Implementation of Industrial Additive Manufacturing: Intelligent Implants and Drug Delivery Systems
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
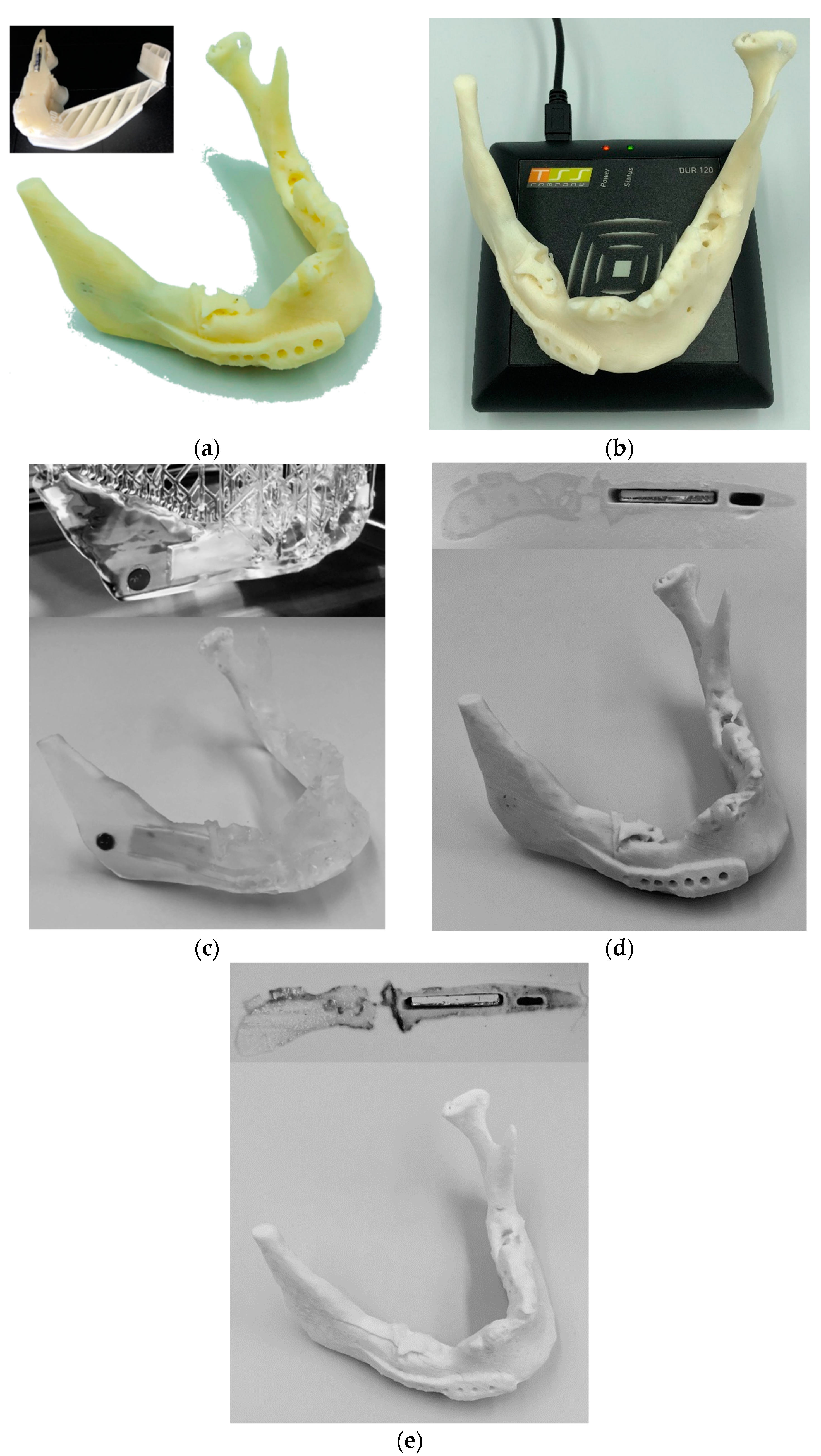
4.1. Mandible Implant
4.2. Parametric Drug Delivery System
4.3. Parametric RFID Systems
4.4. Additive Manufacturing
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ISO/TC 261; ASTM F42. ISO/ASTM 52900:2015(en) Additive Manufacturing–General principles–Terminology; ISO/ASME International: Geneva, Switzerland, 2015. [Google Scholar]
- Kruth, J.P. Material Incress Manufacturing by Rapid Prototyping Techniques. CIRP Ann. 1991, 40, 603–614. [Google Scholar] [CrossRef]
- Guo, N.; Leu, M.C. Additive manufacturing: Technology, applications and research needs. Front. Mech. Eng. 2013, 8, 215–243. [Google Scholar] [CrossRef]
- Attaran, M. The rise of 3-D printing: The advantages of additive manufacturing over traditional manufacturing. Bus. Horiz. 2017, 60, 677–688. [Google Scholar] [CrossRef]
- Tuomi, J.; Paloheimo, K.-S.; Vehviläinen, J.; Björkstrand, R.; Salmi, M. A Novel Classification and Online Platform for Planning and Documentation of Medical Applications of Additive Manufacturing. Surg. Innov. 2014, 21, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Tappa, K.; Jammalamadaka, U. Novel Biomaterials Used in Medical 3D Printing Techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-J.; Tseng, C.-S.; Hsu, S.; Tsai, C. Evaluation of chondrocyte growth in the highly porous scaffolds made by fused deposition manufacturing (FDM) filled with type II collagen. Biomed. Microdevices 2009, 11, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Zein, I.; Hutmacher, D.; Tan, K.; Teoh, S. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef]
- Mäkitie, A.A.; Korpela, J.; Elomaa, L.; Reivonen, M.; Kokkari, A.; Malin, M.; Korhonen, H.; Wang, X.; Salo, J.; Sihvo, E.; et al. Novel additive manufactured scaffolds for tissue engineered trachea research. Acta Oto-Laryngol. 2013, 133, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.M.; Borland, S.W.; Giordano, R.A.; Cima, L.G.; Sachs, E.M.; Cima, M.J. Solid free-form fabrication of drug delivery devices. J. Control. Release 1996, 40, 77–87. [Google Scholar] [CrossRef]
- Kim, S.S.; Utsunomiya, H.; Koski, J.A.; Wu, B.M.; Cima, M.J.; Sohn, J.; Mukai, K.; Griffith, L.G.; Vacanti, J.P. Survival and Function of Hepatocytes on a Novel Three-Dimensional Synthetic Biodegradable Polymer Scaffold With an Intrinsic Network of Channels. Ann. Surg. 1998, 228, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeltinger, J.; Sherwood, J.K.; Graham, D.A.; Mueller, R.; Griffith, L.G. Effect of Pore Size and Void Fraction on Cellular Adhesion, Proliferation, and Matrix Deposition. Tissue Eng. 2001, 7, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.; Mo, X.; Teoh, S.H.; Hutmacher, D.W. Scaffold development using 3D printing with a starch-based polymer. Mater. Sci. Eng. C 2002, 20, 49–56. [Google Scholar] [CrossRef]
- Seitz, H.; Rieder, W.; Irsen, S.; Leukers, B.; Tille, C. Three-Dimensional Printing of Porous Ceramic Scaffolds for Bone Tissue Engineering. J. Biomed. Mater. Res. 2005, 74B, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.N.; Wu, M.B. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-W.; Wang, S.; Fox, B.; Ritman, E.; Yaszemski, M.; Lu, L. Poly (propylene fumarate) bone tissue engineering scaffold fabrication using stereolithography: Effects of resin formulations and laser parameters. Biomacromolecules 2007, 8, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Formlabs Professional Materials for Digital Dentistry. 2018. Available online: https://formlabs.com/media/upload/DentalSG-DataSheet.pdf (accessed on 22 February 2018).
- Lohfeld, S.; Tyndyk, M.; Cahill, S.; Flaherty, N.; Barron, V.; McHugh, P.E. A method to fabricate small features on scaffolds for tissue engineering via selective laser sintering. J. Biomed. Sci. Eng. 2010, 3, 138–147. [Google Scholar] [CrossRef]
- Wiria, F.E.; Leong, K.F.; Chua, C.; Liu, Y. Poly-ε-caprolactone/hydroxyapatite for tissue engineering scaffold fabrication via selective laser sintering. Acta Biomater. 2007, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H.; Chua, C.K.; Leong, K.F.; Cheah, C.M.; Cheang, P.; Bakar, M.S.A.; Cha, S.W. Scaffold development using selective laser sintering of polyetheretherketone–hydroxyapatite biocomposite blends. Biomaterials 2003, 24, 3115–3123. [Google Scholar] [CrossRef]
- Yeong, W.Y.; Sudarmadji, N.; Yu, H.Y.; Chua, C.K.; Leong, K.F.; Venkatraman, S.S.; Boey, Y.C.F.; Tan, L.P. Porous polycaprolactone scaffold for cardiac tissue engineering fabricated by selective laser sintering. Acta Biomater. 2010, 6, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Chua, C.; Leong, K.; Cheah, C.; Gui, W.; Tan, W.; Wiria, F. Selective laser sintering of biocompatible polymers for applications in tissue engineering. Bio-Med. Mater. Eng. 2005, 15, 113–124. [Google Scholar]
- Chua, C.K.; Leong, K.F.; Tan, K.H.; Wiria, F.E.; Cheah, C.M. Development of tissue scaffolds using selective laser sintering of polyvinyl alcohol/hydroxyapatite biocomposite for craniofacial and joint defects. J. Mater. Sci. Mater. Med. 2004, 15, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Nickels, L. World’s first patient-specific jaw implant. Met. Powder Rep. 2012, 67, 12–14. [Google Scholar] [CrossRef]
- Tuomi, J.T.; Björkstrand, R.V.; Pernu, M.L.; Salmi, M.V.J.; Huotilainen, E.I.; Wolff, J.E.H.; Vallittu, P.K.; Mäkitie, A.A. In vitro cytotoxicity and surface topography evaluation of additive manufacturing titanium implant materials. J. Mater. Sci. Mater. Med. 2017, 28, 53. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.A. Drug Delivery; Nova Science Publishers, Inc.: Hauppauge NY, USA, 2011. [Google Scholar]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef] [PubMed]
- Langer, R. New Methods of Drug Delivery. Science 1990, 249, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.K.; MacBarb, R.F.; Wong, M.E.; Athanasiou, K.A. Temporomandibular Joint Disorders: A Review of Etiology, Clinical Management, and Tissue Engineering Strategies. Int. J. Oral Maxillofac. Implants 2013, 28, e393–e414. [Google Scholar] [CrossRef] [PubMed]
- Hieu, L.; Zlatov, N.; Sloten, J.V.; Bohez, E. Medical rapid prototyping applications and methods. Assem. Autom. 2005, 25, 284–292. [Google Scholar] [CrossRef]
- Chougule, V.; Mulay, A.; Ahuja, B.B. Three dimensional point cloud generations from CT scan images for bio-cad modeling. In Proceedings of the International Conference on Additive Manufacturing Technologies, Banglore, India, 16–17 September 2013. [Google Scholar]
- Salmi, M. Possibilities of preoperative medical models made by 3D printing or additive manufacturing. J. Med. Eng. 2016, 2016, 6191526. [Google Scholar] [CrossRef] [PubMed]
- Domdouzis, K.; Kumar, B.; Anumba, C. Radio-Frequency Identification (RFID) applications: A brief introduction. Adv. Eng. Inf. 2007, 21, 350–355. [Google Scholar] [CrossRef]
- Dobkin, D.M. The RF in RFID; Elsevier Science & Technology: Oxford, UK, 2007. [Google Scholar]
- Ngai, E.; Moon, K.K.; Riggins, F.J.; Yi, C.Y. RFID research: An academic literature review (1995–2005) and future research directions. Int. J. Prod. Econ. 2008, 112, 510–520. [Google Scholar] [CrossRef]
- Streit, S.; Bock, F.; Pirk, C.W.; Tautz, J. Automatic life-long monitoring of individual insect behaviour now possible. Zoology 2003, 106, 169–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wismans, W. Identification and registration of animals in the European Union. Comput. Electron. Agric. 1999, 24, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Artmann, R. Electronic identification systems: State of the art and their further development. Comput. Electron. Agric. 1999, 24, 5–26. [Google Scholar] [CrossRef]
- Jones, P.; Clarke-Hill, C.; Comfort, D.; Hillier, D.; Shears, P. Radio frequency identification and food retailing in the UK. Br. Food J. 2005, 107, 356–360. [Google Scholar] [CrossRef]
- Venkatesan, M.; Grauer, Z. Leveraging radio frequency identification (RFID) technology to improve laboratory information management. Am. Lab. 2004, 36, 11–14. [Google Scholar]
- Harmon, C.K. RFID Standards: Opening a World of Possibilities; International Organization for Standardization: Genève, Switzerland, 2010. [Google Scholar]
- Nath, B.; Reynolds, F.; Want, R. RFID Technology and Applications. IEEE Pervasive Comput. 2006, 5, 22–24. [Google Scholar] [CrossRef]








| ISO/ASTM Additive Manufacturing Method | Compatible Biomaterials | Source |
|---|---|---|
| Material Extrusion (ME) | Polycaprolactone (PCL) Polylactide (PLA) Polycarbonate (PC) Polyvinyl alcohol (PVA) Polylactic-co-glycolic acid (PLGA) | [6,7,8,9] |
| Binder Jetting (BJ) | Poly--caprolactone Polylactide-coglycolide Poly-l-lactic acid Peptides Proteins (e.g., fibrinogen, collagen) Polysaccharides (e.g., hyaluronan, alginate) DNA plasmids Living cells | [10,11,12,13,14,15] |
| Vat Photopolymerization (VP) | Polypropylene fumarate (PPF) Polycaprolactone (PCL) Dental SG Resin | [9,16,17] |
| Powder Bed Fusion (PBF) | PCL Polyether ether ketone (PEEK) PVA + Hydroxyapatite (HA) PEEK + HA Calcium phosphate Titanium TiAL6V4 ELI | [18,19,20,21,22,23,24,25] |
| ISO/ASTM AM Method | Embedded ISO HF RFID Data Communicated [Yes/No] | Embedded ISO UHF RFID Data Communicated [Yes/No] |
|---|---|---|
| ME | Yes | Yes |
| VP | Yes | Yes |
| BJ | Yes | Yes |
| PBF | Yes | Yes |
| ISO/ASTM AM Method | Machine | Software | Material | Layer Thickness | Printing Time | Other Parameters | Post Processing |
|---|---|---|---|---|---|---|---|
| ME | Stratasys Uprint SE Plus | GrabCAD Print | ABS+ | 0.254 mm | 8 h 44 min | Model interior fill: solid Model support fill: SMART | Manual and dissolvable support removal |
| VP | Formlabs Form 2 | Preform | Clear Resin V4 | 0.1 mm | 6 h 17 min | Support density: 1.00 Support point size: 0.60 mm | Manual support removal |
| BJ | 3D Systems Zprinter 450 | ZPrint | ZP 150 | 0.1 mm | 4 h 22 min | Bleed compensation: on | Compressed air depowdering and drizzle infiltration |
| PBF | Academic Printer | RepliSLS3D | PP | 0.2 mm | 2 h 54 min | Laser power: 16.5 W Scan speed: 2250 mm/s Hatch distance: 0.4 mm Energy density: 0.092 J/mm3 | Compressed air depowdering |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akmal, J.S.; Salmi, M.; Mäkitie, A.; Björkstrand, R.; Partanen, J. Implementation of Industrial Additive Manufacturing: Intelligent Implants and Drug Delivery Systems. J. Funct. Biomater. 2018, 9, 41. https://doi.org/10.3390/jfb9030041
Akmal JS, Salmi M, Mäkitie A, Björkstrand R, Partanen J. Implementation of Industrial Additive Manufacturing: Intelligent Implants and Drug Delivery Systems. Journal of Functional Biomaterials. 2018; 9(3):41. https://doi.org/10.3390/jfb9030041
Chicago/Turabian StyleAkmal, Jan Sher, Mika Salmi, Antti Mäkitie, Roy Björkstrand, and Jouni Partanen. 2018. "Implementation of Industrial Additive Manufacturing: Intelligent Implants and Drug Delivery Systems" Journal of Functional Biomaterials 9, no. 3: 41. https://doi.org/10.3390/jfb9030041
APA StyleAkmal, J. S., Salmi, M., Mäkitie, A., Björkstrand, R., & Partanen, J. (2018). Implementation of Industrial Additive Manufacturing: Intelligent Implants and Drug Delivery Systems. Journal of Functional Biomaterials, 9(3), 41. https://doi.org/10.3390/jfb9030041








