Redeker, J.; Kuhbier, J.W.; Strauss, S.; Allmeling, C.; Kasper, C.; Reimers, K.; Vogt, P.M. Bundles of spider silk, braided into sutures, resist basic cyclic tests: Potential use for flexor tendon repair. PLoS ONE 2013, 8, e61100. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Wang, X.Y.; Kaplan, D.L.; Garlick, J.A.; Egles, C. Biofunctionalized electrospun silk mats as a topical bioactive dressing for accelerated wound healing. Acta Biomater. 2009, 5, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- Scheibel, T. Spider silks: Recombinant synthesis, assembly, spinning, and engineering of synthetic proteins. Microb. Cell Fact. 2004, 3, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.F.Y.; McGhie, A.J.; Tang, S.L. New internal structure of spider dragline silk revealed by atomic force microscopy. Biophys. J. 1994, 66, 1209–1212. [Google Scholar] [CrossRef]
- Buehler, M.J.; Keten, S.; Ackbarow, T. Theoretical and computational hierarchical nanomechanics of protein materials: Deformation and fracture. Prog. Mater. Sci. 2008, 53, 1101–1241. [Google Scholar] [CrossRef]
- Andrade, M.C.B. Female hunger can explain variation in cannibalistic behavior despite male sacrifice in redback spiders. Behav. Ecol. 1988, 9, 33–42. [Google Scholar] [CrossRef]
- Work, R.W.; Emerson, P.D. An apparatus and technique for the forcible silking of spiders. J. Arachnol. 1982, 10, 1–10. [Google Scholar]
- Perz-Rigueiro, J.; Elices, M.; Plaza, G.; Real, J.I.; Guinea, G.V. The effect of spinning forces on spider silk properties. J. Exp. Biol. 2005, 208, 2633–2639. [Google Scholar] [CrossRef] [PubMed]
- Ortlepp, C.S.; Gosline, J.M. Consequences of forced silking. Biomacromolecules 2004, 5, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Elices, M.; Pérez-Rigueiro, J.; Plaza, G.R.; Guinea, G.V. Finding inspiration in argiope trifasciata spider silk fibers. J. Miner. Met. Mater. Soc. 2005, 57, 60–66. [Google Scholar] [CrossRef]
- Goh, K.; Chen, S.; Liao, K. A thermomechanical framework for reconciling the effects of ultraviolet radiation exposure time and wavelength on connective tissue elasticity. Biomech. Model. Mechanobiol. 2014, 13, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P. Biomedical applications of photochemistry. Tissue Eng. Part B 2010, 16, 509–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermanson, K.D.; Huemmerich, D.; Scheibel, T.; Bausch, A.R. Engineered microcapsules fabricated from feconstituted spider silk. Adv. Mater. 2007, 19, 1810–1815. [Google Scholar] [CrossRef]
- Osaki, S. Ultraviolet rays mechanically strengthen spider’s silks. Polym. J. 2004, 36, 657–660. [Google Scholar] [CrossRef]
- Osaki, S. Spiders’ mechanical lifelines provide a key for the study of trust in the quality of materials. Polym. J. 2011, 43, 194–199. [Google Scholar] [CrossRef]
- Osaki, S. Effects of ultraviolet rays and temperature on spider silks. Acta Arachnol. 1997, 46, 1–4. [Google Scholar] [CrossRef]
- Kitagawa, M.; Kitayama, T. Mechanical properties of dragline and capture thread for the spider Nephila clavata. J. Mater. Sci. 1997, 32, 2005–2012. [Google Scholar] [CrossRef]
- Chirgadze, Y.N.; Fedorov, O.V.; Trushina, N.P. Estimation of amino acid residue side-chain absorption in the infrared spectra of protein solutions in heavy water. Biopolymers 1975, 14, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, M.E.; Cruz, D.H.; West, M.M.; Hitchcock, A.P.; Pézolet, M. Nephila clavipes spider dragline silk microstructure studied by scanning transmission X-ray microscopy. J. Am. Chem. Soc. 2007, 129, 3897–3905. [Google Scholar] [CrossRef] [PubMed]
- Singha, K.; Maity, S.; Singha, M. Spinning and applications of spider silk. Front. Sci. 2012, 2, 92–100. [Google Scholar] [CrossRef]
- Van Nimmen, E.; Gellynck, K.; van Langenhove, L. The tensile behaviour of spider silk. Autex Res. J. 2005, 5, 120–126. [Google Scholar]
- Chew, S.L.; Wang, K.; Chai, S.P.; Goh, K.L. Elasticity, thermal stability and bioactivity of polyhedral oligomeric silsesquioxanes reinforced chitosan-based microfibres. J. Mater. Sci. Mater. Med. 2011, 22, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.Z.; Hein, S.; Wang, K.; Liao, K.; Goh, K.L. Influence of hydroxyapatite crystallization temperature and concentration on stress transfer in wet-spun nanohydroxyapatite-chitosan composite fibres. Biomed. Mater. 2008, 3, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.L.; Listrat, A.; Béchet, D. Hierarchical mechanics of connective tissues: Integrating insights from nano to macroscopic studies. J. Biomed. Nanotechnol. 2014, 10, 2464–2507. [Google Scholar] [CrossRef]
- Chirgadze, Y.N.; Brazhnikov, E.V. Intensities and other spectral parameters of infrared amide bands of polypeptides in the α-helical form. Biopolymers 1974, 13, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Bramanti, E.; Catalano, D.; Forte, C.; Giovanneschi, M.; Masetti, M.; Veracini, A.C. Solid state 13C NMR and FT-IR spectroscopy of the cocoon silk of two common spiders. Spectrochim. Acta Part A 2005, 62, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Valluzzi, R.; Kaplan, D. Conformational transitions in model silk peptides. Biophys. J. 2000, 78, 2690–2701. [Google Scholar] [CrossRef]
- Semmrich, C.; Bausch, A.R. Protein crystals: How the weak become strong. Nat. Mater. 2010, 9, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Riekel, C. Comparative architecture of silks, fibrous proteins and their encoding genes in insects and spiders. Comp. Biochem. Physiol. Part B 2002, 133, 493–507. [Google Scholar] [CrossRef]
- Goh, K.L.; Chen, Y.; Chou, S.M.; Listrat, A.; Bechet, D.; Wess, T.J. Effects of frozen storage temperature on the elasticity of tendons from a small murine model. Animal 2010, 4, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.L.; Holmes, D.F.; Lu, H.Y.; Richardson, S.; Kadler, K.E.; Purslow, P.P.; Wess, T.J. Ageing changes in the tensile properties of tendons: Influence of collagen fibril volume. J. Biomech. Eng. 2008, 130. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.L.; Holmes, D.F.; Lu, Y.; Purslow, P.P.; Kadler, K.E.; Bechet, D.; Wess, T.J. Bimodal collagen fibril diameter distributions direct age-related variations in tendon resilience and resistance to rupture. J. Appl. Physiol. 2012, 113, 878–888. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, W.L.; Goh, K.L. Consequences of Ultra-Violet Irradiation on the Mechanical Properties of Spider Silk. J. Funct. Biomater. 2015, 6, 901-916. https://doi.org/10.3390/jfb6030901
Lai WL, Goh KL. Consequences of Ultra-Violet Irradiation on the Mechanical Properties of Spider Silk. Journal of Functional Biomaterials. 2015; 6(3):901-916. https://doi.org/10.3390/jfb6030901
Chicago/Turabian StyleLai, Wee Loong, and Kheng Lim Goh. 2015. "Consequences of Ultra-Violet Irradiation on the Mechanical Properties of Spider Silk" Journal of Functional Biomaterials 6, no. 3: 901-916. https://doi.org/10.3390/jfb6030901
APA StyleLai, W. L., & Goh, K. L. (2015). Consequences of Ultra-Violet Irradiation on the Mechanical Properties of Spider Silk. Journal of Functional Biomaterials, 6(3), 901-916. https://doi.org/10.3390/jfb6030901






