Effect of Nano-Sized Heat Transfer Enhancers on PCM-Based Heat Sink Performance at Various Heat Loads
Abstract
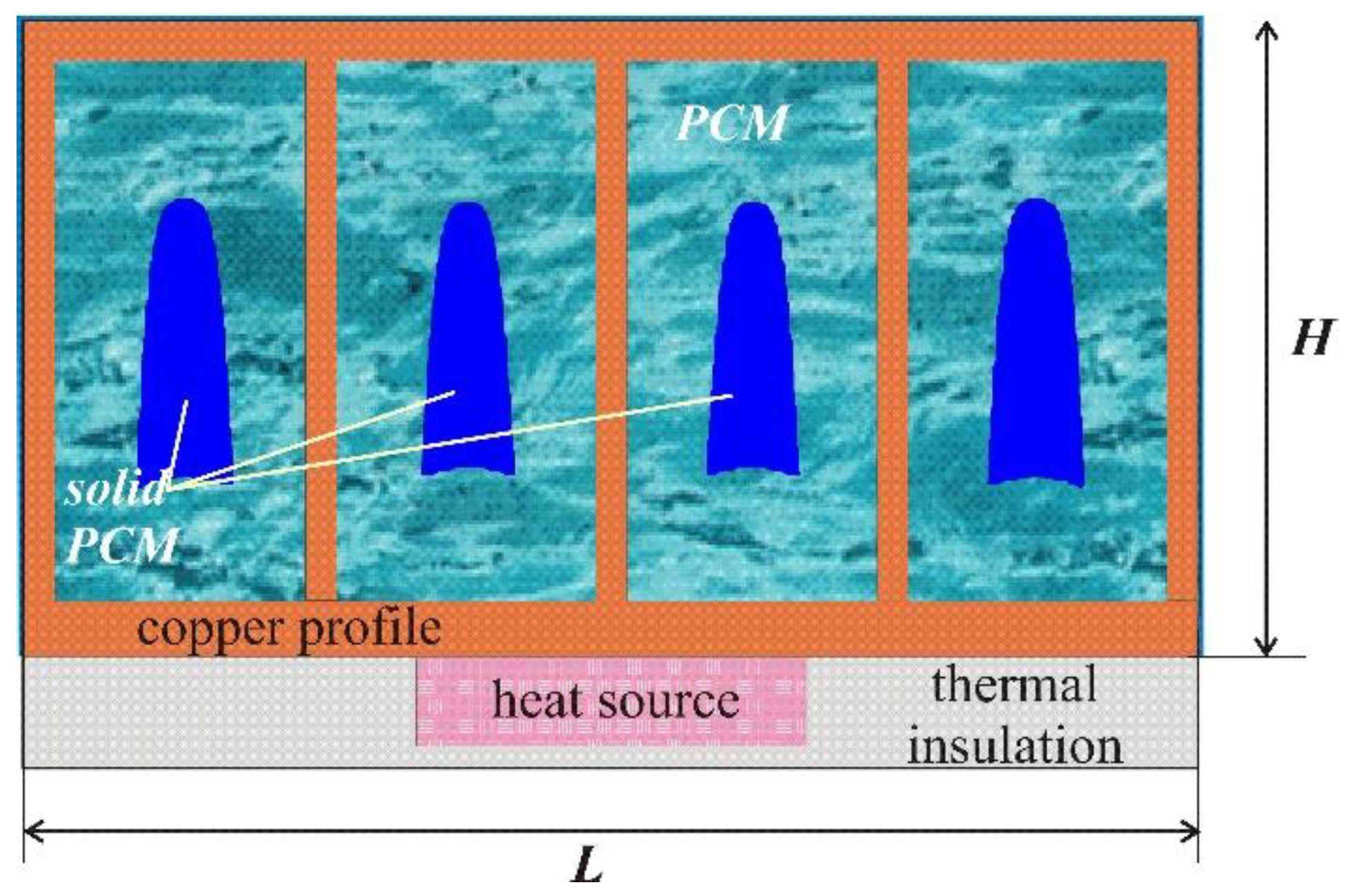
:1. Introduction
2. Mathematical Model
- Rayleigh number—;
- Prandtl number—;
- Stefan number—, characterizing the latent heat effect;
- Ostrogradsky number—, characterizing the volumetric heat flux effect;
- Biot number—, characterizing the intensity of external cooling.
- τ = 0: Θ = Θout, ψ = 0, Ω = 0;
- X = 0 and X = 2, 0 ≤ Y ≤ 1: and ;
- 0 ≤ X ≤ 0.6 and 1.4 ≤ X ≤ 2, Y = 0: ;
- 0.6 ≤ X ≤ 1.4, Y = 0: ;
- 0 ≤ X ≤ 2, Y = 1: ;
- at the profile surface: ;
- at the solid-liquid interface: Θ = 0;
- at X = 0.6 and X = 1.4, −0.2 ≤ Y ≤ 0: ;
- at 0.6 ≤ X ≤ 1.4, Y = −0.2: .
3. Thermophysical Properties of NePCM
- Using the experimental data [43], a correlation for the thermal conductivity was obtained taking into account the Brownian diffusion influence:In this relation, for thermal conductivity of NePCM, we have [43] and as the Boltzmann constant, while r(T,Φ) can be defined as:The first term in correlation for (kl)nm and for (ks)nm is the Maxwell model. The second term was determined experimentally by Vajjha for liquids with the addition of Al2O3 nanoparticles at a concentration range Φ ≤ 10% and this term determines the Brownian motion of nanoparticles [43]. Since the particle motion also depends on the diameter of nanoparticles and temperature, the considered model includes the functions r(T,Φ) and βλ, which depend on these parameters.
- Dynamic viscosity [33]
- The coefficient of thermal volume expansion
- Volumetric heat capacity
- Density
- Heat capacity
- Latent heat
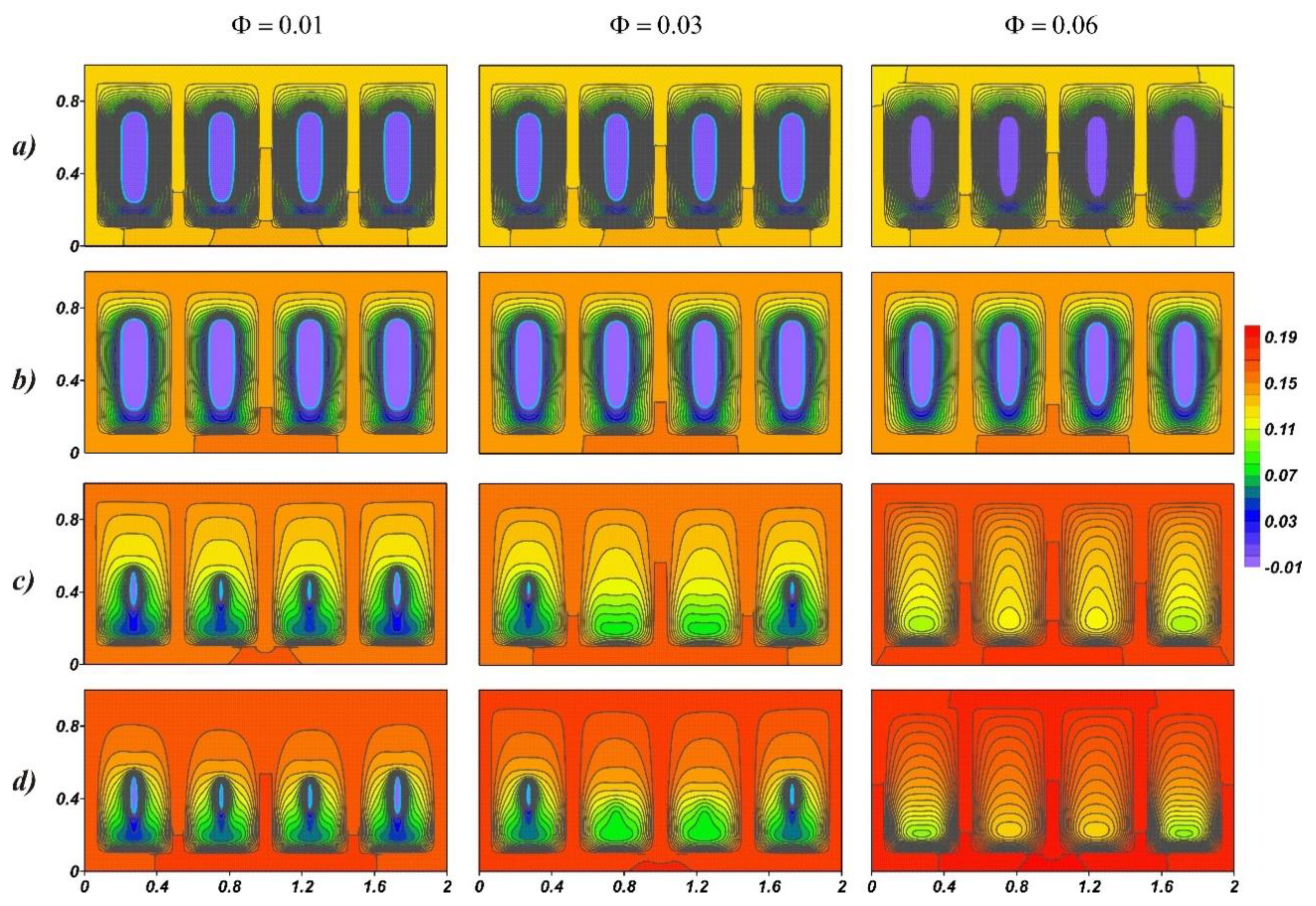
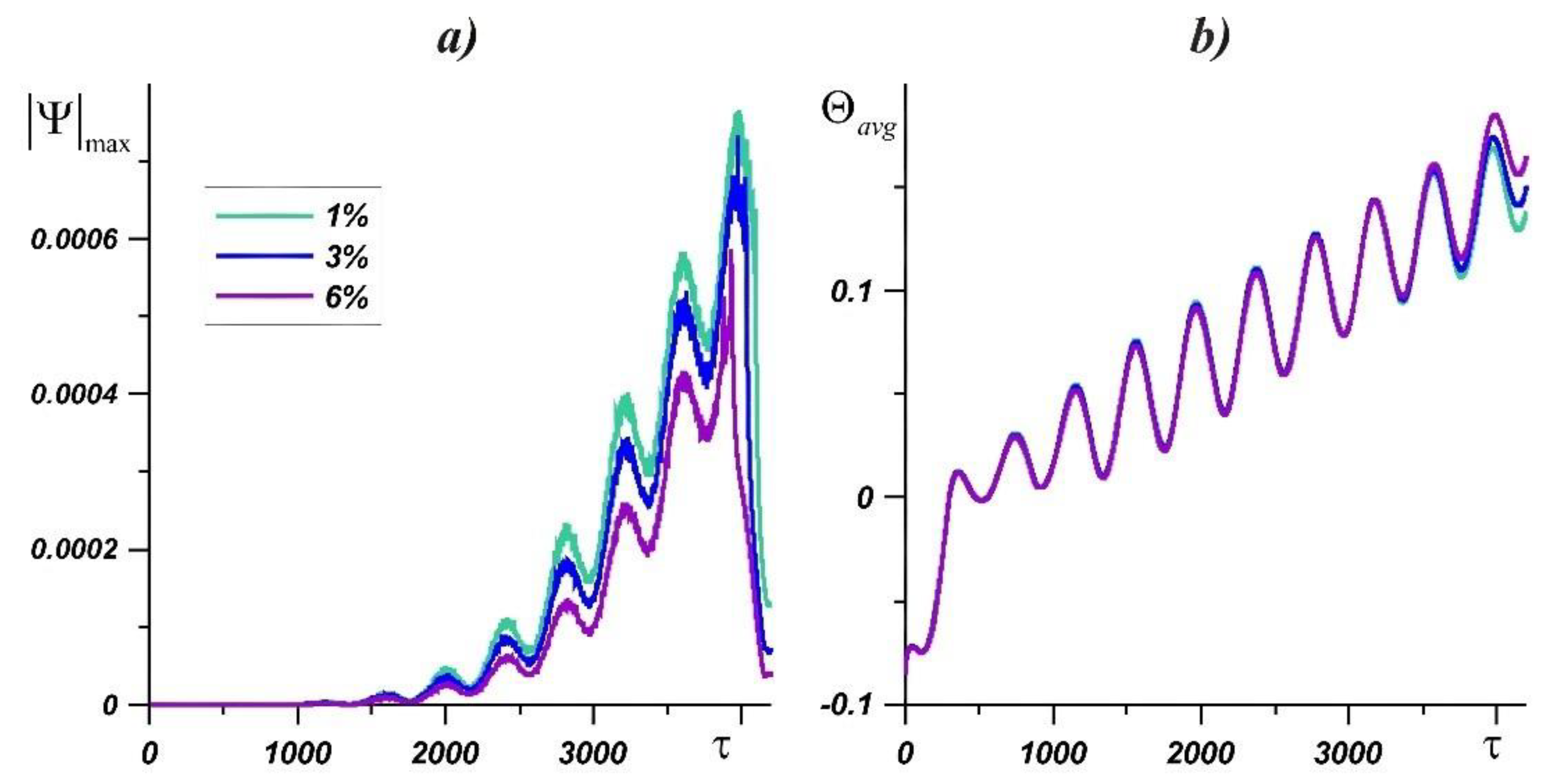
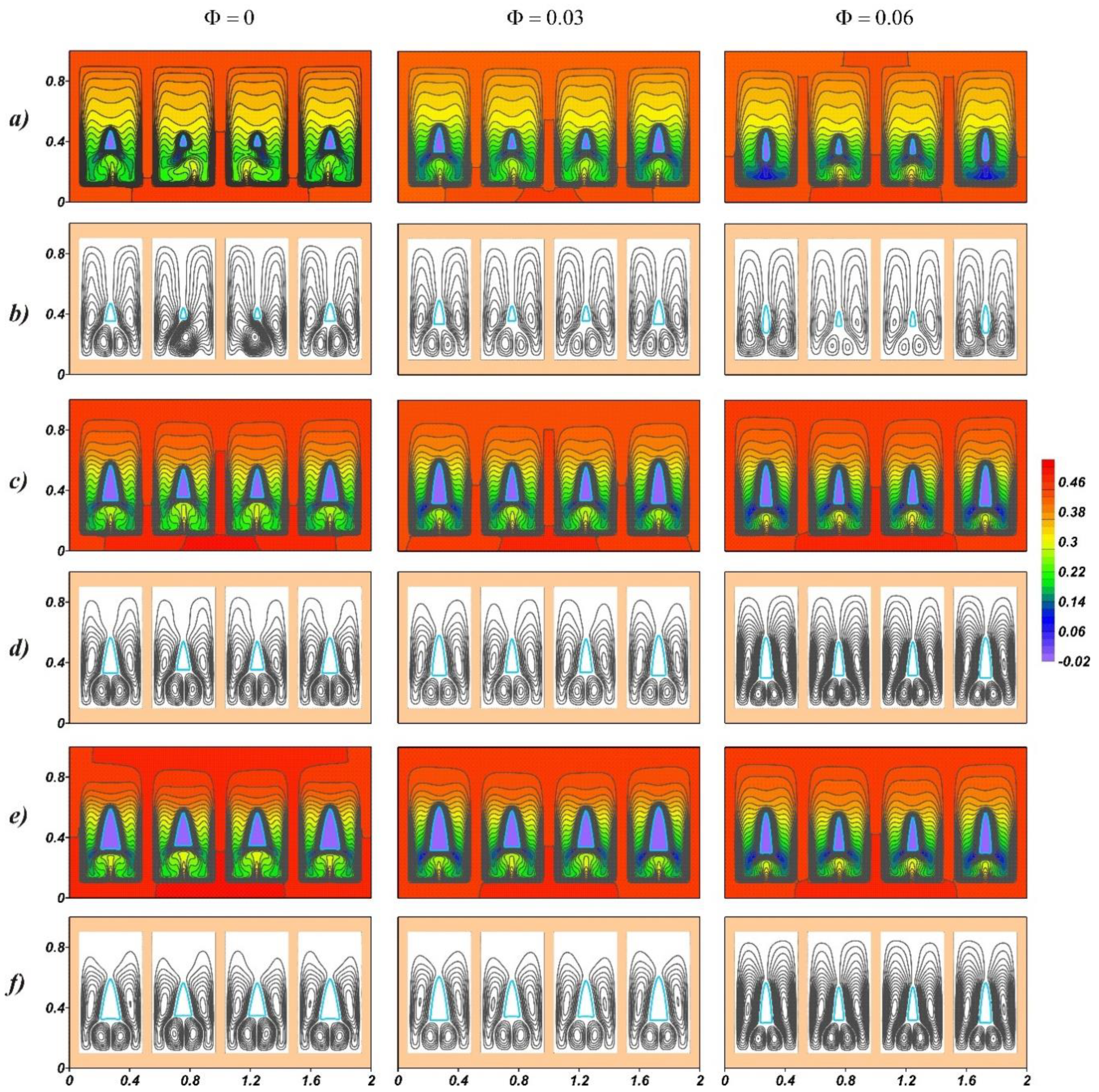
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| b | dimensional volumetric heat flux oscillation frequency, s−1 |
| Bi | the Biot number |
| c | specific heat, JK−1 kg−1 |
| d | diameter, m |
| f | non-dimensional volumetric heat flux oscillation frequency |
| g | gravitational acceleration, ms−2 |
| H | cavity height, m |
| h | specific enthalpy, Jkg−1 |
| k | thermal conductivity, WK−1m−1 |
| L | cavity length, m |
| Lm | fusion energy or latent heat of melting, Jkg−1 |
| normal to the surface | |
| Os | Ostrogradsky number |
| p | pressure, Pa |
| Pr | Prandtl number |
| Q | heat transfer rate per unit of volume, W m−3 |
| Ra | Rayleigh number |
| Ste | Stefan number |
| t | time, s |
| T | temperature, K |
| Tm | melting temperature |
| u, v | velocity components in Cartesian coordinate along x and y, ms−1 |
| U, V | dimensionless velocity components |
| x, y | Cartesian coordinate, m |
| X, Y | dimensionless Cartesian coordinates |
| Greek symbols | |
| α | thermal diffusivity, m2 s−1 |
| β | coefficient of thermal expansion, K−1 |
| γ | heat transfer coefficient, WK−1m−2 |
| η | smoothing parameter (or melting temperature range), K |
| Θ | dimensionless temperature |
| μ | dynamic viscosity, Pa s |
| ν | kinematic viscosity, m2 s−1 |
| ρ | density, kgm−3 |
| τ | dimensionless time |
| Φ | nanoparticles volume fraction |
| φ | volume fraction of the melt |
| ψ | stream function, m2 s−1 |
| Ψ | dimensionless stream function |
| ω | vorticity, s−1 |
| Ω | dimensionless vorticity |
| Subscripts | |
| 0 | initial condition or ambient |
| 1 | radiator |
| 2 | heater |
| l | liquid |
| m | melting |
| nm | nanomaterial |
| np | nanoparticle |
| s | solid |
References
- Gharbi, S.; Harmand, S.; Jabrallah, S.B. Experimental comparison between different configurations of PCM based heat sinks for cooling electronic components. Appl. Therm. Eng. 2015, 87, 454–462. [Google Scholar] [CrossRef]
- Walsh, E.; Walsh, P.; Grimes, R.; Egan, V. Thermal management of low profile electronic equipment using radial fans and heat sinks. J. Heat Transf. 2008, 130. [Google Scholar] [CrossRef]
- Baby, R.; Balaji, C. Experimental investigations on thermal performance enhancement and effect of orientation on porous matrix filled PCM based heat sink. Int. Commun. Heat Mass Transf. 2013, 46, 27–30. [Google Scholar] [CrossRef]
- Li, Z.-W.; Lv, L.-C.; Li, J. Combination of heat storage and thermal spreading for high power portable electronics cooling. Int. J. Heat Mass Transf. 2016, 98, 550–557. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, Z.; Ghafoor, A. A review of performance enhancement of PCM based latent heat storage system within the context of materials, thermal stability and compatibility. Energy Convers. Manag. 2016, 115, 132–158. [Google Scholar] [CrossRef]
- Abdelrahman, H.E.; Wahba, M.H.; Refaey, H.A.; Moawad, M.; Berbish, N.S. Performance enhancement of photovoltaic cells by changing configuration and using PCM (RT35HC) with nanoparticles Al2O3. Sol. Energy 2019, 177, 665–671. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, Z.A.; Sewell, P. Heat transfer evaluation of metal oxides based nano-PCMs for latent heat storage system application. Int. J. Heat Mass Transf. 2019, 144. [Google Scholar] [CrossRef]
- Salem, M.R.; Elsayed, M.M.; Abd-Elaziz, A.A.; Elshazly, K.M. Performance enhancement of the photovoltaic cells using Al2O3/PCM mixture and/or water cooling-techniques. Renew. Energy 2019, 138, 876–890. [Google Scholar] [CrossRef]
- Motahar, S.; Nikkam, N.; Alemrajabi, A.A.; Khodabandeh, R.; Toprak, M.S.; Muhammed, M. A novel phase change material containing mesoporous silica nanoparticles for thermal storage: A study on thermal conductivity and viscosity. Int. Commun. Heat Mass Transf. 2014, 56, 114–120. [Google Scholar] [CrossRef]
- Warzoha, R.J.; Weigand, R.M.; Fleischer, A.S. Temperature-dependent thermal properties of a paraffin phase change material embedded with herringbone style graphite nanofibers. Appl. Energy 2015, 137, 716–725. [Google Scholar] [CrossRef]
- Ho, C.J.; Gao, J.Y. Preparation and thermophysical properties of nanoparticle-in-paraffin emulsion as phase change material. Int. Commun. Heat Mass Transf. 2009, 36, 467–470. [Google Scholar] [CrossRef]
- Saydam, V.; Duan, X. Dispersing different nanoparticles in paraffin wax as enhanced phase change materials. J. Therm. Anal. Calorim. 2019, 135, 1135–1144. [Google Scholar] [CrossRef]
- Nourani, M.; Hamdami, N.; Keramat, J.; Moheb, A.; Shahedi, M. Thermal behavior of paraffin-nano-Al2O3 stabilized by sodium stearoyl lactylate as a stable phase change material with high thermal conductivity. Renew. Energy 2016, 88, 474–482. [Google Scholar] [CrossRef]
- Choi, D.H.; Lee, J.; Hong, H.; Kang, Y.T. Thermal conductivity and heat transfer performance enhancement of phase change materials (PCM) containing carbon additives for heat storage application. Int. J. Refrig. 2014, 42, 112–120. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Pendyala, R.; Marneni, N. Stability of Nanofluids. In Engineering Applications of Nanotechnology. Topics in Mining, Metallurgy and Materials Engineering; Korada, V., Hisham, B., Hamid, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Che Sidik, N.A.; Jamil, M.M.; Japar, W.M.A.A.; Adamua, I.M. A review on preparation methods, stability and applications of hybrid nanofluids. Renew. Sustain. Energy Rev. 2017, 80, 1112–1122. [Google Scholar] [CrossRef]
- Xian, H.W.; Che Sidik, N.A.; Saidur, R. Impact of different surfactants and ultrasonication time on the stability and thermophysical properties of hybrid nanofluids. Int. Commun. Heat Mass Transf. 2020, 110. [Google Scholar] [CrossRef]
- Li, D.; Fang, W.; Feng, Y.; Geng, Q.; Song, M. Stability properties of water-based gold and silver nanofluids stabilized by cationic gemini surfactants. J. Taiwan Ins. Chem. Eng. 2019, 97, 458–465. [Google Scholar] [CrossRef]
- Zhao, M.; Lv, W.; Li, Y.; Dai, C.; Zhou, H.; Song, X.; Wu, Y. A Study on preparation and stabilizing mechanism of hydrophobic silica nanofluids. Materials 2018, 11, 1385. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Luo, J.; Song, G.; Liu, Y.; Tang, G. The experimental exploration of nano-Si3N4/paraffin on thermal behavior of phase change materials. Thermochim. Acta 2014, 597, 101–106. [Google Scholar] [CrossRef]
- Motahar, S.; Nikkamb, N.; Alemrajabi, A.A.; Khodabandeh, R.; Toprak, M.S.; Muhammed, M. Experimental investigation on thermal and rheological properties of n-octadecane with dispersed TiO2 nanoparticles. Int. Commun. Heat Mass Transf. 2014, 59, 68–74. [Google Scholar] [CrossRef]
- Kumar, K.R.S.; Dinesha, R.; Roseline, A.A.; Kalaiselvam, S. Performance analysis of heat pipe aided NEPCM heat sink for transient electronic cooling. Microelectron. Reliab. 2017, 73, 1–13. [Google Scholar] [CrossRef]
- Khodadadi, J.M.; Hosseinizadeh, S.F. Nanoparticle-enhanced phase change materials (NEPCM) with great potential for improved thermal energy storage. Int. Commun. Heat Mass Transf. 2007, 34, 534–543. [Google Scholar] [CrossRef]
- John, M.R.W.; Mamidi, T.; Subendran, S.; Subramanian, L.R.G. Experimental investigation of low-temperature latent heat thermal energy storage system using PCM and NEPCM. IOP Conf. Ser. Mater. Sci. Eng. 2018, 402. [Google Scholar] [CrossRef]
- Barreneche, C.; Martín, M.; Calvo-de la Rosa, J.; Majó, M.; Fernández, A.I. Own-synthetize nanoparticles to develop nano-enhanced phase change materials (NEPCM) to improve the energy efficiency in buildings. Molecules 2019, 24, 1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.; Villalba, A.; Fernández, A.I.; Barreneche, C. Development of new nano-enhanced phase change materials (NEPCM) to improve energy efficiency in buildings: Lab-scale characterization. Energy Build. 2019, 192, 75–83. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Smith, S.; Xu, J.; Yu, X. Review of R&D progress and practical application of the solar photovoltaic/thermal (PV/T) technologies. Renew. Sustain. Energy Rev. 2012, 16, 599–617. [Google Scholar]
- Bakan, G.; Gerislioglu, B.; Dirisaglik, F.; Jurado, Z.; Sullivan, L.; Dana, A.; Lam, C.; Gokirmak, A.; Silva, H. Extracting the temperature distribution on a phase-change memory cell during crystallization. J. Appl. Phys. 2016, 120. [Google Scholar] [CrossRef] [Green Version]
- Ding, F.; Yang, Y.; Bozhevolnyi, S.I. Dynamic metasurfaces using phase-change chalcogenides. Adv. Opt. Mater. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Gerislioglu, B.; Ahmadivand, A. The role of electron transfer in the nonlinear response of Ge2Sb2Te5-mediated plasmonic dimers. Photonics 2019, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Gerislioglu, B.; Ahmadivand, A.; Karabiyik, M.; Sinha, R.; Pala, N. Reconfigurable antennae: VO2-based reconfigurable antenna platform with addressable microheater matrix. Adv. Electron. Mater. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Aguila, B.; Vasco, D.A.; Galvez, P.; Zapata, P.A. Effect of temperature and CuO-nanoparticle concentration on the thermal conductivity and viscosity of an organic phase-change material. Int. J. Heat Mass Transf. 2018, 120, 1009–1019. [Google Scholar] [CrossRef]
- Bayat, M.; Faridzadeh, M.R.; Toghraie, D. Investigation of finned heat sink performance with nano enhanced phase change material (NePCM). Therm. Sci. Eng. Prog. 2018, 5, 50–59. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K.; Namburu, P.K. Numerical study of fluid dynamic and heat transfer performance of Al2O3 and CuO nanofluids in the flat tubes of a radiator. Int. J. Heat Fluid Flow 2010, 31, 613–621. [Google Scholar] [CrossRef]
- Bondareva, N.S.; Buonomo, B.; Manca, O.; Sheremet, M.A. Performance of the finned nano-enhanced phase change material system under the inclination influence. Int. J. Heat Mass Transf. 2019, 135, 1063–1072. [Google Scholar] [CrossRef]
- Bondareva, N.S.; Sheremet, M.A. Numerical investigation of the two-dimensional natural convection inside the system based on phase change material with a source of volumetric heat generation. Thermophys. Aeromech. 2018, 25, 525–537. [Google Scholar] [CrossRef]
- Yang, Y.-T.; Wang, Y.-H. Numerical simulation of three-dimensional transient cooling application on a portable electronic device using phase change material. Int. J. Therm. Sci. 2012, 51, 155–162. [Google Scholar] [CrossRef]
- Bondareva, N.S.; Sheremet, M.A. Conjugate heat transfer in the PCM-based heat storage system with finned copper profile: Application in electronics cooling. Int. J. Heat Mass Transf. 2018, 124, 1275–1284. [Google Scholar] [CrossRef]
- Bondareva, N.S.; Sheremet, M.A. Effect of inclined magnetic field on natural convection melting in a square cavity with a local heat source. J. Magn. Magn. Mater. 2016, 419, 476–484. [Google Scholar] [CrossRef]
- Gau, C.; Viskanta, R. Melting and solidification of a pure metal on a vertical wall. J. Heat Transf. 1986, 108, 174–181. [Google Scholar] [CrossRef]
- Gong, Z.X.; Mujumdar, A.S. Flow and heat transfer in convection-dominated melting in a rectangular cavity heated from below. Int. J. Heat Mass Transf. 1997, 41, 2573–2580. [Google Scholar] [CrossRef]
- Casano, G.; Piva, S. Experimental and numerical investigation of a phase change energy storage system. J. Phys. Conf. Ser. 2014, 501. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K. Experimental determination of thermal conductivity of three nanofluids and development of new correlations. Int. J. Heat Mass Transf. 2009, 52, 4675–4682. [Google Scholar] [CrossRef]

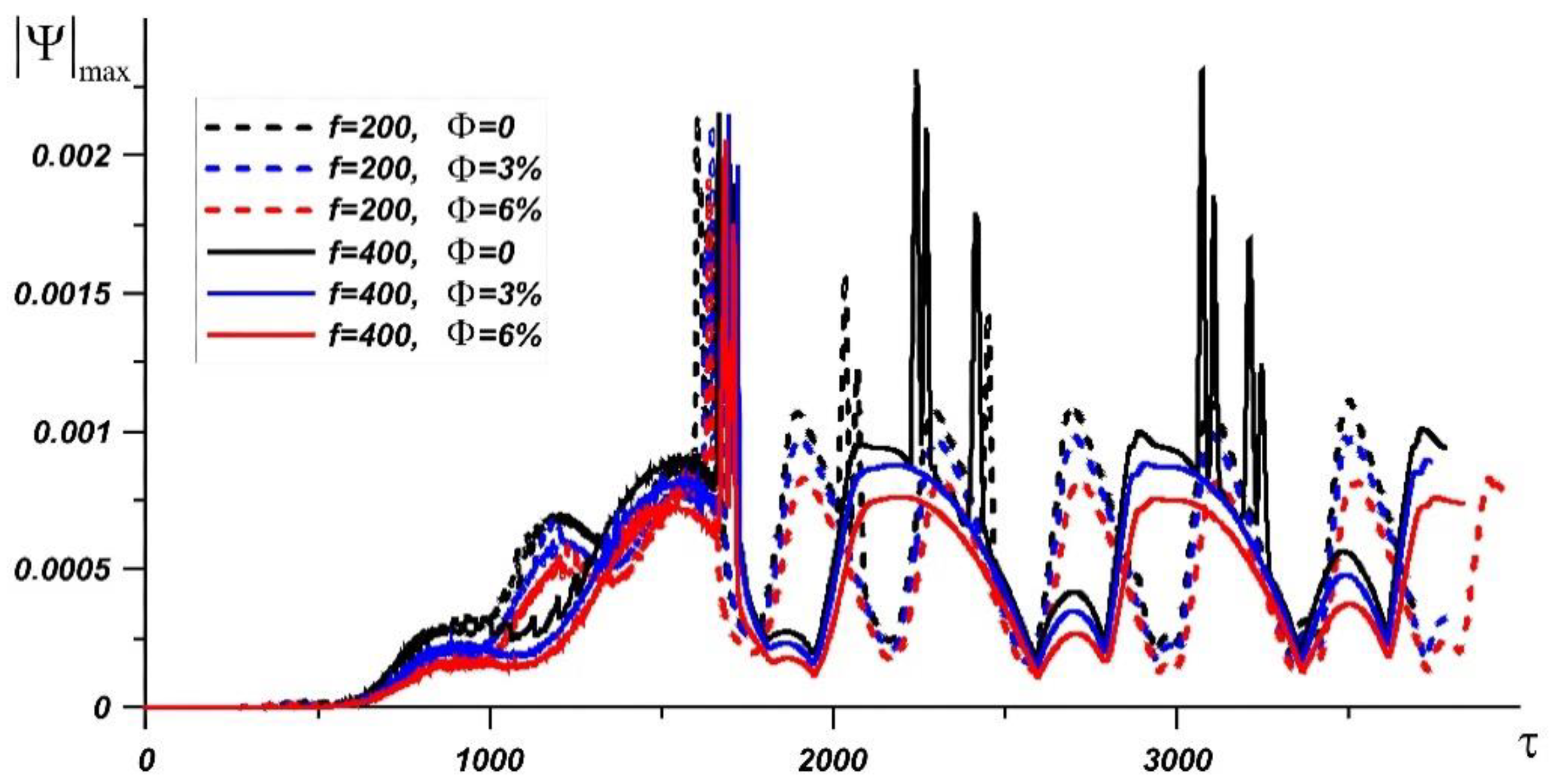
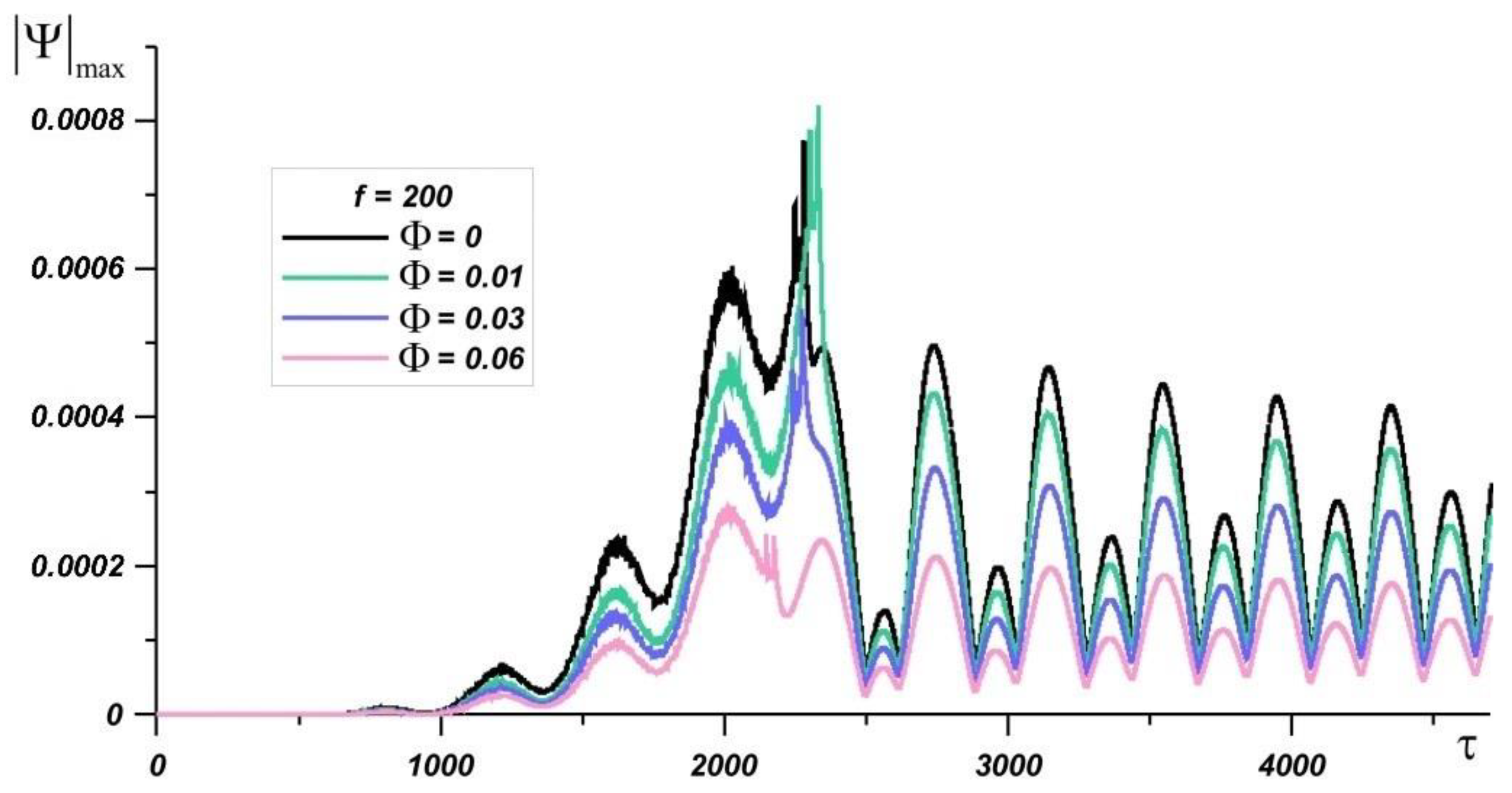
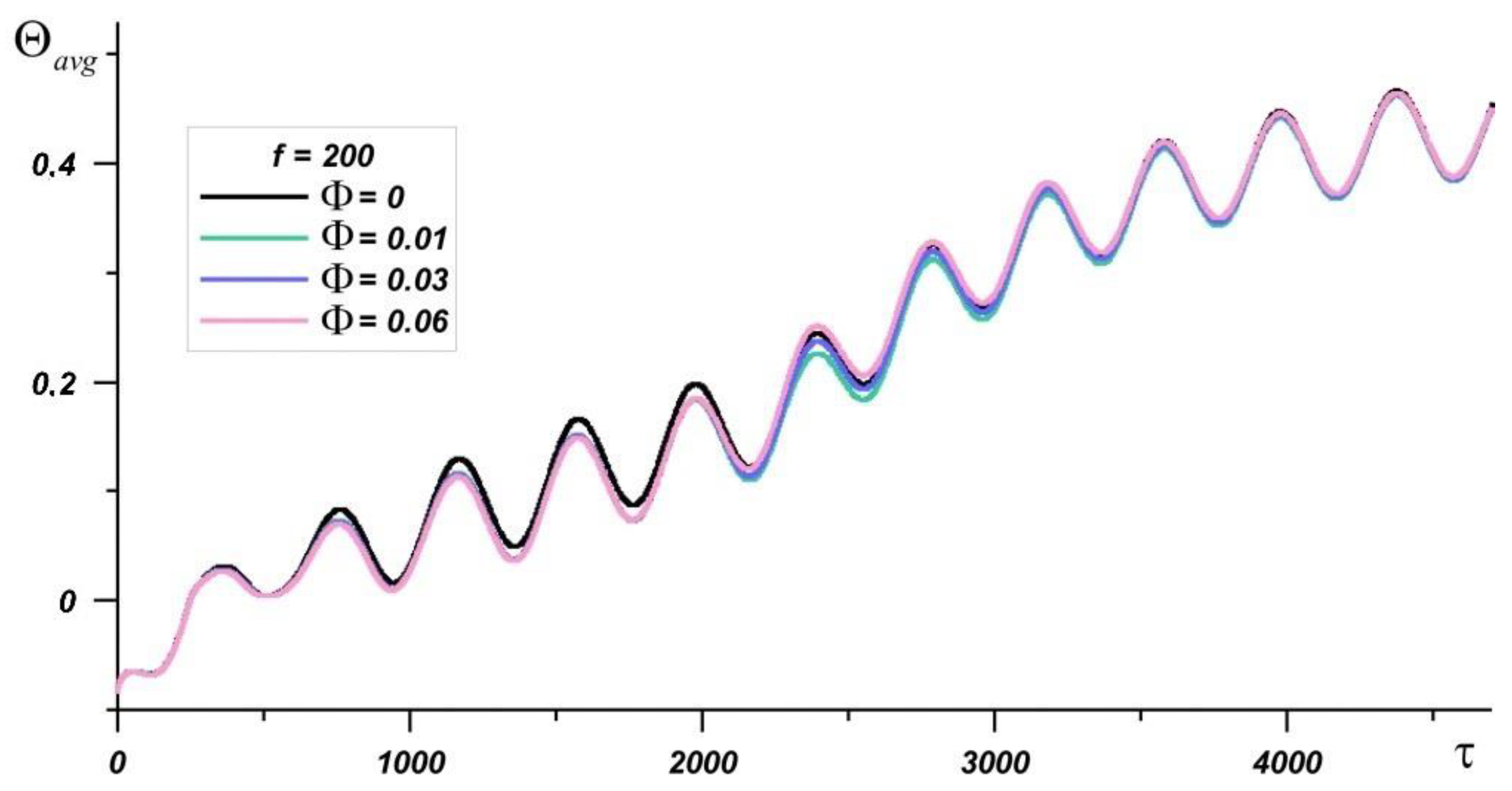
| Material | k, W/(m·K) | c, J/(kg·K) | μ, Pa·s | ρ, kg/m3 | β, K−1 | |
|---|---|---|---|---|---|---|
| Paraffin, n-octadecane (Tm = 301.05 K, Lf = 2.41·105 J/kg) [42] | solid | 0.39 | 1900 | – | 814 | 8.5·10−4 |
| liquid | 0.157 | 2200 | 3.8·10−3 | 770 | ||
| Aluminum oxide (nanoparticles) (d = 59·10−9 m) [33] | 36 | 765 | – | 3600 | 7.8·10−6 | |
| Copper (radiator) | 401 | 385 | – | 8900 | – | |
| Silicon (heat source) | 148 | 714 | – | 2330 | – | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondareva, N.S.; Sheremet, M.A. Effect of Nano-Sized Heat Transfer Enhancers on PCM-Based Heat Sink Performance at Various Heat Loads. Nanomaterials 2020, 10, 17. https://doi.org/10.3390/nano10010017
Bondareva NS, Sheremet MA. Effect of Nano-Sized Heat Transfer Enhancers on PCM-Based Heat Sink Performance at Various Heat Loads. Nanomaterials. 2020; 10(1):17. https://doi.org/10.3390/nano10010017
Chicago/Turabian StyleBondareva, Nadezhda S., and Mikhail A. Sheremet. 2020. "Effect of Nano-Sized Heat Transfer Enhancers on PCM-Based Heat Sink Performance at Various Heat Loads" Nanomaterials 10, no. 1: 17. https://doi.org/10.3390/nano10010017






