Catalytic Performance of Palladium Supported on Sheaf-Like Ceria in the Lean Methane Combustion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Catalysts
2.3. Catalytic Activity Measurements
2.4. Catalyst Characterization
3. Results
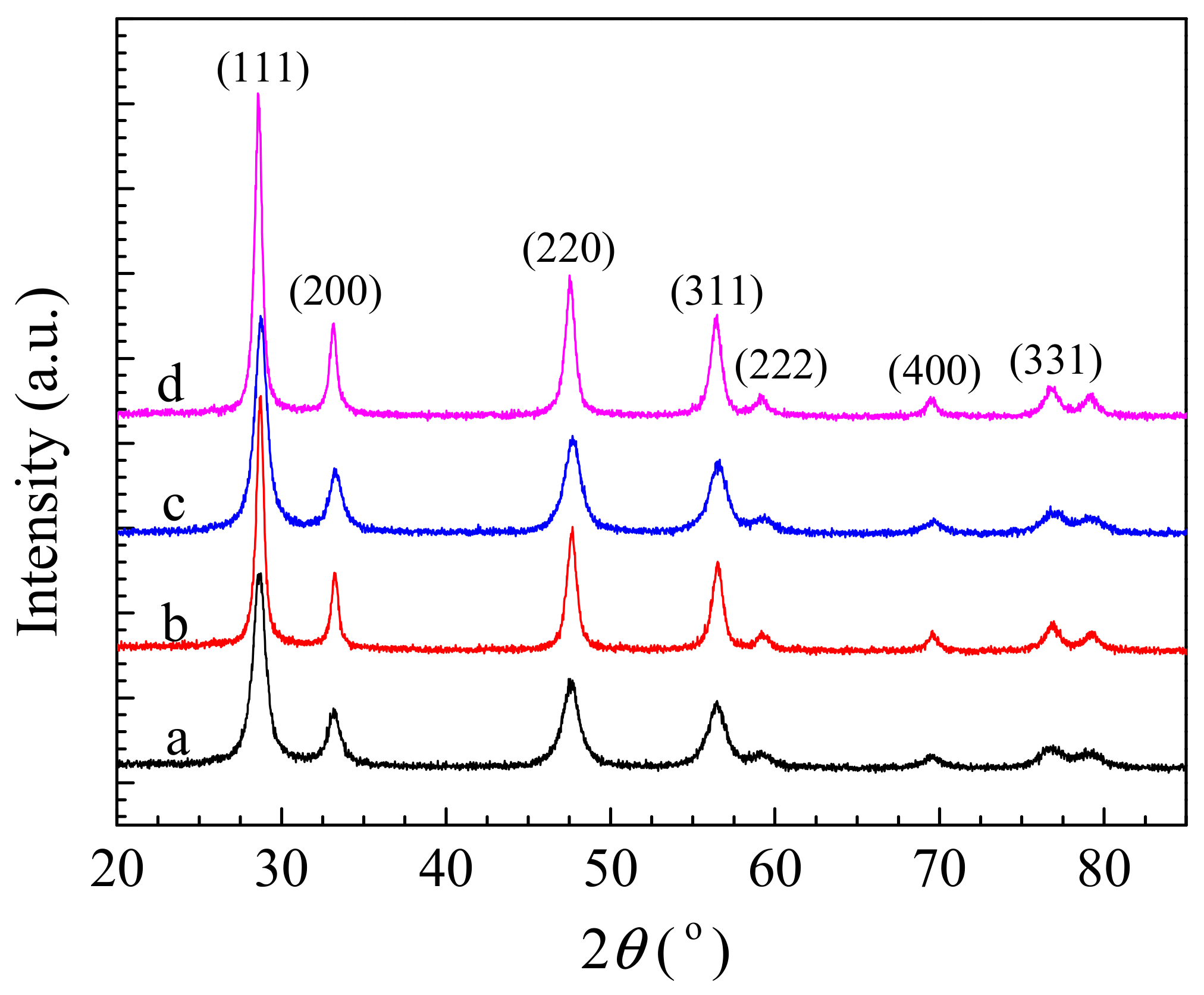
3.1. XRD Results and Textural Properties
3.2. SEM Results
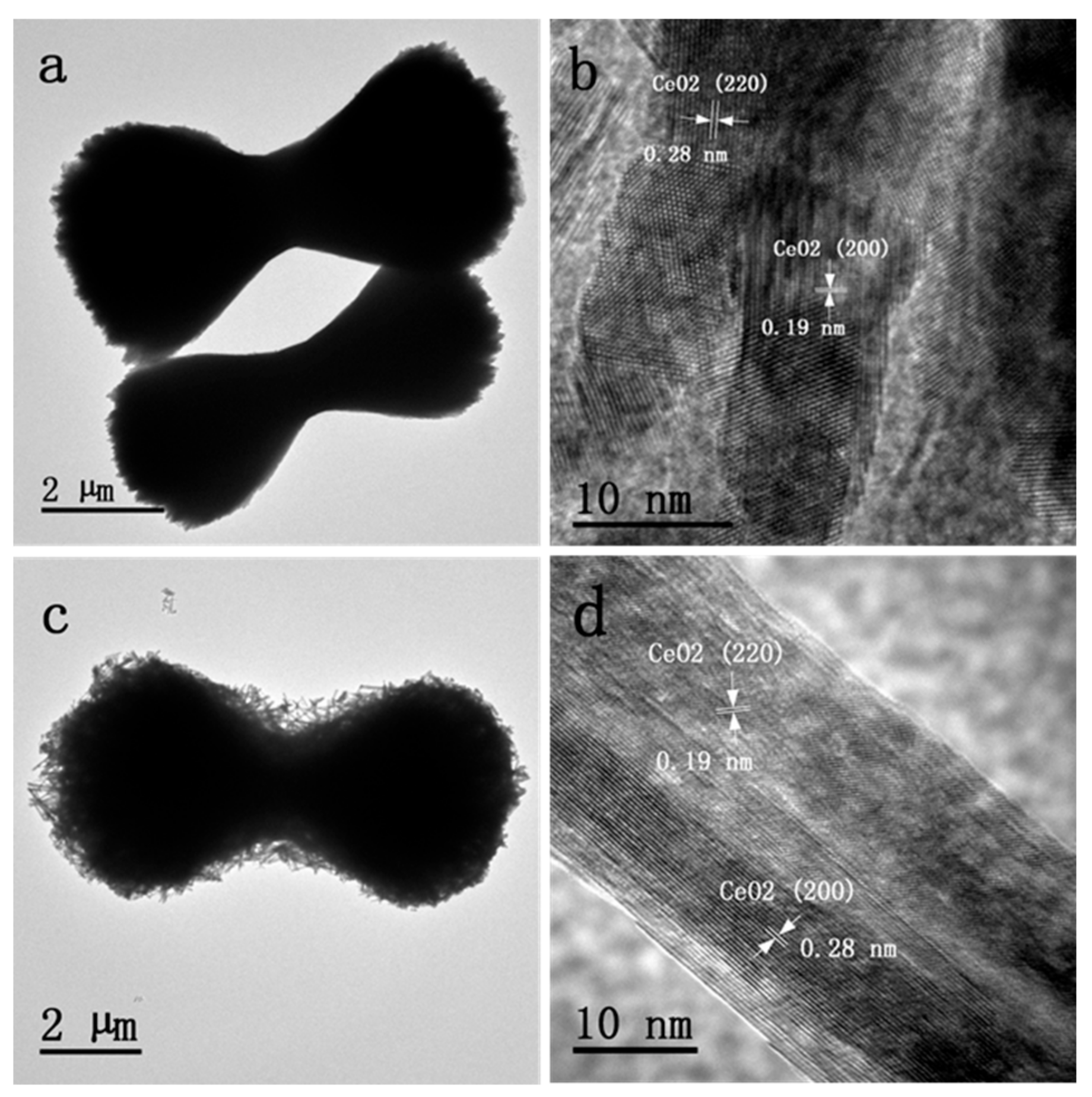
3.3. TEM and HRTEM Results
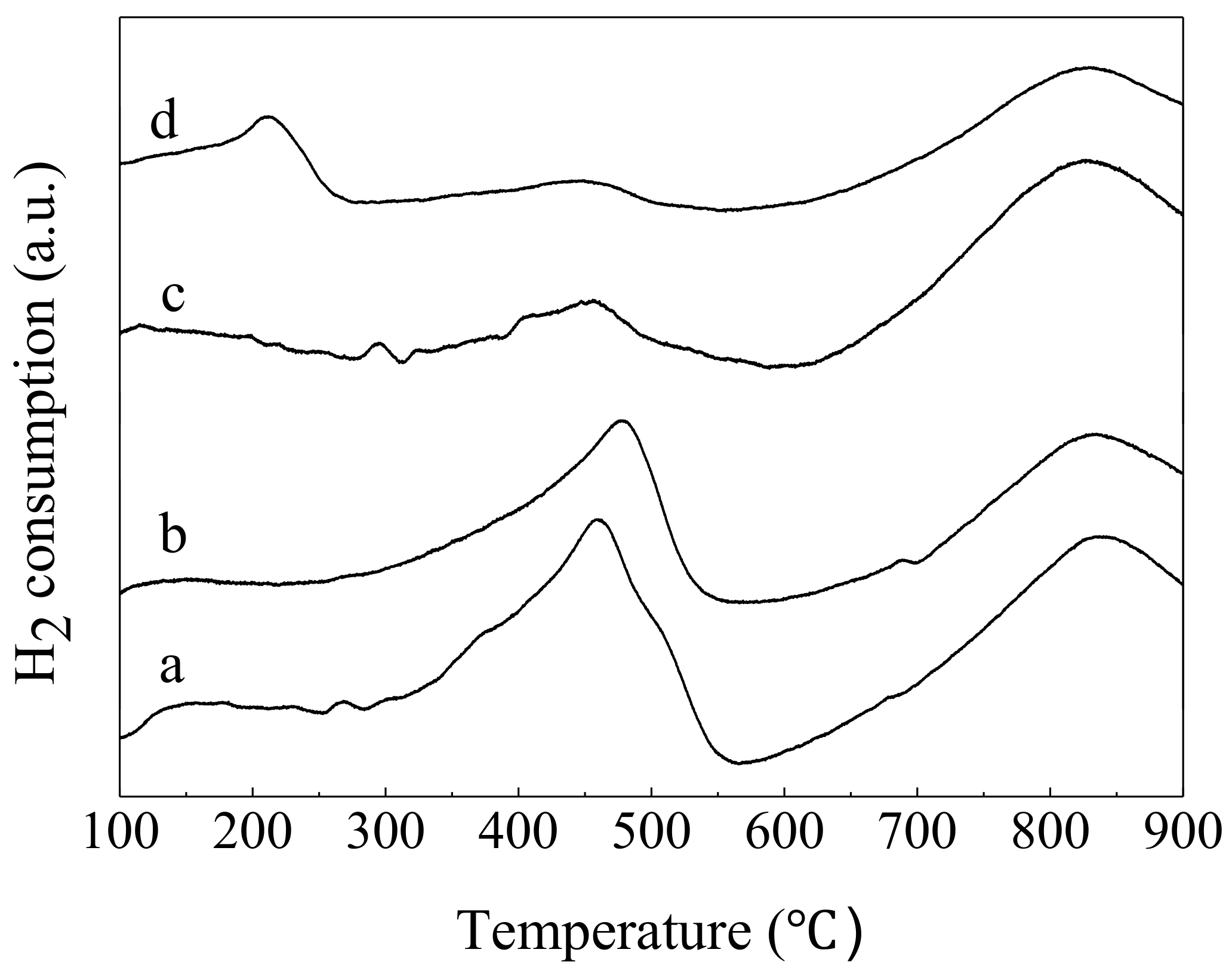
3.4. H2-TPR Results
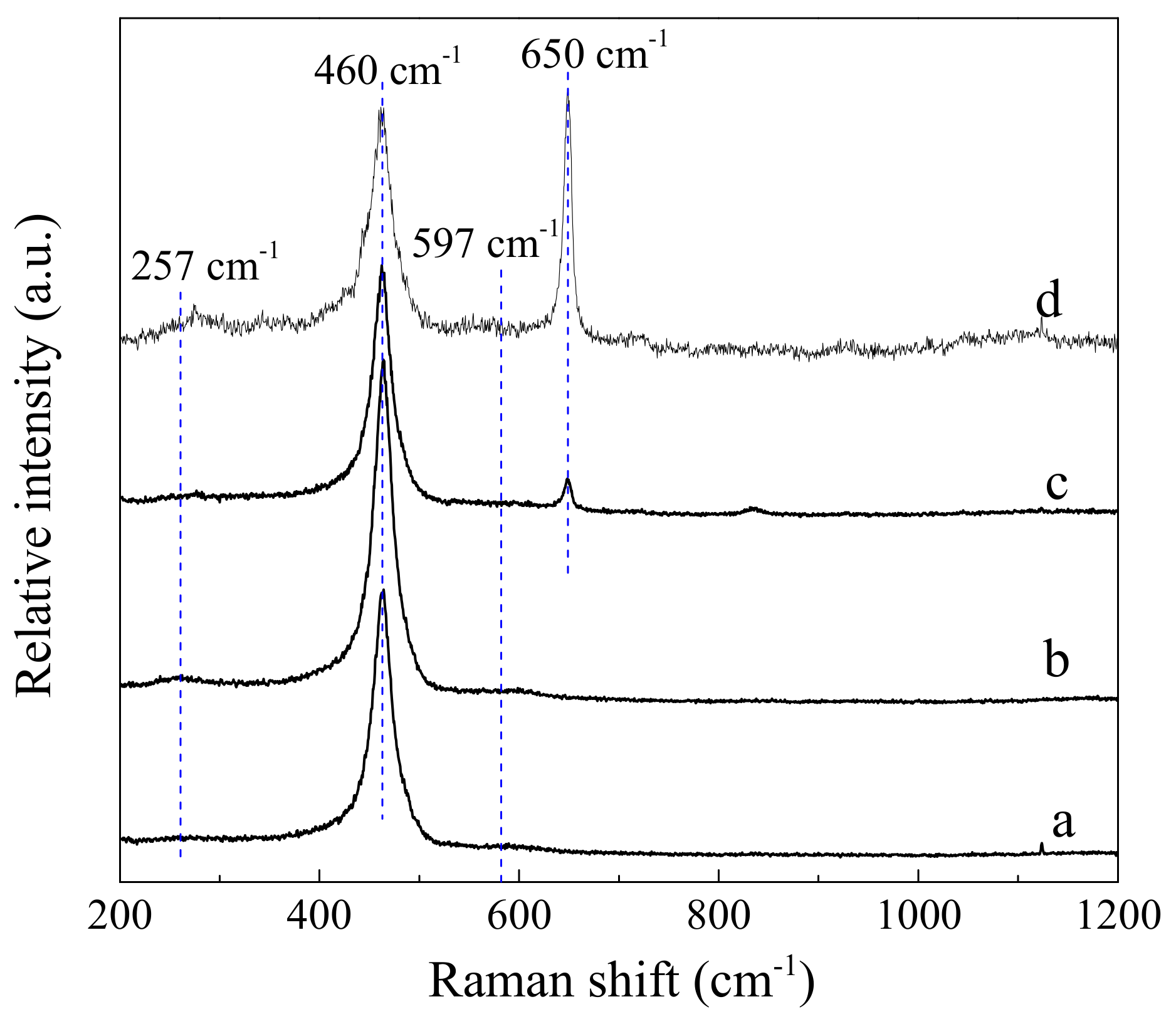
3.5. Raman Spectroscopy
3.6. XPS Results
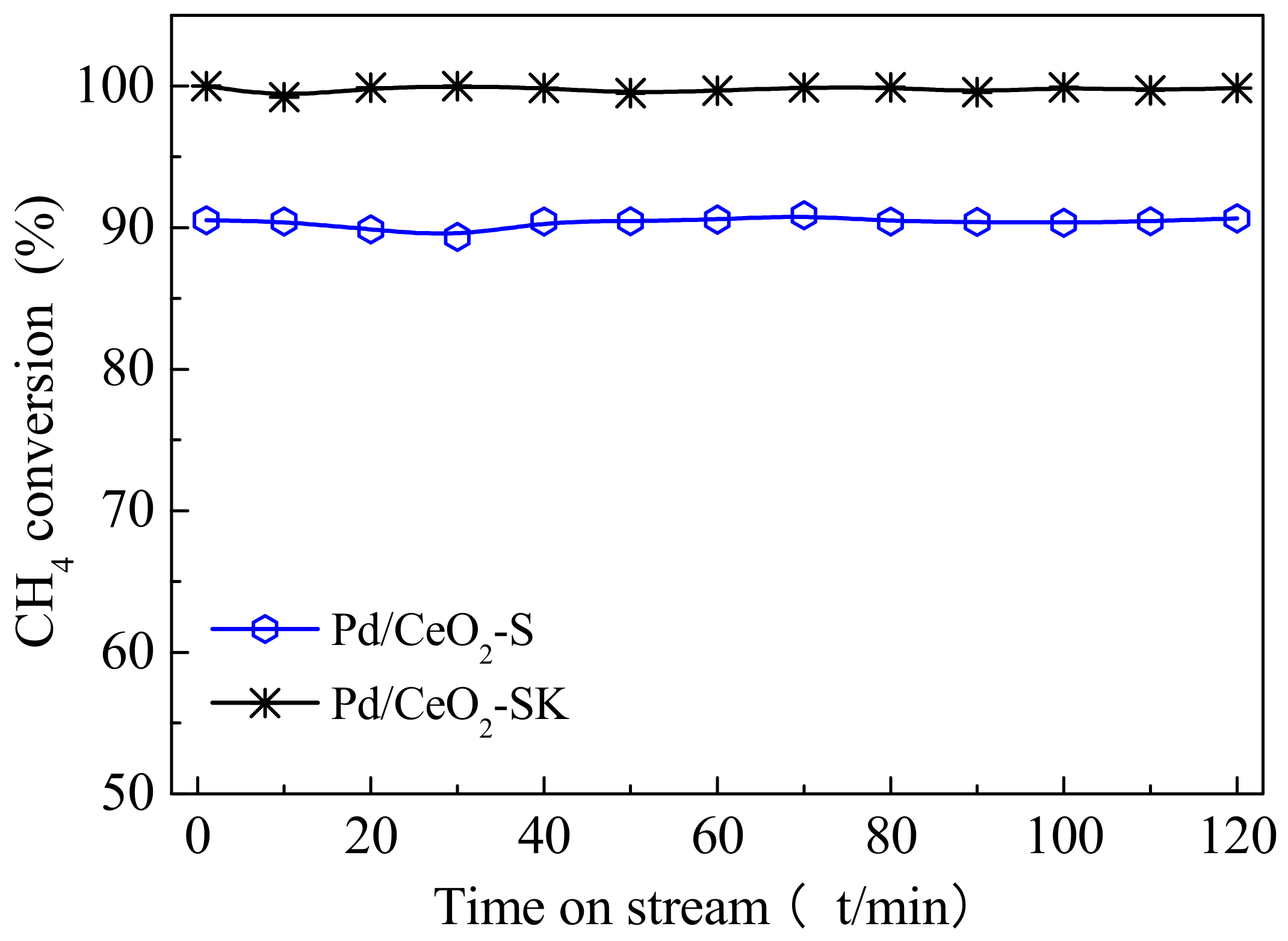
3.7. CH4 Combustion Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, F.X.; Sang, Y.Y.; Ma, H.W.; Li, Z.P.; Gao, Z.M. Nickel oxide as an effective catalyst for catalytic combustion of methane. J. Nat. Gas Sci. Eng. 2017, 41, 1–6. [Google Scholar] [CrossRef]
- Zhou, F.B.; Xia, T.Q.; Wang, X.X.; Zhang, Y.F.; Sun, Y.N.; Liu, J.S. Recent developments of coal mine methane extraction and utilization in China: A review. J. Nat. Gas Sci. Eng. 2016, 31, 437–458. [Google Scholar] [CrossRef]
- Guo, T.; Du, J.; Wu, J.; Wang, S.; Li, J. Structure and kinetic investigations of surfacestepped CeO2-supported Pd catalysts for low-concentration methane oxidation. Chem. Eng. J. 2016, 306, 745–753. [Google Scholar] [CrossRef]
- Zhang, H.; Li, P.; Hui, N.; Liang, J.; Ding, Y.; Liu, T. The microstructure and methane catalytic combustion of ceria composite materials modified with tourmaline particles. J. Alloys Compd. 2017, 712, 567–572. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, Z.; Wang, G.; Zhu, H.; Dong, M.; Li, S.; Wu, Z.; Li, Z.; Wu, Z.; Zhang, J.; et al. Catalytic performance of MnOx-NiO composite oxide in lean methane combustion at low temperature. Appl. Catal. B Environ. 2013, 129, 172–181. [Google Scholar] [CrossRef]
- Ercolino, G.; Stelmachowski, P.; Kotarba, A.; Specchia, S. Reactivity of mixed iron-cobalt spinels in the lean methane combustion. Top. Catal. 2017, 60, 1370–1379. [Google Scholar] [CrossRef]
- Wang, B.; Qin, Z.; Wang, G.; Wu, Z.; Fan, W.; Zhu, H.; Li, S.; Zhang, Y.; Li, Z.; Wang, J. Catalytic combustion of lean methane at low temperature over palladium on a CoOx-SiO2 composite support. Catal. Lett. 2017, 143, 411–417. [Google Scholar] [CrossRef]
- Ercolino, G.; Karimi, S.; Stelmachowski, P.; Specchia, S. Catalytic combustion of residual methane on alumina monoliths and open cell foams coated with Pd/Co3O4. Chem. Eng. J. 2017, 326, 339–349. [Google Scholar] [CrossRef]
- Guo, T.; Du, J.; Li, J. The effects of ceria morphology on the properties of Pd/ceria catalyst for catalytic oxidation of low-concentration methane. J. Mater. Sci. 2016, 51, 10917–10925. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Pfefferle, L.D. Combustion of methane over palladium-based catalysts: support interactions. J. Phys. Chem. C 2012, 116, 8571–8578. [Google Scholar] [CrossRef]
- Fujimoto, K.; Ribeiro, F.H.; Avalos-Borja, M.; Iglesia, E. Structure and reactivity of PdOx/ZrO2 catalysts for methane oxidation at low temperatures. J. Catal. 1998, 179, 431–442. [Google Scholar] [CrossRef] [Green Version]
- Xin, Y.; Lieb, S.; Wang, H.; Law, C.K. Kinetics of catalytic oxidation of methane over palladium oxide by wire microcalorimetry. J. Phys. Chem. C 2013, 117, 19499–19507. [Google Scholar] [CrossRef]
- Chenakin, S.P.; Melaet, G.; Szukiewicz, R.; Kruse, N. XPS study of the surface chemical state of a Pd/(SiO2+TiO2) catalyst after methane oxidation and SO2 treatment. J. Catal. 2014, 312, 1–11. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Lampert, J.K.; Hobson, M.C.; Waterman, E.M. Thermal decomposition and reformation of PdO catalysts; support effects. Appl. Catal. B Environ. 1995, 6, 263–270. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, K.; Zong, L.; Tong, S.; Wang, X.; Feng, G. Gold supported on ceria nanotubes for CO oxidation. Appl. Surf. Sci. 2017, 416, 183–190. [Google Scholar] [CrossRef]
- Li, S.; Zhu, H.; Qin, Z.; Wang, G.; Zhang, Y.; Wu, Z.; Li, Z.; Chen, G.; Wu, Z.; Zheng, L.; et al. Morphologic effects of nano CeO2-TiO2 on the performance of Au/CeO2-TiO2 catalysts in low-temperature CO oxidation. Appl. Catal. B Environ. 2014, 144, 498–506. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Mallesham, B.; Reddy, P.S.; Großmann, D.; Grünert, W.; Reddy, B.M. Nano-Au/CeO2 catalysts for CO oxidation: Influence of dopants (Fe, Laand Zr) on the physicochemical properties and catalytic activity. Appl. Catal. B Environ. 2014, 144, 900–908. [Google Scholar] [CrossRef]
- Cordatos, H.; Bunluesin, T.; Stubenrauch, J.; Vohs, J.M.; Gorte, R.J. Effect of ceria structure on oxygen migration for Rh/ceria catalysts. J. Phys. Chem. 1996, 100, 785–789. [Google Scholar] [CrossRef]
- Ma, J.; Lou, Y.; Cai, Y.; Zhao, Z.; Wang, L.; Zhan, W.; Guo, Y.L.; Guo, Y. The relationship between the chemical state of Pd species and the catalytic activity for methane combustion on Pd/CeO2. Catal. Sci. Technol. 2018, 8, 2567–2577. [Google Scholar] [CrossRef]
- Dai, Q.; Bai, S.; Lou, Y.; Wang, X.; Guo, Y.; Lu, G. Sandwich-like PdO/CeO2 nanosheet@HZSM-5 membrane hybrid composite for methane combustion: Self-redispersion, sintering-resistance and oxygen, water-tolerance. Nanoscale 2016, 8, 9621–9628. [Google Scholar] [CrossRef]
- Mayernick, A.D.; Janik, M.J. Methane oxidation on Pd-Ceria: A DFT study of the mechanism over PdxCe1-xO2, Pd, and PdO. J. Catal. 2011, 278, 16–25. [Google Scholar] [CrossRef]
- Tan, H.; Wang, J.; Yu, S.; Zhou, K. Support morphology-dependent catalytic activity of Pd/CeO2 for formaldehyde oxidation. Environ. Sci. Technol. 2015, 49, 8675–8682. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Li, W.; Liu, Q.; Lin, Q.; Zheng, X.; Huang, Q.; Guan, S.; Wang, X.; Wang, C.; Li, F. Typical crystal face effects of different morphology ceria on the activity of Pd/CeO2 catalysts for lean methane combustion. Fuel 2018, 233, 10–20. [Google Scholar] [CrossRef]
- Guo, H.; He, Y.; Wang, Y.; Liu, L.; Yang, X.; Wang, S.; Huang, Z.; Wei, Q. Morphology-controlled synthesis of cage-bell Pd@CeO2 structured nanoparticle aggregates as catalysts for the low-temperature oxidation of CO. J. Mater. Chem. A 2013, 1, 7494–7499. [Google Scholar] [CrossRef]
- Guo, T.Y.; Du, J.P.; Wu, J.T.; Li, J.P. Palladium catalyst supported on stair-like microstructural CeO2 provides enhanced activity and stability for low-concentration methane oxidation. Appl. Cata. A General. 2016, 524, 237–242. [Google Scholar] [CrossRef]
- Comotti, M.; Li, W.C.; Spliethoff, B.; Schüth, F. Support effect in high activity gold catalysts for CO oxidation. J. Am. Chem. Soc. 2006, 128, 917–924. [Google Scholar] [CrossRef]
- Wang, G.; Li, W.; Jia, K.; Spliethoff, B.; Schüth, F.; Lu, A. Shape and size controlled α-Fe2O3 nanoparticles as supports for gold-catalysts: Synthesis and influence of support shape and size on catalytic performance. Appl. Catal. B Environ. 2009, 364, 42–47. [Google Scholar] [CrossRef]
- Bernal, S.; Calvino, J.J.; Cifredo, G.A.; Gatica, J.M.; Pérez Omil, J.A.; Laachir, A.; Perrichon, V. Influence of the nature of the metal precursor salt on the redox behaviour of ceria in Rh/CeO2 catalysts. Surf. Sci. Catal. 1995, 96, 419–429. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, X.D.; Zhang, M.L. Hydrothermal synthesis of sheaf-like CuO via ionic liquids. Mater. Lett. 2008, 62, 385–388. [Google Scholar] [CrossRef]
- Tang, J.; Paul Alivisatos, A. Crystal splitting in the growth of Bi2S3. Nano Lett. 2006, 6, 2701–2706. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Qin, Z.; Shan, W.; Shen, W.; Wang, J. Pd/CeO2-TiO2 catalyst for CO oxidation at low temperature: a TPR study with H2 and CO as reducing agents. J. Catal. 2004, 225, 267–277. [Google Scholar] [CrossRef]
- Hu, F.; Chen, J.; Peng, Y.; Song, H.; Li, K.; Li, J. Novel nanowire self-assembled hierarchical CeO2 microspheres for low temperature toluene catalytic combustion. Chem. Eng. J. 2018, 331, 425–434. [Google Scholar] [CrossRef]
- Fu, Q.; Wagner, T. Interaction of nanostructured metal overlayers with oxide surfaces. Surf. Sci. Rep. 2007, 62, 431–498. [Google Scholar] [CrossRef]
- Mai, H.X.; Sun, L.D.; Zhang, Y.W.; Si, R.; Feng, W.; Zhang, H.P.; Liu, H.C.; Yan, C.H. Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes. J. Phys. Chem. B 2005, 109, 24380–24385. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Li, X.; Yang, X.; Li, Z.; Wang, R.; Zhu, H. Preferential oxidation of CO in H2-rich stream over Au/CeO2-NiO catalysts: effect of the preparation method. Catal. Lett. 2018, 148, 328–340. [Google Scholar] [CrossRef]
- Francisco, M.S.P.; Mastelaro, V.; Nascente, P.A.P.; Florentino, A. Activity and characterization by XPS, HR-TEM, Raman spectroscopy, and bet surface area of CuO/CeO2-TiO2 catalysts. J. Phys. Chem. B 2001, 105, 10515–10522. [Google Scholar] [CrossRef]
- Peng, C.; Lia, H.; Liaw, B.; Chen, Y. Removal of CO in excess hydrogen over CuO/Ce1−xMnxO2 catalysts. Chem. Eng. J. 2011, 172, 452–458. [Google Scholar] [CrossRef]
- Reddy, B.M.; Khan, A.; Yamada, Y.; Kobayashi, T.; Loridant, S.; Volta, J. Structural characterization of CeO2-MO2 (M = Si4+, Ti4+ and Zr4+) mixed oxides by Raman spectroscopy, X-ray photoelectron spectroscopy, and other techniques. J. Phys. Chem. B 2003, 107, 11475–11484. [Google Scholar] [CrossRef]
- Hernández, W.Y.; Centeno, M.A.; Romero-Sarria, F.; Odriozola, J.A. Synthesis and characterization of Ce1-xEuxO2-x/2 mixed oxides and their catalytic activities for CO oxidation. J. Phys. Chem. C 2009, 113, 5629–5635. [Google Scholar] [CrossRef]
- Demoulin, O.; Navez, M.; Gaigneaux, E.M.; Ruiz, P.; Mamede, A.S.; Granger, P.; Payen, E. Operando resonance Raman spectroscopic characterisation of the oxidation state of palladium in Pd/γ-Al2O3 catalysts during the combustion of methane. Phys. Chem. Chem. Phys. 2003, 5, 4394–4401. [Google Scholar] [CrossRef]
- McBride, J.R.; Hass, K.C.; Weber, W.H. Resonance-Raman and lattice-dynamics studies of single-crystal PdO. Phys. Rev. B 1991, 44, 5016–5028. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Deng, J.; Liu, Y.; Xie, S.; Jiang, Y.; Zhao, X.; Yang, J.; Arandiyan, H.; Guo, G.; Dai, H. Three-dimensionally ordered mesoporous Co3O4-supported Au-Pd alloy nanoparticles: High-performance catalysts for methane combustion. J. Catal. 2015, 332, 13–24. [Google Scholar] [CrossRef]
- Lou, Y.; Ma, J.; Hu, W.; Dai, Q.; Wang, L.; Zhan, W.; Guo, Y.; Cao, X.M.; Guo, Y.; Hu, P.; et al. Low-temperature methane combustion over Pd/H-ZSM-5: active Pd sites with specific electronic properties modulated by acidic sites of H-ZSM-5. ACS Catal. 2016, 6, 8127–8139. [Google Scholar] [CrossRef]
- Specchia, S.; Conti, F.; Specchia, V. Kinetic studies on Pd/CexZr1-xO2 Catalyst for methane combustion. Ind. Eng. Chem. Res. 2010, 49, 11101–11111. [Google Scholar] [CrossRef]
- Misch, L.M.; Kurzman, J.A.; Derk, A.R.; Kim, Y.; Seshadri, R.; Metiu, H.; McFarland, E.W.; Stucky, G.D. C-H bond activation by Pd-substituted CeO2: substituted ions versus reduced species. Chem. Mater. 2011, 23, 5432–5439. [Google Scholar] [CrossRef]
- Gholami, R.; Smith, K.J. Activity of PdO/SiO2 catalysts for CH4 oxidation following thermal treatments. Appl. Catal. B Environ. 2015, 168, 156–163. [Google Scholar] [CrossRef]
- Sayle, T.X.T.; Parker, S.C.; Sayle, D.C. Oxidising CO to CO2 using ceria nanoparticles. Phys. Chem. Chem. Phys. 2005, 7, 2936–2941. [Google Scholar] [CrossRef]
- Qian, K.; Huang, W.X. Au–Pd alloying-promoted thermal decomposition of PdO supported on SiO2 and its effect on the catalytic performance in CO oxidation. Catal. Today 2011, 164, 320–324. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, F.; Liu, Y.; Zhang, L.; Chen, Y.; Wang, H.; Tian, Y.; Zhang, C.; Liu, D. Morphology effect of ceria on the performance of CuO/CeO2 catalysts for hydrogen production by methanol steam reforming. Int. J. Hydrogen Energy 2019, 44, 7252–7261. [Google Scholar] [CrossRef]
- Scirè, S.; Crisafulli, C.; Riccobene, P.M.; Patanè, G.; Pistone, A. Selective oxidation of CO in H2-rich stream over Au/CeO2 and Cu/CeO2 catalysts: An insight on the effect of preparation method and catalyst pretreatment. Appl. Catal. A Gen. 2012, 417, 66–75. [Google Scholar] [CrossRef]
- Wang, B.; Chi, C.; Xu, M.; Wang, C.; Meng, D. Plasma-catalytic removal of toluene over CeO2-MnOx catalysts in an atmosphere dielectric barrier discharge. Chem. Eng. J. 2017, 322, 679–692. [Google Scholar] [CrossRef]
- Bêche, E.; Charvin, P.; Perarnau, D.; Abanades, S.; Flamant, G. Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (CexTiyOz). Surf. Interface Anal. 2008, 40, 264–267. [Google Scholar] [CrossRef]




| Samples | Pd Loading (wt.%) | SBET (m2/g) | Average Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|---|
| CeO2-S | - | 66.5 | 4.2 | 0.0626 |
| CeO2-SK | - | 70.6 | 5.0 | 0.0654 |
| Pd/CeO2-S | 0.94 | 65.6 | 4.1 | 0.0602 |
| Pd/CeO2-SK | 0.93 | 68.7 | 4.6 | 0.0636 |
| Samples | Peak Position (°C) | H2 Uptake (μmol/g) | Theoretical H2 Uptake (µmol/g) a |
|---|---|---|---|
| CeO2-S | 460 | 1170 | 2904 |
| CeO2-SK | 466 | 985 | 2904 |
| Pd/CeO2-S | 450 | 448 | 2954 |
| Pd/CeO2-SK | 210; 450 | 110; 332 | 2954 |
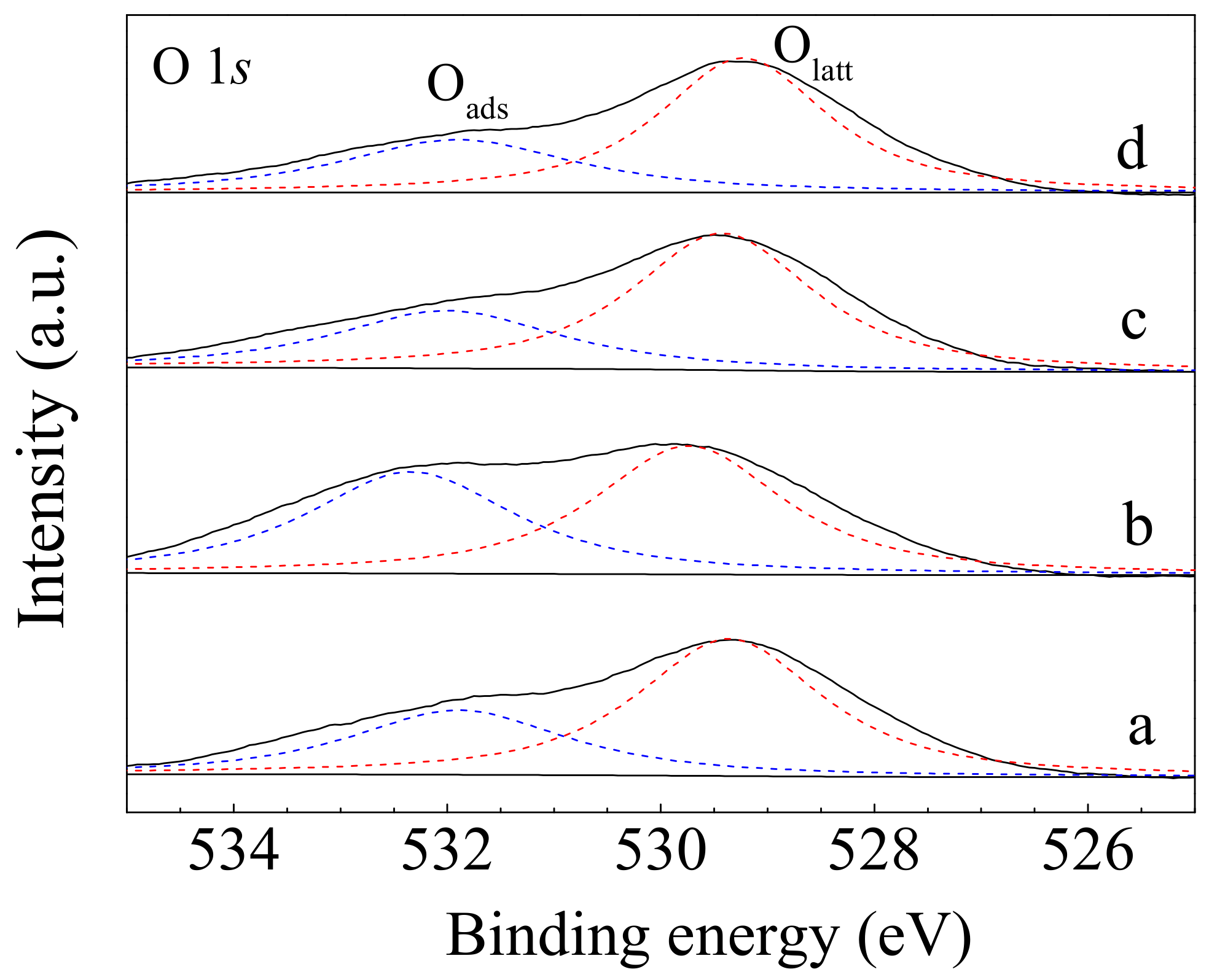
| Samples | D/F2g | Pd0 Content (%) | Ce3+ Content (%) | Oads/Olatt Ratio |
|---|---|---|---|---|
| CeO2-S | 0.068 | - | 16.5 | 0.55 |
| CeO2-SK | 0.104 | - | 19.3 | 0.97 |
| Pd/CeO2-S | 0.093 | 50.0 | 17.4 | 0.48 |
| Pd/CeO2-SK | 0.136 | 45.8 | 21.0 | 0.63 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhang, Y.; Shi, J.; Zhu, G.; Xie, Y.; Li, Z.; Wang, R.; Zhu, H. Catalytic Performance of Palladium Supported on Sheaf-Like Ceria in the Lean Methane Combustion. Nanomaterials 2020, 10, 31. https://doi.org/10.3390/nano10010031
Li S, Zhang Y, Shi J, Zhu G, Xie Y, Li Z, Wang R, Zhu H. Catalytic Performance of Palladium Supported on Sheaf-Like Ceria in the Lean Methane Combustion. Nanomaterials. 2020; 10(1):31. https://doi.org/10.3390/nano10010031
Chicago/Turabian StyleLi, Shuna, Yagang Zhang, Jing Shi, Gang Zhu, Yanxiang Xie, Zhikai Li, Ruiyi Wang, and Huaqing Zhu. 2020. "Catalytic Performance of Palladium Supported on Sheaf-Like Ceria in the Lean Methane Combustion" Nanomaterials 10, no. 1: 31. https://doi.org/10.3390/nano10010031





