ToxTracker Reporter Cell Lines as a Tool for Mechanism-Based (Geno)Toxicity Screening of Nanoparticles—Metals, Oxides and Quantum Dots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles
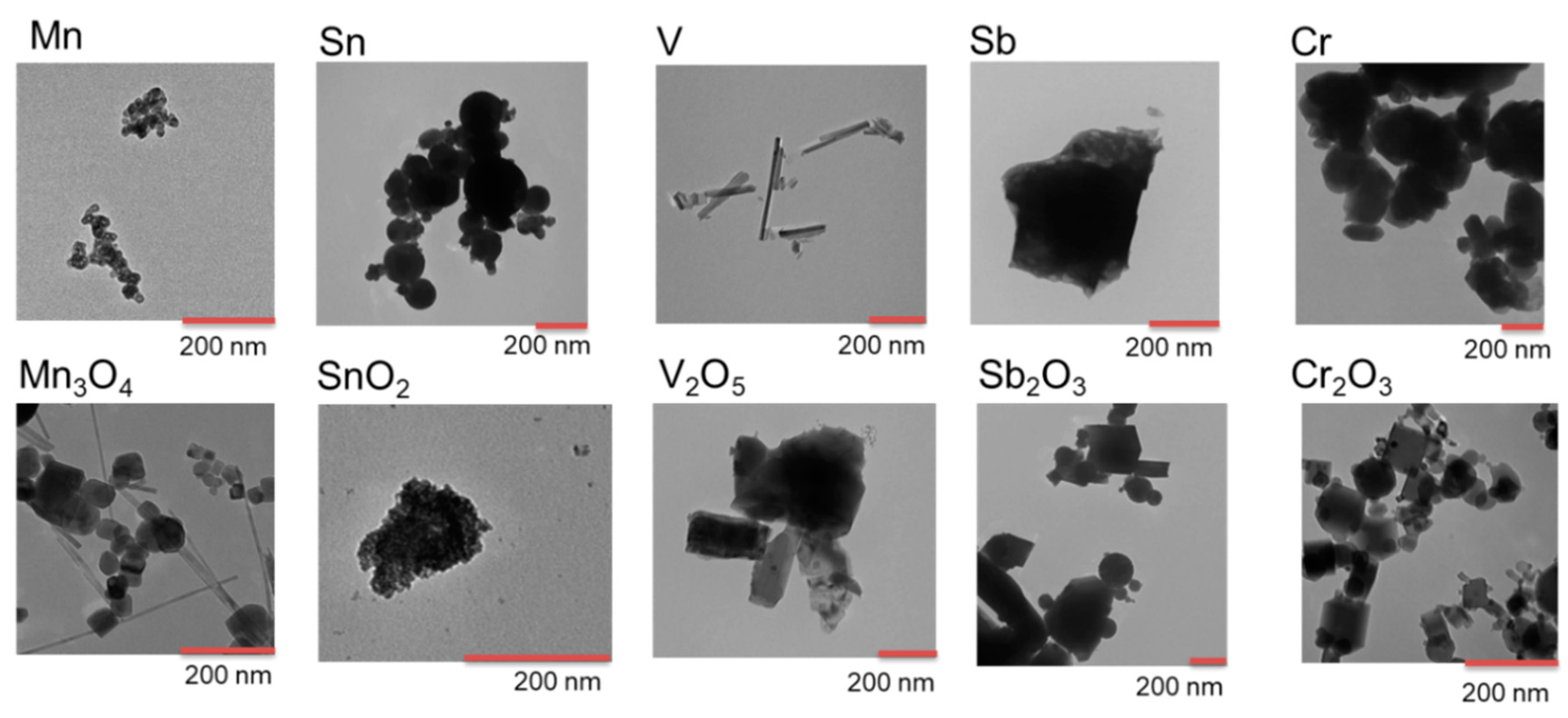
2.2. Particle Morphology
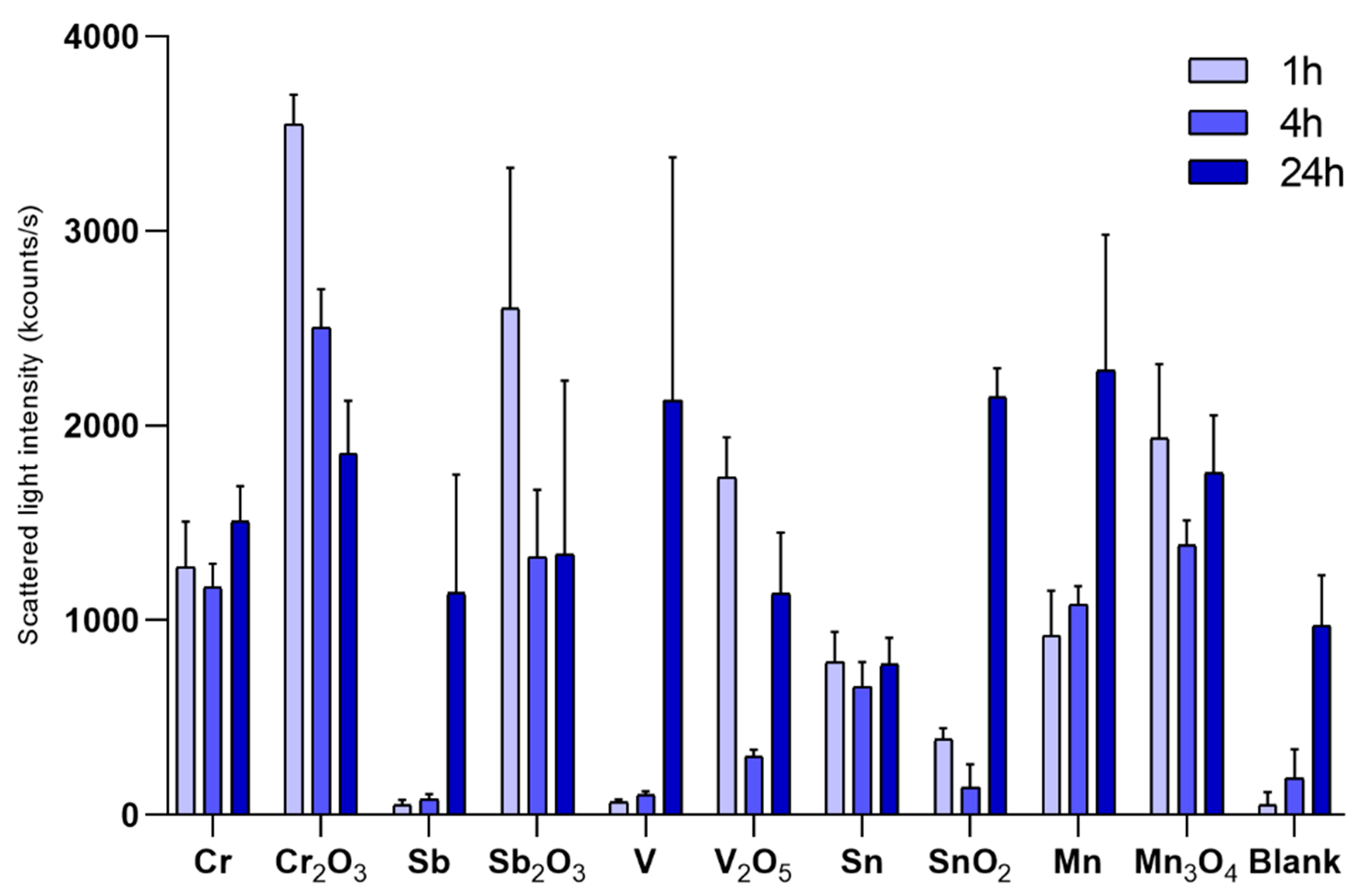
2.3. Characterization of Particle Agglomeration
2.4. Cell Culture and Reagents
2.5. ToxTracker Assay (mES Cells)
2.6. Dose Metric Modelling Analysis
2.7. Statistical Analysis
3. Results
3.1. Particle Characterization
3.2. Nontoxic NPs—Several NPs Cause No Toxicity or ToxTracker Activation
3.3. Cytotoxicity—Several NPs and QDs Affect Cell Viability
3.4. Oxidative Stress—Many NPs and All QDs Induce Reporters Related to Oxidative Stress
3.5. DNA Damage—Few NPs Induce Reporters Related to DNA Damage
3.6. Protein Unfolding and p53 Related Stress—Few NPs and QDs Induce These Reporters
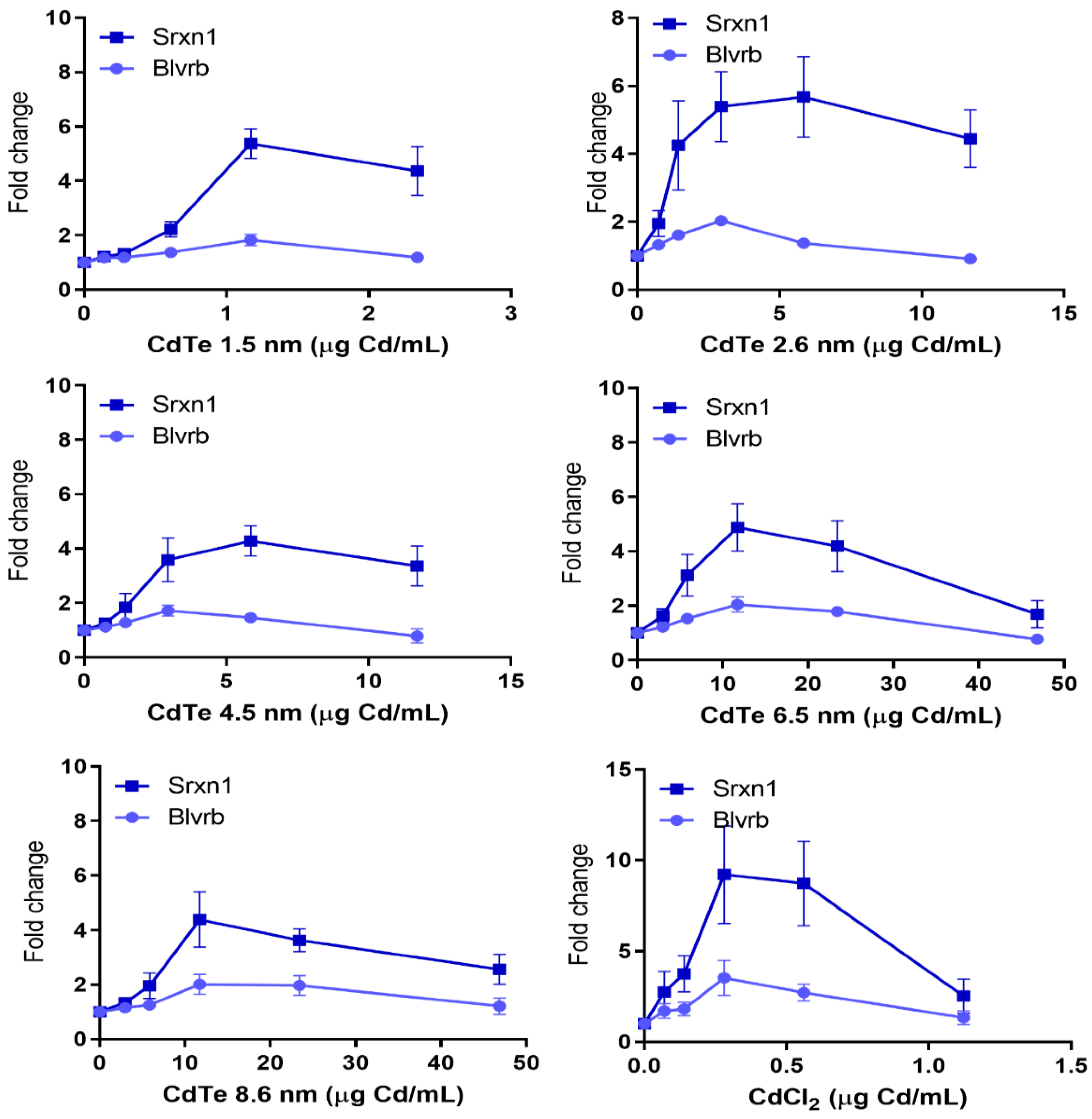
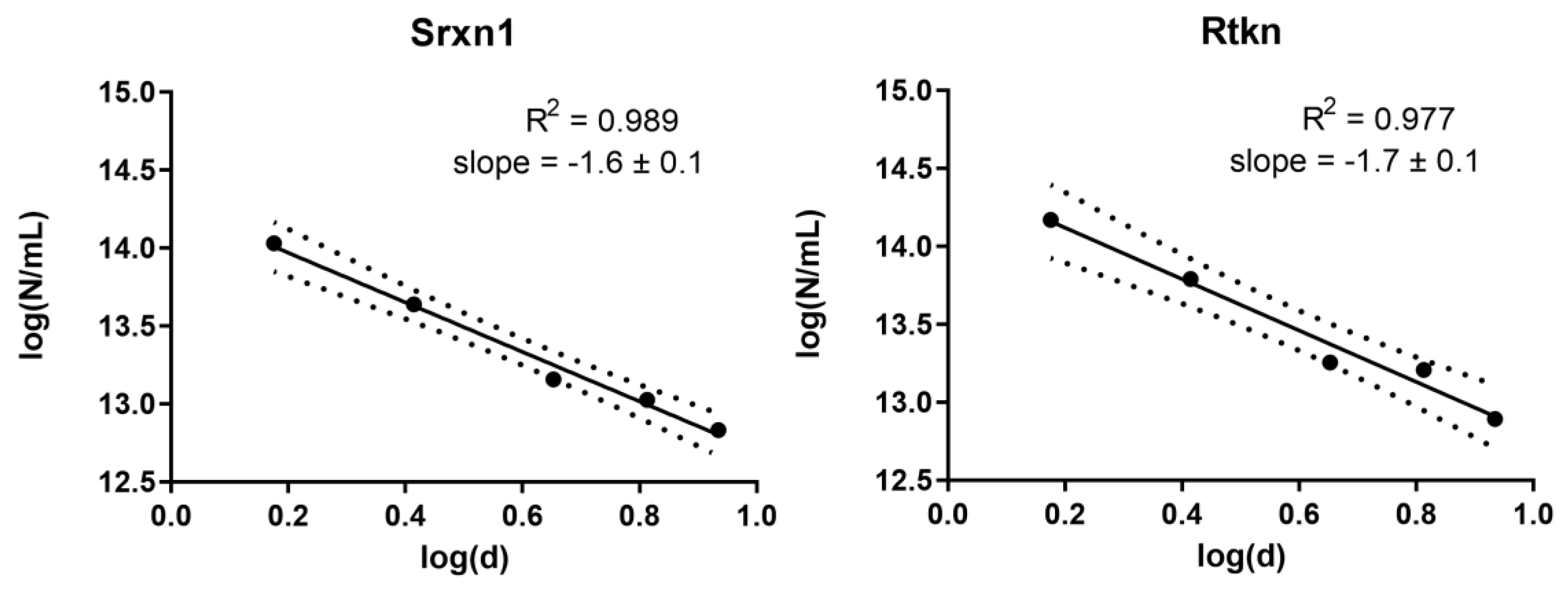
3.7. Dose Metric: Surface Area Appears to be the Most Suitable Dose Metric for CdTe QD Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References and Notes
- Karlsson, H.L. The comet assay in nanotoxicology research. Anal. Bioanal. Chem. 2010, 398, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.; Sanderson, B.J.; Kirsch-Volders, M. Adaptations of the in vitro MN assay for the genotoxicity assessment of nanomaterials. Mutagenesis 2011, 26, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Magdolenova, Z.; Collins, A.; Kumar, A.; Dhawan, A.; Stone, V.; Dusinska, M. Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology 2014, 8, 233–278. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Meng, H.; Wang, X.; Lin, S.; Ji, Z.; Zhang, H. Nanomaterial toxicity testing in the 21st century: Use of a predictive toxicological approach and high-throughput screening. Acc. Chem. Res. 2013, 46, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.C.; Wright, C.W.; Ibuki, Y.; Moreno-Villanueva, M.; Karlsson, H.L.; Hendriks, G.; Sims, C.M.; Singh, N.; Doak, S.H. Emerging metrology for high-throughput nanomaterial genotoxicology. Mutagenesis 2017, 32, 215–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hastwell, P.W.; Chai, L.L.; Roberts, K.J.; Webster, T.W.; Harvey, J.S.; Rees, R.W.; Walmsley, R.M. High-specificity and high-sensitivity genotoxicity assessment in a human cell line: Validation of the GreenScreen HC GADD45a-GFP genotoxicity assay. Mutat. Res. 2006, 607, 160–175. [Google Scholar] [CrossRef]
- Hughes, C.; Rabinowitz, A.; Tate, M.; Birrell, L.; Allsup, J.; Billinton, N.; Walmsley, R.M. Development of a high-throughput Gaussia luciferase reporter assay for the activation of the GADD45a gene by mutagens, promutagens, clastogens, and aneugens. J. Biomol. Screen. 2012, 17, 1302–1315. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, G.; Derr, R.S.; Misovic, B.; Morolli, B.; Calleja, F.M.; Vrieling, H. The extended ToxTracker assay discriminates between induction of DNA damage, oxidative stress, and protein misfolding. Toxicol. Sci. 2016, 150, 190–203. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, G.; Atallah, M.; Morolli, B.; Calleja, F.; Ras-Verloop, N.; Huijskens, I.; Raamsman, M.; van de Water, B.; Vrieling, H. The ToxTracker assay: Novel GFP reporter systems that provide mechanistic insight into the genotoxic properties of chemicals. Toxicol. Sci. 2012, 125, 285–298. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Gliga, A.R.; Calléja, F.M.; Gonçalves, C.S.; Odnevall Wallinder, I.; Vrieling, H.; Fadeel, B.; Hendriks, G. Mechanism-based genotoxicity screening of metal oxide nanoparticles using the ToxTracker panel of reporter cell lines. Part. Fibre Toxicol. 2014, 11, 41. [Google Scholar] [CrossRef] [Green Version]
- Akerlund, E.; Cappellini, F.; Di Bucchianico, S.; Islam, S.; Skoglund, S.; Derr, R.; Odnevall Wallinder, I.; Hendriks, G.; Karlsson, H.L. Genotoxic and mutagenic properties of Ni and NiO nanoparticles investigated by comet assay, gamma-H2AX staining, Hprt mutation assay and ToxTracker reporter cell lines. Environ. Mol. Mutagenesis 2018, 59, 211–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappellini, F.; Hedberg, Y.; McCarrick, S.; Hedberg, J.; Derr, R.; Hendriks, G.; Odnevall Wallinder, I.; Karlsson, H.L. Mechanistic insight into reactivity and (geno)toxicity of well-characterized nanoparticles of cobalt metal and oxides. Nanotoxicology 2018, 12, 602–620. [Google Scholar] [CrossRef]
- McCarrick, S.; Wei, Z.; Moelijker, N.; Derr, R.; Persson, K.-A.; Hendriks, G.; Odnevall Wallinder, I.; Hedberg, Y.; Karlsson, H.L. High variability in toxicity of welding fume nanoparticles from stainless steel in lung cells and reporter cell lines: The role of particle reactivity and solubility. Nanotoxicology 2019, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial applications of nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klebowski, B.; Depciuch, J.; Parlinska-Wojtan, M.; Baran, J. Applications of noble metal-based nanoparticles in medicine. Int. J. Mol. Sci. 2018, 19, 4031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [Green Version]
- Rzigalinski, B.A.; Strobl, J.S. Cadmium-containing nanoparticles: Perspectives on pharmacology and toxicology of quantum dots. Toxicol. Appl. Pharmacol. 2009, 238, 280–288. [Google Scholar] [CrossRef] [Green Version]
- Hardman, R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef]
- Wang, F.; Shu, L.; Wang, J.; Pan, X.; Huang, R.; Lin, Y.; Cai, X. Perspectives on the toxicology of cadmium-based quantum dots. Curr. Drug Metab. 2013, 14, 847–856. [Google Scholar] [CrossRef]
- Lovric, J.; Bazzi, H.S.; Cuie, Y.; Fortin, G.R.; Winnik, F.M.; Maysinger, D. Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. J. Mol. Med. 2005, 83, 377–385. [Google Scholar] [CrossRef]
- Lovric, J.; Cho, S.J.; Winnik, F.M.; Maysinger, D. Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chem. Biol. 2005, 12, 1227–1234. [Google Scholar] [CrossRef] [Green Version]
- Golbamaki, N.; Rasulev, B.; Cassano, A.; Marchese Robinson, R.L.; Benfenati, E.; Leszczynski, J.; Cronin, M.T. Genotoxicity of metal oxide nanomaterials: Review of recent data and discussion of possible mechanisms. Nanoscale 2015, 7, 2154–2198. [Google Scholar] [CrossRef] [PubMed]
- Lebedova, J.; Hedberg, Y.S.; Odnevall Wallinder, I.; Karlsson, H.L. Size-dependent genotoxicity of silver, gold and platinum nanoparticles studied using the mini-gel comet assay and micronucleus scoring with flow cytometry. Mutagenesis 2018, 33, 77–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delmaar, C.J.; Peijnenburg, W.J.; Oomen, A.G.; Chen, J.; de Jong, W.H.; Sips, A.J.; Wang, Z.; Park, M.V. A practical approach to determine dose metrics for nanomaterials. Environ. Toxicol. Chem. 2015, 34, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Zahn, D.; Buciuman, F.; Patcas, F.; Craciun, R. Vibrational spectroscopy of bulk and supported manganese oxides. Phys. Chem. Chem. Phys. 1999, 1, 185–190. [Google Scholar]
- Hedberg, Y.S.; Pradhan, S.; Cappellini, F.; Karlsson, M.E.; Blomberg, E.; Karlsson, H.L.; Odnevall Wallinder, I.; Hedberg, J.F. Electrochemical surface oxide characteristics of metal nanoparticles (Mn, Cu and Al) and the relation to toxicity. Electrochim. Acta 2016, 212, 360–371. [Google Scholar] [CrossRef] [Green Version]
- Lin, A.W.C.; Armstrong, N.R.; Kuwana, T. X-ray photoelectron/Auger electron spectroscopic studies of tin and indium metal foils and oxides. Anal. Chem. 1977, 49, 1228–1235. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron. Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- ICDD. JCPDS no 35-732.
- ICDD. JCPDS card No. 11-689.
- Pereira, A.L.J.; Gracia, L.; Santamaría-Pérez, D.; Vilaplana, R.; Manjón, F.J.; Errandonea, D.; Nalin, M.; Beltrán, A. Structural and vibrational study of cubic Sb2O3 under high pressure. Phys. Rev. B 2012, 85, 174108. [Google Scholar] [CrossRef] [Green Version]
- Pérez, O.E.L.; Sánchez, M.D.; López Teijelo, M. Characterization of growth of anodic antimony oxide films by ellipsometry and XPS. J. Electroanal. Chem. 2010, 645, 143–148. [Google Scholar] [CrossRef]
- Spanier, J.E.; Robinson, R.D.; Zhang, F.; Chan, S.-W.; Herman, I.P. Size-dependent properties of CeO 2-y nanoparticles as studied by Raman scattering. Phys. Rev. B 2001, 64, 245407. [Google Scholar] [CrossRef] [Green Version]
- Limbach, L.K.; Wick, P.; Manser, P.; Grass, R.N.; Bruinink, A.; Stark, W.J. Exposure of engineered nanoparticles to human lung epithelial cells: Influence of chemical composition and catalytic activity on oxidative stress. Environ. Sci. Technol. 2007, 41, 4158–4163. [Google Scholar] [CrossRef] [PubMed]
- Urner, M.; Schlicker, A.; Z’Graggen, B.R.; Stepuk, A.; Booy, C.; Buehler, K.P.; Limbach, L.; Chmiel, C.; Stark, W.J.; Beck-Schimmer, B. Inflammatory response of lung macrophages and epithelial cells after exposure to redox active nanoparticles: Effect of solubility and antioxidant treatment. Environ. Sci. Technol. 2014, 48, 13960–13968. [Google Scholar] [CrossRef] [Green Version]
- Ivask, A.; Titma, T.; Visnapuu, M.; Vija, H.; Kakinen, A.; Sihtmae, M.; Pokhrel, S.; Madler, L.; Heinlaan, M.; Kisand, V.; et al. Toxicity of 11 metal oxide nanoparticles to three mammalian cell types in vitro. Curr. Top. Med. Chem. 2015, 15, 1914–1929. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, D.; Alkahtani, S. Oxidative stress-induced DNA damage by manganese dioxide nanoparticles in human neuronal cells. Biomed. Res. Int. 2017, 2017, 5478790. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.P.; Kumari, M.; Kumari, S.I.; Rahman, M.F.; Mahboob, M.; Grover, P. Toxicity assessment of manganese oxide micro and nanoparticles in Wistar rats after 28 days of repeated oral exposure. J. Appl. Toxicol. 2013, 33, 1165–1179. [Google Scholar] [CrossRef]
- Cao, Y.; Long, J.; Liu, L.; He, T.; Jiang, L.; Zhao, C.; Li, Z. A review of endoplasmic reticulum (ER) stress and nanoparticle (NP) exposure. Life Sci. 2017, 186, 33–42. [Google Scholar] [CrossRef]
- Simard, J.-C.; Durocher, I.; Girard, D. Silver nanoparticles induce irremediable endoplasmic reticulum stress leading to unfolded protein response dependent apoptosis in breast cancer cells. Apoptosis 2016, 21, 1279–1290. [Google Scholar] [CrossRef]
- Noel, C.; Simard, J.C.; Girard, D. Gold nanoparticles induce apoptosis, endoplasmic reticulum stress events and cleavage of cytoskeletal proteins in human neutrophils. Toxicol. Vitr. 2016, 31, 12–22. [Google Scholar] [CrossRef]
- Christen, V.; Fent, K. Silica nanoparticles induce endoplasmic reticulum stress response and activate mitogen activated kinase (MAPK) signalling. Toxicol. Rep. 2016, 3, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Huo, L.; Shi, X.; Bai, R.; Zhang, Z.; Zhao, Y.; Chang, Y.; Chen, C. Endoplasmic reticulum stress induced by zinc oxide nanoparticles is an earlier biomarker for nanotoxicological evaluation. ACS Nano 2014, 8, 2562–2574. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.S.; Lee, H.; Son, J.H. Manganese induces endoplasmic reticulum (ER) stress and activates multiple caspases in nigral dopaminergic neuronal cells, SN4741. Neurosci. Lett. 2001, 316, 5–8. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, Y.M.; Wu, D.D.; Yao, Y.; Liang, Z.L.; Tan, H.W.; Lau, A.T.Y. Acute and chronic cadmium telluride quantum dots-exposed human bronchial epithelial cells: The effects of particle sizes on their cytotoxicity and carcinogenicity. Biochem. Biophys. Res. Commun. 2018, 495, 899–903. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Kong, L.; Xue, Y.; Tang, M. Threshold dose of three types of quantum dots (QDs) induces oxidative stress triggers DNA damage and apoptosis in mouse fibroblast L929 cells. Int. J. Environ. Res. Public Health 2015, 12, 13435–13454. [Google Scholar] [CrossRef]
- Nguyen, K.C.; Willmore, W.G.; Tayabali, A.F. Cadmium telluride quantum dots cause oxidative stress leading to extrinsic and intrinsic apoptosis in hepatocellular carcinoma HepG2 cells. Toxicology 2013, 306, 114–123. [Google Scholar] [CrossRef]
- Katubi, K.M.; Alzahrani, F.M.; Ali, D.; Alarifi, S. Dose- and duration-dependent cytotoxicity and genotoxicity in human hepato carcinoma cells due to CdTe QDs exposure. Hum. Exp. Toxicol. 2019, 38, 914–926. [Google Scholar] [CrossRef]
- Jiang, S.; Lin, Y.; Yao, H.; Yang, C.; Zhang, L.; Luo, B.; Lei, Z.; Cao, L.; Lin, N.; Liu, X.; et al. The role of unfolded protein response and ER-phagy in quantum dots-induced nephrotoxicity: An in vitro and in vivo study. Arch. Toxicol. 2018, 92, 1421–1434. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, Y.; Qin, H.; Liu, K.; Guo, M.; Ge, Y.; Xu, M.; Sun, Y.; Zheng, X. Cytotoxicity of CdTe quantum dots in human umbilical vein endothelial cells: The involvement of cellular uptake and induction of pro-apoptotic endoplasmic reticulum stress. Int. J. Nanomed. 2016, 11, 529–542. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.C.; Rippstein, P.; Tayabali, A.F.; Willmore, W.G. Mitochondrial toxicity of cadmium telluride quantum dot nanoparticles in mammalian hepatocytes. Toxicol. Sci. 2015, 146, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.J.; Maysinger, D.; Jain, M.; Röder, B.; Hackbarth, S.; Winnik, F.M. Long-term exposure to CdTe quantum dots causes functional impairments in live cells. Langmuir 2007, 23, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Pfuhler, S.; Elespuru, R.; Aardema, M.J.; Doak, S.H.; Maria Donner, E.; Honma, M.; Kirsch-Volders, M.; Landsiedel, R.; Manjanatha, M.; Singer, T.; et al. Genotoxicity of nanomaterials: Refining strategies and tests for hazard identification. Environ. Mol. Mutagenesis 2013, 54, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Di Bucchianico, S.; Collins, A.R.; Dusinska, M. Can the comet assay be used reliably to detect nanoparticle-induced genotoxicity? Environ. Mol. Mutagenesis 2015, 56, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Doak, S.H.; Yan, J.; Chen, D.H.; Zhou, M.; Mittelstaedt, R.A.; Chen, Y.; Li, C.; Chen, T. Factors affecting the in vitro micronucleus assay for evaluation of nanomaterials. Mutagenesis 2017, 32, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Biological Damage | Cellular Pathway | Biomarker Gene |
|---|---|---|
| Oxidative stress | NRF2 antioxidant response | Srxn1 |
| NRF2 independent | Blvrb | |
| DNA damage | NF-kB signaling | Rtkn |
| ATR/Chk1 DNA damage signaling | Bscl2 | |
| Protein damage | Unfolded protein response | Ddit3 |
| Cellular stress | P53 signaling | Btg2 |
| Particle | Primary Size (TEM)/nm | Oxide Composition |
|---|---|---|
| Ag | Spherical, 5.2 ± 1.1 a | N/A |
| Au | Spherical, 5.2 ± 0.9 a | N/A |
| CdTe QDs | Spherical, 1.6, 2.6, 4.5, 6.5, 8.6 b | N/A |
| Cr | Cubic and spherical, 10–115 | Cr */Cr2O3 |
| Cr2O3 | Oval, 90–460 | Cr2O3 |
| Mn | Spherical, 15–50 | Mn */MnO/MnO2/Mn2O3/Mn3O4 |
| Mn3O4 | Cubic 20–180, rods 8000 × 400 | Mn3O4/MnO |
| Pt | Spherical, 4.8 ± 0.8 a | N/A |
| Sb | Irregular, 90–1200 | Sb */Sb2O3 |
| Sb2O3 | Spherical, 70–250 | Sb2O3 |
| Sn | Spherical, 20–250 | Sn */SnO/SnO2 |
| SnO2 | Spherical, 2–3 (as aggregates) | SnO2 |
| V | Rods 20 × 20 to 400 × 40 | V2O5/VO2 |
| V2O5 | Irregular 40–450 | V2O5/VO2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCarrick, S.; Cappellini, F.; Kessler, A.; Moelijker, N.; Derr, R.; Hedberg, J.; Wold, S.; Blomberg, E.; Odnevall Wallinder, I.; Hendriks, G.; et al. ToxTracker Reporter Cell Lines as a Tool for Mechanism-Based (Geno)Toxicity Screening of Nanoparticles—Metals, Oxides and Quantum Dots. Nanomaterials 2020, 10, 110. https://doi.org/10.3390/nano10010110
McCarrick S, Cappellini F, Kessler A, Moelijker N, Derr R, Hedberg J, Wold S, Blomberg E, Odnevall Wallinder I, Hendriks G, et al. ToxTracker Reporter Cell Lines as a Tool for Mechanism-Based (Geno)Toxicity Screening of Nanoparticles—Metals, Oxides and Quantum Dots. Nanomaterials. 2020; 10(1):110. https://doi.org/10.3390/nano10010110
Chicago/Turabian StyleMcCarrick, Sarah, Francesca Cappellini, Amanda Kessler, Nynke Moelijker, Remco Derr, Jonas Hedberg, Susanna Wold, Eva Blomberg, Inger Odnevall Wallinder, Giel Hendriks, and et al. 2020. "ToxTracker Reporter Cell Lines as a Tool for Mechanism-Based (Geno)Toxicity Screening of Nanoparticles—Metals, Oxides and Quantum Dots" Nanomaterials 10, no. 1: 110. https://doi.org/10.3390/nano10010110
APA StyleMcCarrick, S., Cappellini, F., Kessler, A., Moelijker, N., Derr, R., Hedberg, J., Wold, S., Blomberg, E., Odnevall Wallinder, I., Hendriks, G., & Karlsson, H. L. (2020). ToxTracker Reporter Cell Lines as a Tool for Mechanism-Based (Geno)Toxicity Screening of Nanoparticles—Metals, Oxides and Quantum Dots. Nanomaterials, 10(1), 110. https://doi.org/10.3390/nano10010110





