Bacterial Disinfection by CuFe2O4 Nanoparticles Enhanced by NH2OH: A Mechanistic Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Copper Ferrite
2.3. E. Coli Inactivation Assay
2.4. ROS Quantification Assay
2.5. ROS Scavenging Assay
2.6. Recycling of Copper Ferrite
2.7. Statistical Analysis
3. Results and Discussion
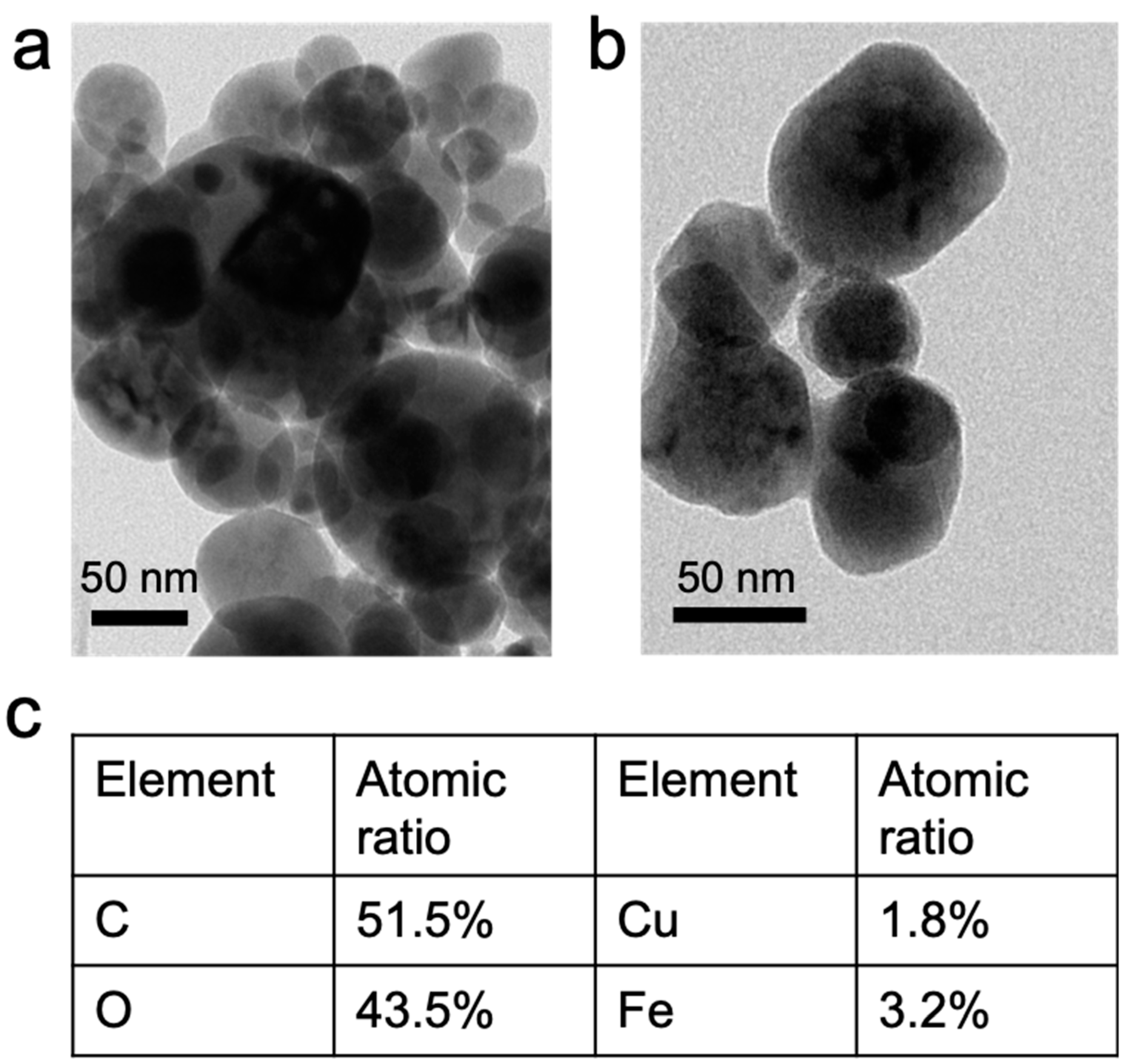
3.1. Characterization of Synthesized Copper Ferrite
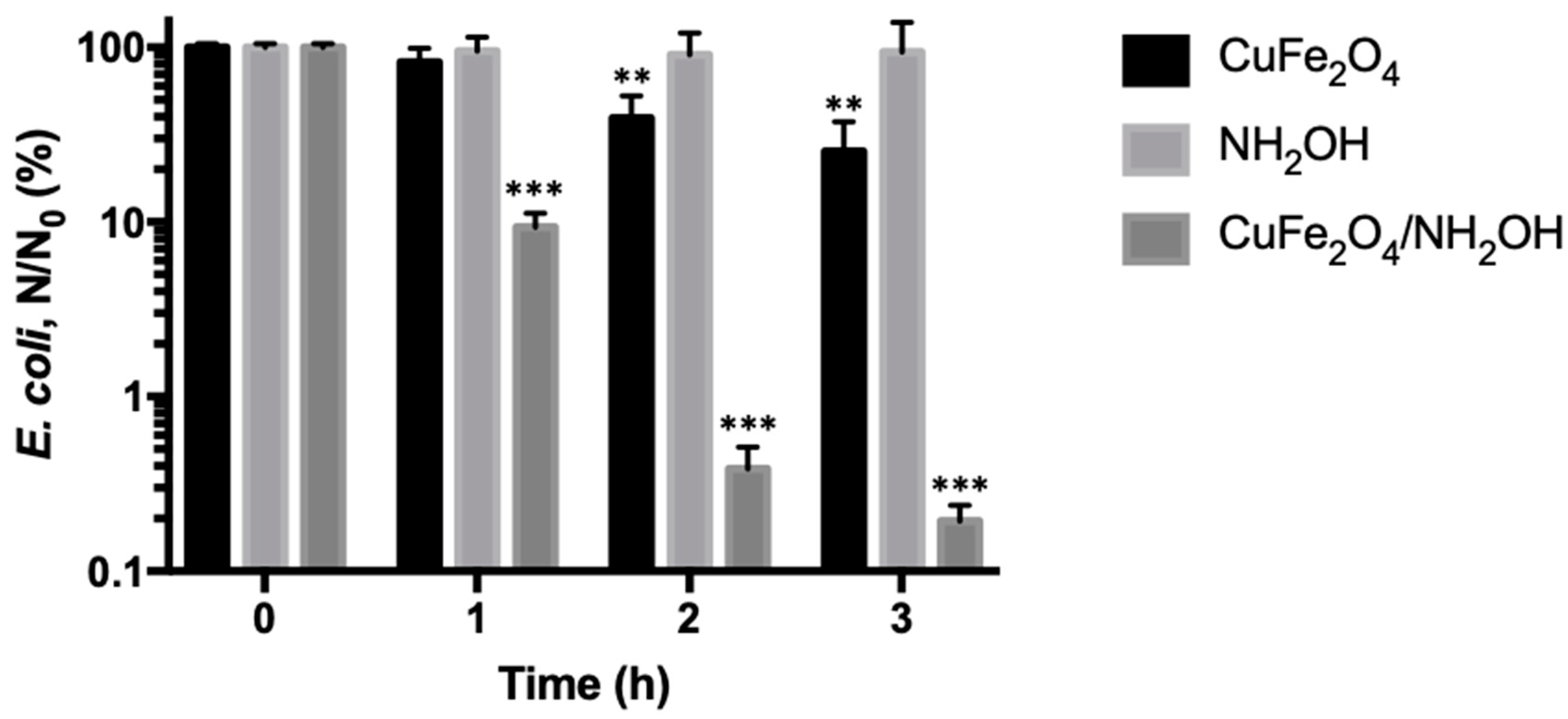
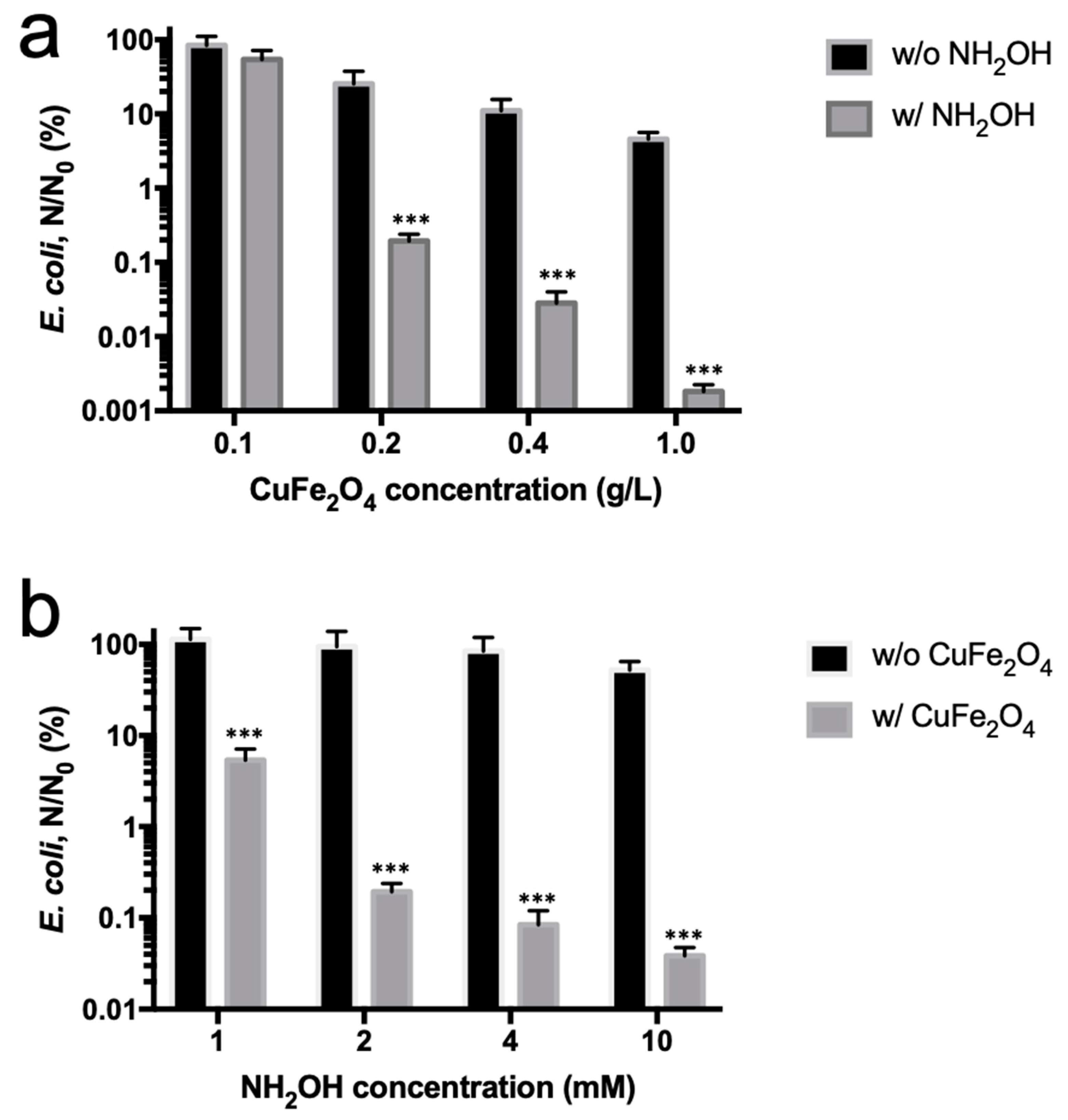
3.2. Enhanced Bactericidal Performance of Copper Ferrite by Hydroxylamine Addition
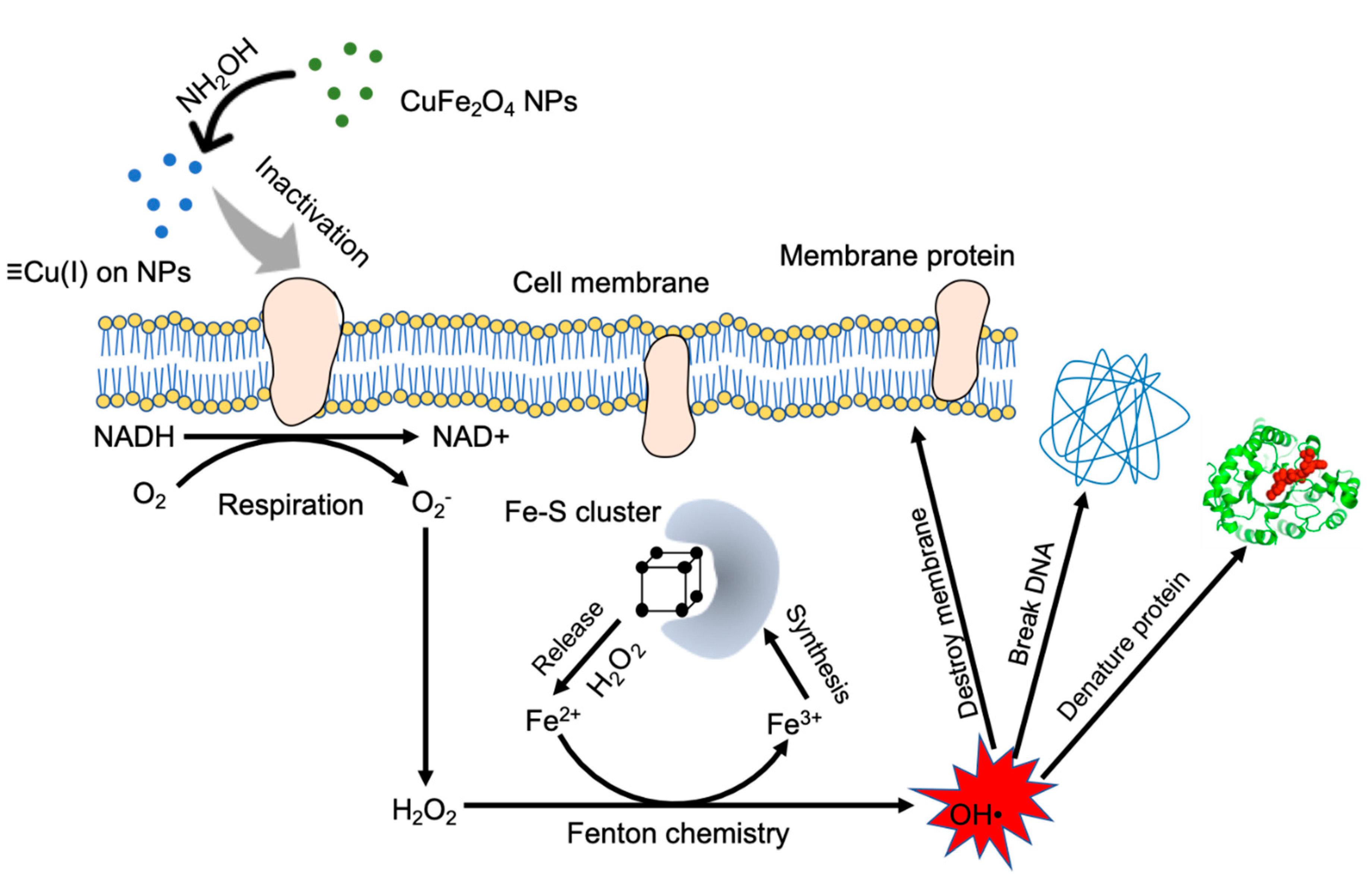
3.3. Reduction of Surface Cu(II) into Cu(I) by Hydroxylamine
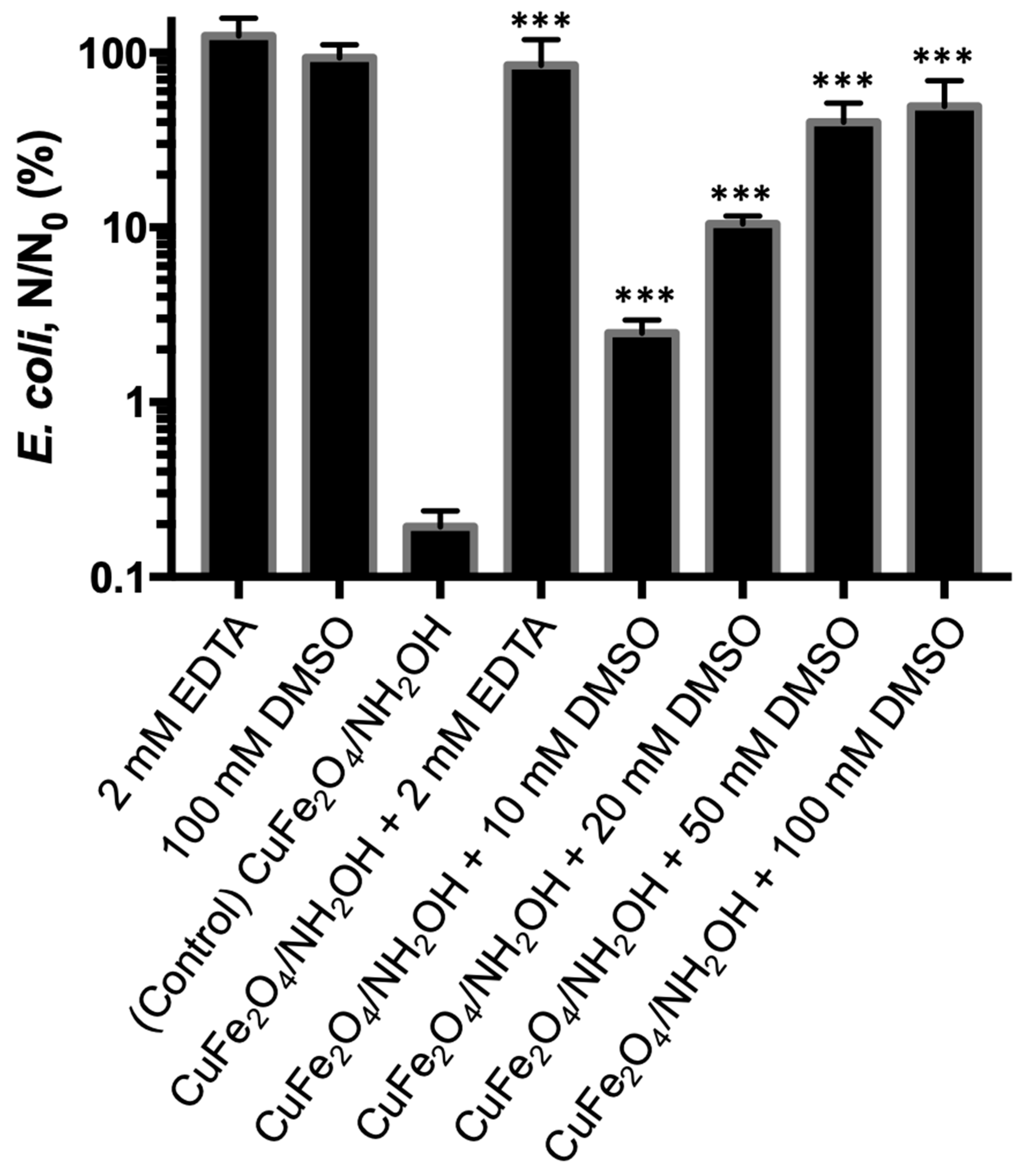
3.4. Bactericidal Action by CuFe2O4/NH2OH Reaction
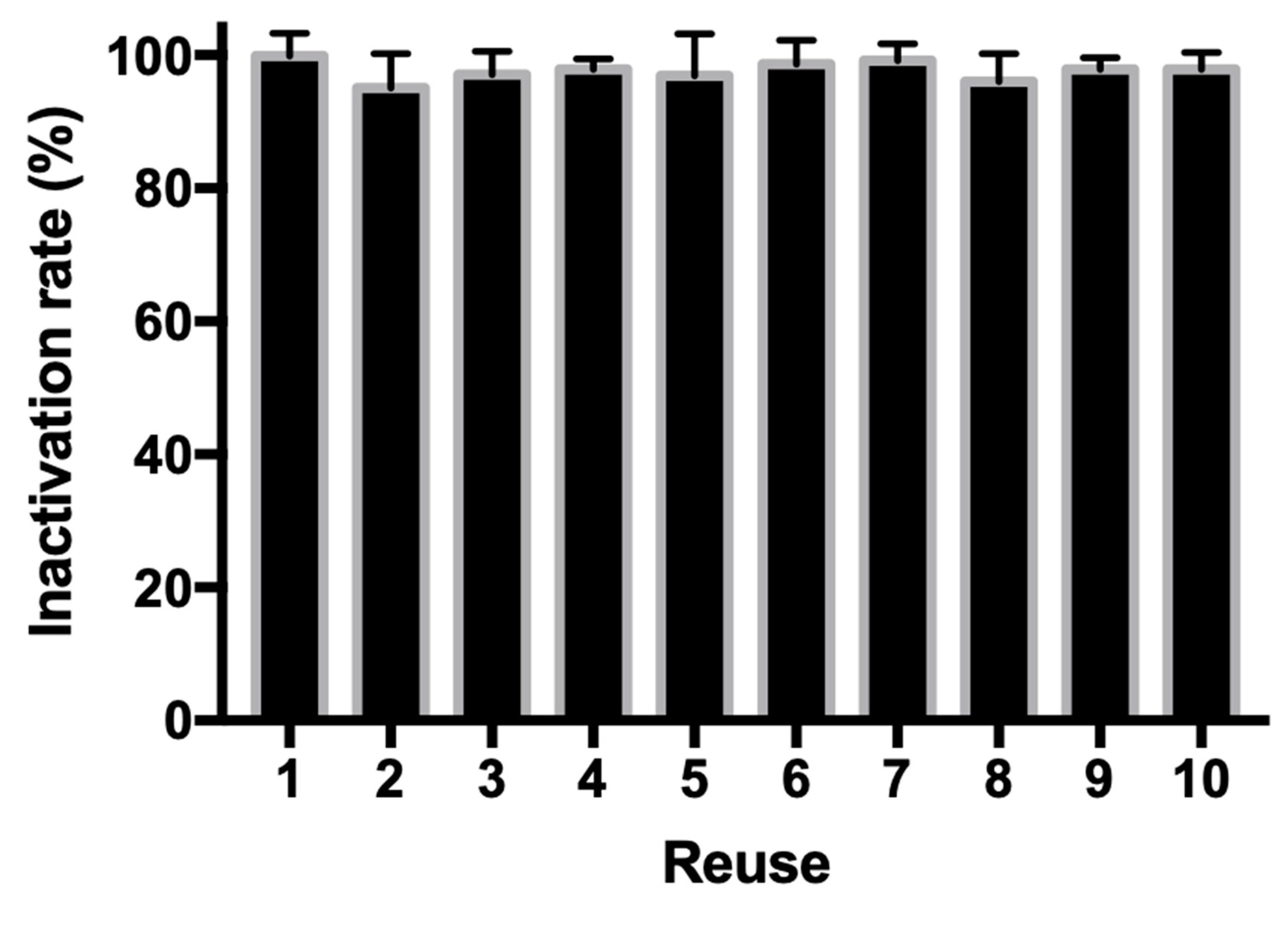
3.5. Recycling Assay of Copper Ferrite Nanoparticle
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tien, J.H.; Earn, D.J. Multiple transmission pathways and disease dynamics in a waterborne pathogen model. Bull. Math. Biol. 2010, 72, 1506–1533. [Google Scholar] [CrossRef]
- Fouz, B.; Toranzo, A.E.; Milan, M.; Amaro, C. Evidence that water transmits the disease caused by the fish pathogen Photobacterium damselae subsp. damselae. J. Appl. Microbiol. 2000, 88, 531–535. [Google Scholar] [CrossRef]
- Ashbolt, N.J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 2004, 198, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Boorman, G.A. Drinking water disinfection byproducts: Review and approach to toxicity evaluation. Environ. Health Perspect. 1999, 107, 207–217. [Google Scholar] [PubMed]
- McGuigan, K.G.; Conroy, R.M.; Mosler, H.J.; du Preez, M.; Ubomba-Jaswa, E.; Fernandez-Ibanez, P. Solar water disinfection (SODIS): A review from bench-top to roof-top. J. Hazard. Mater. 2012, 235, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Mohseni, M.; Taghipour, F. Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: A review. Water Res. 2016, 94, 341–349. [Google Scholar] [CrossRef]
- Chen, L.; Tang, M.; Chen, C.; Chen, M.; Luo, K.; Xu, J.; Zhou, D.; Wu, F. Efficient bacterial inactivation by transition metal catalyzed auto-oxidation of sulfite. Environ. Sci. Technol. 2017, 51, 12663–12671. [Google Scholar] [CrossRef]
- Chen, L.; Pinto, A.; Alshawabkeh, A.N. Activated Carbon as a Cathode for Water Disinfection through the Electro-Fenton Process. Catalysts 2019, 9, 601. [Google Scholar] [CrossRef]
- Morris, R.D.; Audet, A.M.; Angelillo, I.F.; Chalmers, T.C.; Mosteller, F. Chlorination, chlorination by-products, and cancer: A meta-analysis. Am. J. Public Health 1992, 82, 955–963. [Google Scholar] [CrossRef]
- Kim, J.; Chung, Y.; Shin, D.; Kim, M.; Lee, Y.; Lim, Y.; Lee, D. Chlorination by-products in surface water treatment process. Desalination 2003, 151, 1–9. [Google Scholar] [CrossRef]
- Jyoti, K.K.; Pandit, A.B. Ozone and cavitation for water disinfection. Biochem. Eng. J. 2004, 18, 9–19. [Google Scholar] [CrossRef]
- Kerwick, M.I.; Reddy, S.M.; Chamberlain, A.H.L.; Holt, D.M. Electrochemical disinfection, an environmentally acceptable method of drinking water disinfection? Electrochim. Acta 2005, 50, 5270–5277. [Google Scholar] [CrossRef]
- Thomas, V.; Bouchez, T.; Nicolas, V.; Robert, S.; Loret, J.F.; Levi, Y. Amoebae in domestic water systems: Resistance to disinfection treatments and implication in Legionella persistence. J. Appl. Microbiol. 2004, 97, 950–963. [Google Scholar] [CrossRef]
- Chiao, T.H.; Clancy, T.M.; Pinto, A.; Xi, C.; Raskin, L. Differential resistance of drinking water bacterial populations to monochloramine disinfection. Environ. Sci. Technol. 2014, 48, 4038–4047. [Google Scholar] [CrossRef]
- Berry, D.; Xi, C.; Raskin, L. Microbial ecology of drinking water distribution systems. Curr. Opin. Biotechnol. 2006, 17, 297–302. [Google Scholar] [CrossRef]
- Colberg, P.J.; Lingg, A.J. Effect of ozonation on microbial fish pathogens, ammonia, nitrate, nitrite, and BOD in simulated reuse hatchery water. J. Fish. Board Can. 1978, 35, 1290–1296. [Google Scholar] [CrossRef]
- Wedemeyer, G.A.; Nelson, N.C. Survival of two bacterial fish pathogens (Aeromonas salmonicida and the enteric redmouth bacterium) in ozonated, chlorinated, and untreated waters. J. Fish. Board Can. 1977, 34, 429–432. [Google Scholar] [CrossRef]
- Liltved, H.; Hektoen, H.; Efraimsen, H. Inactivation of bacterial and viral fish pathogens by ozonation or UV irradiation in water of different salinity. Aquacult. Eng. 1995, 14, 107–122. [Google Scholar] [CrossRef]
- Zyara, A.; Torvinen, E.; Veijalainen, A.M.; Heinonen-Tanski, H. The effect of UV and combined chlorine/UV treatment on coliphages in drinking water disinfection. Water 2016, 8, 130. [Google Scholar] [CrossRef]
- Guerrero-Latorre, L.; Gonzales-Gustavson, E.; Hundesa, A.; Sommer, R.; Rosina, G. UV disinfection and flocculation-chlorination sachets to reduce hepatitis E virus in drinking water. Int. J. Hyg. Environ. Health 2016, 219, 405–411. [Google Scholar] [CrossRef]
- Hull, N.M.; Isola, M.R.; Petri, B.; Chan, P.S.; Linden, K.G. Algal DNA repair kinetics support culture-based enumeration for validation of ultraviolet disinfection ballast water treatment systems. Environ. Sci. Technol. Lett. 2017, 4, 192–196. [Google Scholar] [CrossRef]
- Ma, S.; Zhan, S.; Jia, Y.; Shi, Q.; Zhou, Q. Enhanced disinfection application of Ag-modified g-C3N4 composite under visible light. Appl. Catal. B 2016, 186, 77–87. [Google Scholar] [CrossRef]
- Teng, Z.; Yang, N.; Lv, H.; Wang, S.; Hu, M.; Wang, C.; Wang, D.; Wang, G. Edge-Functionalized g-C3N4 Nanosheets as a Highly Efficient Metal-free Photocatalyst for Safe Drinking Water. Chem 2019, 5, 664–680. [Google Scholar] [CrossRef]
- Chen, M.; Li, Z.; Chen, L. Highly antibacterial rGO/Cu2O nanocomposite from a biomass precursor: Synthesis, performance, and mechanism. Nano Mater. Sci. 2019. [Google Scholar] [CrossRef]
- Chen, L.; Li, Z.; Chen, M. Facile production of silver-reduced graphene oxide nanocomposite with highly effective antibacterial performance. J. Environ. Chem. Eng. 2019, 7, 103160. [Google Scholar] [CrossRef]
- Jin, X.; Li, M.; Wang, J.; Marambio-Jones, C.; Peng, F.; Huang, X.; Damoiseaux, R.; Hoek, E.M. High-throughput screening of silver nanoparticle stability and bacterial inactivation in aquatic media: Influence of specific ions. Environ. Sci. Technol. 2010, 44, 7321–7328. [Google Scholar] [CrossRef]
- Thurman, R.B.; Gerba, C.P.; Bitton, G. The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. Crit. Rev. Environ. Sci. Technol. 1989, 18, 295–315. [Google Scholar] [CrossRef]
- Wang, L.; Luo, J.; Shan, S.; Crew, E.; Yin, J.; Zhong, C.J.; Wallek, B.; Wong, S.S. Bacterial inactivation using silver-coated magnetic nanoparticles as functional antimicrobial agents. Anal. Chem. 2011, 83, 8688–8695. [Google Scholar] [CrossRef]
- Mikolay, A.; Huggett, S.; Tikana, L.; Grass, G.; Braun, J.; Nies, D.H. Survival of bacteria on metallic copper surfaces in a hospital trial. Appl. Microbiol. Biotechnol. 2010, 87, 1875–1879. [Google Scholar] [CrossRef]
- Santo, C.E.; Lam, E.W.; Elowsky, C.G.; Quaranta, D.; Domaille, D.W.; Chang, C.J.; Grass, G. Bacterial killing by dry metallic copper surfaces. Appl. Environ. Microbiol. 2011, 77, 794–802. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Putting copper into action: Copper-impregnated products with potent biocidal activities. FASEB J. 2004, 18, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, H.E.; Lee, C. Combination of cupric ion with hydroxylamine and hydrogen peroxide for the control of bacterial biofilms on RO membranes. Water Res. 2017, 110, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.E.; Nguyen, T.T.; Lee, H.; Lee, C. Enhanced inactivation of Escherichia coli and MS2 coliphage by cupric ion in the presence of hydroxylamine: Dual microbicidal effects. Environ. Sci. Technol. 2015, 49, 14416–14423. [Google Scholar] [CrossRef]
- Morgada, M.N.; Abriata, L.A.; Cefaro, C.; Gajda, K.; Banci, L.; Vila, A.J. Loop recognition and copper-mediated disulfide reduction underpin metal site assembly of CuA in human cytochrome oxidase. Proc. Natl. Acad. Sci. USA 2015, 112, 11771–11776. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J. Risks of copper and iron toxicity during aging in humans. Chem. Res. Toxicol. 2009, 23, 319–326. [Google Scholar] [CrossRef] [PubMed]
- US EPA Standard for Copper in Drinking Water. Available online: https://www.epa.gov/dwreginfo/lead-and-copper-rule (accessed on 30 November 2019).
- Brenner, K.P.; Rankin, C.C.; Roybal, Y.R.; Stelma, G.N.; Scarpino, P.V.; Dufour, A.P. New medium for the simultaneous detection of total coliforms and Escherichia coli in water. Appl. Environ. Microbiol. 1993, 59, 3534–3544. [Google Scholar]
- Heijnen, L.; Medema, G. Quantitative detection of E. coli, E. coli O157 and other shiga toxin producing E. coli in water samples using a culture method combined with real-time PCR. J. Water Health 2006, 4, 487–498. [Google Scholar] [CrossRef]
- Atia, T.A.; Altimari, P.; Moscardini, E.; Pettiti, I.; Toro, L.; Pagnanelli, F. Synthesis and characterization of copper ferrite magnetic nanoparticles by hydrothermal route. Chem. Eng. Trans. 2016, 47, 151–156. [Google Scholar]
- Petersen, E.J.; Hirsch, C.; Elliott, J.T.; Krug, H.F.; Aengenheister, L.; Arif, A.T.; Bogni, A.; Kinsner-Ovaskainen, A.; May, S.; Walser, T.; et al. Cause-and-Effect Analysis as a Tool To Improve the Reproducibility of Nanobioassays: Four Case Studies. Chem. Res. Toxicol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gilchrist, A.; Zhang, J.; Li, X.F. Detection of viable but nonculturable Escherichia coli O157: H7 bacteria in drinking water and river water. Appl. Environ. Microbiol. 2008, 74, 1502–1507. [Google Scholar] [CrossRef]
- Joyce, T.M.; McGuigan, K.G.; Elmore-Meegan, M.; Conroy, R.M. Inactivation of fecal bacteria in drinking water by solar heating. Appl. Environ. Microbiol. 1996, 62, 399–402. [Google Scholar]
- Wolfe, R.L.; Ward, N.R.; Olson, B.H. Inactivation of heterotrophic bacterial populations in finished drinking water by chlorine and chloramines. Water Res. 1985, 19, 1393–1403. [Google Scholar] [CrossRef]
- Santo, C.E.; Quaranta, D.; Grass, G. Antimicrobial metallic copper surfaces kill Staphylococcus haemolyticus via membrane damage. Microbiologyopen 2012, 1, 46–52. [Google Scholar] [CrossRef]
- Silver, S. Bacterial resistances to toxic metal ions—A review. Gene 1996, 179, 9–19. [Google Scholar] [CrossRef]
- Bondarczuk, K.; Piotrowska-Seget, Z. Molecular basis of active copper resistance mechanisms in Gram-negative bacteria. Cell Biol. Toxicol. 2013, 29, 397–405. [Google Scholar] [CrossRef]
- Saphier, M.; Silberstein, E.; Shotland, Y.; Popov, S.; Saphier, O. Prevalence of Monovalent Copper Over Divalent in Killing Escherichia coli and Staphylococcus Aureus. Curr. Microbiol. 2018, 75, 426–430. [Google Scholar] [CrossRef]
- Borkow, G. Using copper to improve the well-being of the skin. Curr. Chem. Biol. 2014, 8, 89–102. [Google Scholar] [CrossRef]
- Rowe, L.A.; Degtyareva, N.; Doetsch, P.W. DNA damage-induced reactive oxygen species (ROS) stress response in Saccharomyces cerevisiae. Free Radic. Biol. Med. 2008, 45, 1167–1177. [Google Scholar] [CrossRef]
- Tomizawa, S.; Imai, H.; Tsukada, S.; Simizu, T.; Honda, F.; Nakamura, M.; Nagano, T.; Urano, Y.; Matsuoka, Y.; Fukasaku, N.; et al. The detection and quantification of highly reactive oxygen species using the novel HPF fluorescence probe in a rat model of focal cerebral ischemia. J. Neurosci. Res. 2005, 53, 304–313. [Google Scholar] [CrossRef]
- Veltwisch, D.; Janata, E.; Asmus, K.D. Primary processes in the reaction of OH -radicals with sulphoxides. J. Chem. Soc. Perkin Trans. I 1980, 146–153. [Google Scholar] [CrossRef]
- Reuvers, A.P.; Greenstock, C.L.; Borsa, J.; Chapman, J.D. Studies on the mechanism of chemical radioprotection by dimethyl sulphoxide. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1973, 24, 533–536. [Google Scholar] [CrossRef]
- Mi, H.; Wang, D.; Xue, Y.; Zhang, Z.; Niu, J.; Hong, Y.; Drlica, K.; Zhao, X. Dimethyl sulfoxide protects Escherichia coli from rapid antimicrobial-mediated killing. Antimicrob. Agents Chemother. 2016, 60, 5054–5058. [Google Scholar] [CrossRef]




| Cu 2p | Fe 2p | O 1s | |||||
|---|---|---|---|---|---|---|---|
| Cu(II) | Cu(I) | O-Site Fe(III) | T-Site Fe(III) | Lattice O | Surface OH | Absorbed H2O | |
| Before | 72.6% | 27.4% | 45.5% | 54.5% | 28.6% | 66% | 47.4% |
| After | 24.8% | 75.2% | 42.4% | 57.6% | 32.5% | 67.5% | n/a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Xiao, F.; Luo, L.; Zhou, X.; Zhou, X.; Li, J.; Li, Z. Bacterial Disinfection by CuFe2O4 Nanoparticles Enhanced by NH2OH: A Mechanistic Study. Nanomaterials 2020, 10, 18. https://doi.org/10.3390/nano10010018
Gu Y, Xiao F, Luo L, Zhou X, Zhou X, Li J, Li Z. Bacterial Disinfection by CuFe2O4 Nanoparticles Enhanced by NH2OH: A Mechanistic Study. Nanomaterials. 2020; 10(1):18. https://doi.org/10.3390/nano10010018
Chicago/Turabian StyleGu, Yu, Furen Xiao, Liumin Luo, Xiaoyu Zhou, Xiaodong Zhou, Jin Li, and Zhi Li. 2020. "Bacterial Disinfection by CuFe2O4 Nanoparticles Enhanced by NH2OH: A Mechanistic Study" Nanomaterials 10, no. 1: 18. https://doi.org/10.3390/nano10010018
APA StyleGu, Y., Xiao, F., Luo, L., Zhou, X., Zhou, X., Li, J., & Li, Z. (2020). Bacterial Disinfection by CuFe2O4 Nanoparticles Enhanced by NH2OH: A Mechanistic Study. Nanomaterials, 10(1), 18. https://doi.org/10.3390/nano10010018





