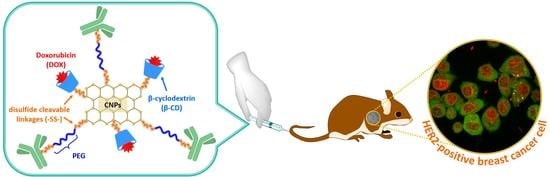
Dual pH- and GSH-Responsive Degradable PEGylated Graphene Quantum Dot-Based Nanoparticles for Enhanced HER2-Positive Breast Cancer Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
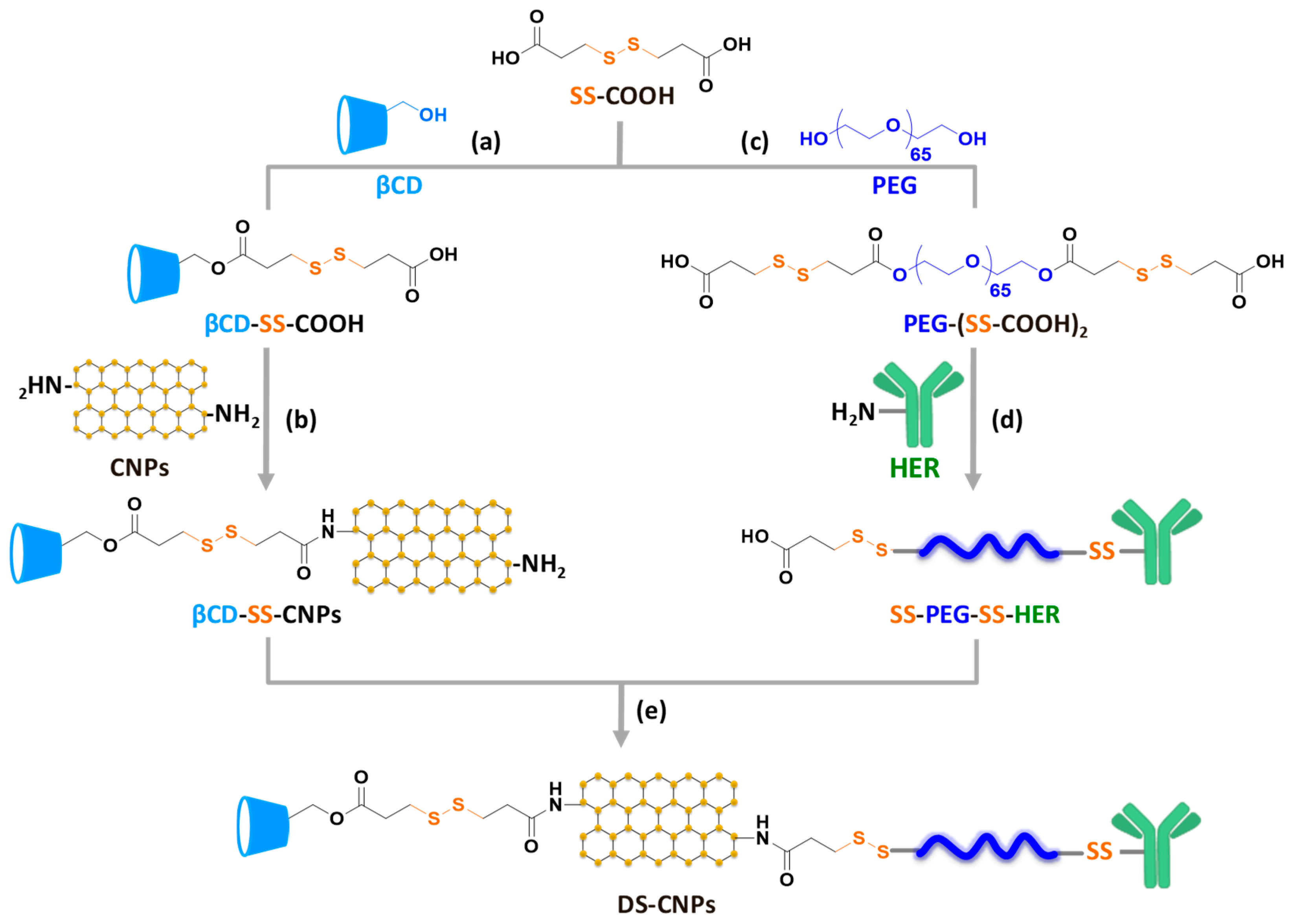
2.3. Carboxylation of βCD and Conjugation with CNPs (βCD-SS-CNPs)
2.4. Carboxylation of PEG and Conjugation with HER (SS-PEG-SS-HER)
2.5. Synthesis of Dual Stimuli-Responsive Degradable CNPs (DS-CNPs)
2.6. Preparation DOX-Loaded DS-CNPs (DL-CNPs)
2.7. pH and GSH-Triggered Release of DOX from DL-CNPs
2.8. Cell Culture
2.9. Protein Preparation and Immunoblot Analysis
2.10. Cell Viability Using the CCK-8 Assay
2.11. Confocal Laser Scanning Microscopy (CLSM)
2.12. Animal Studies
3. Results and Discussions
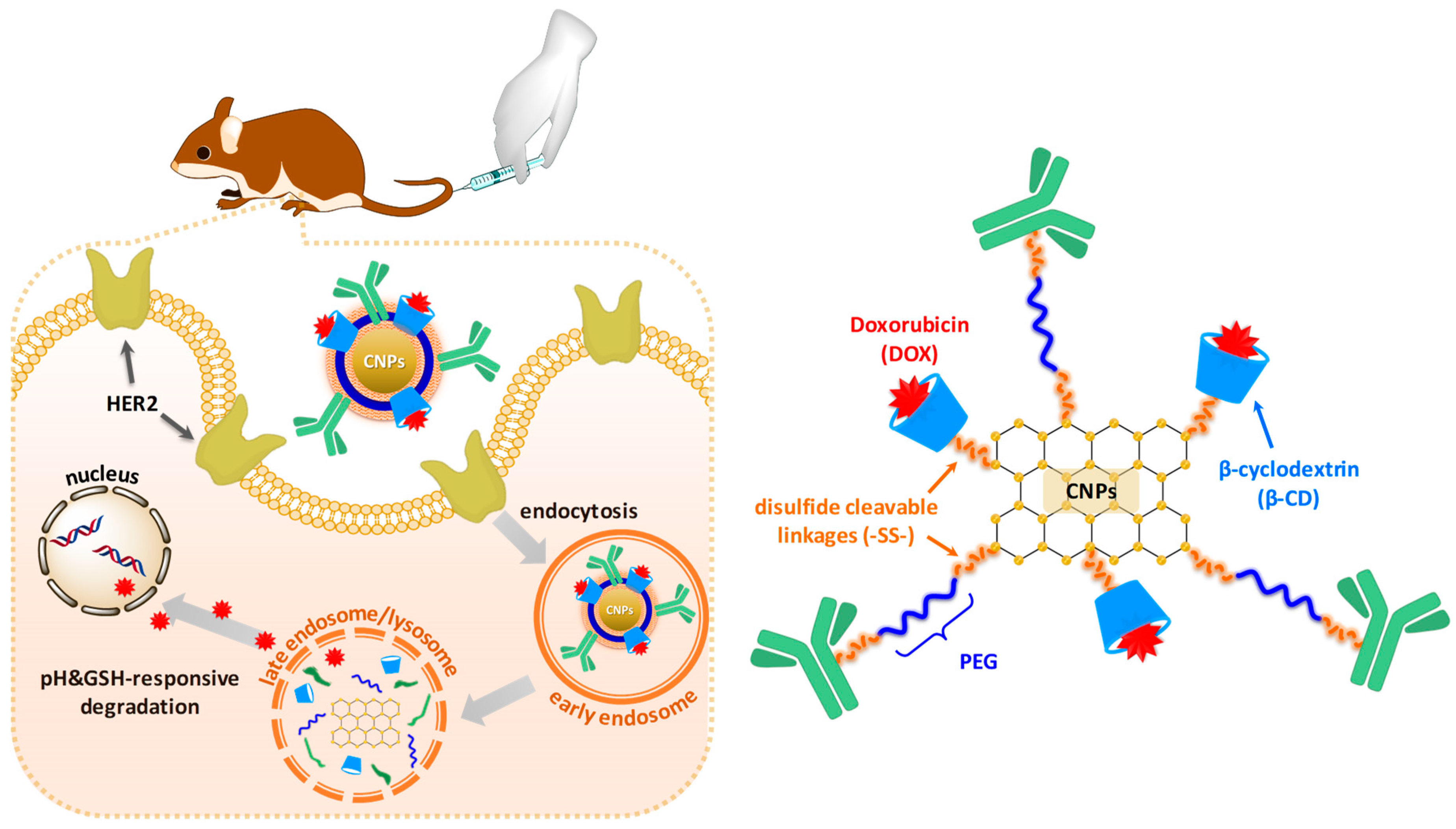
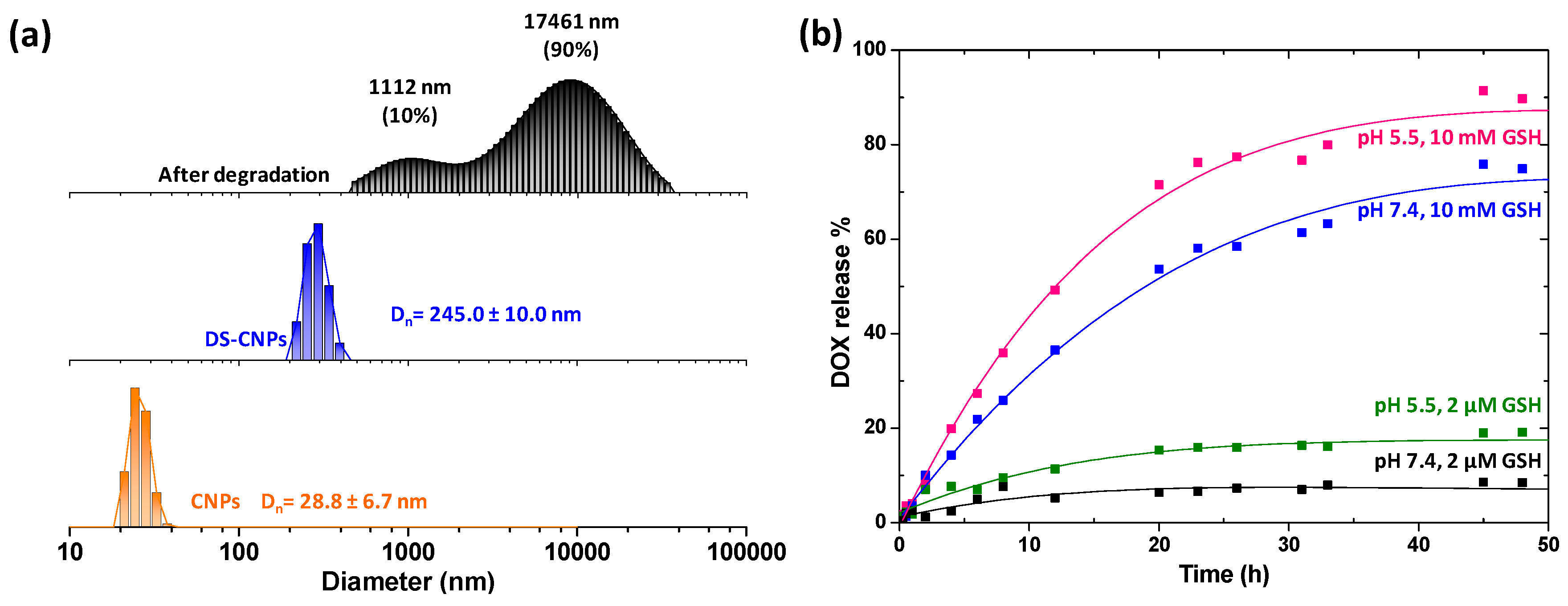
3.1. Synthesis and Characterization of DS-CNPs
3.2. Loading and External Stimuli-Trigger Release of DOX
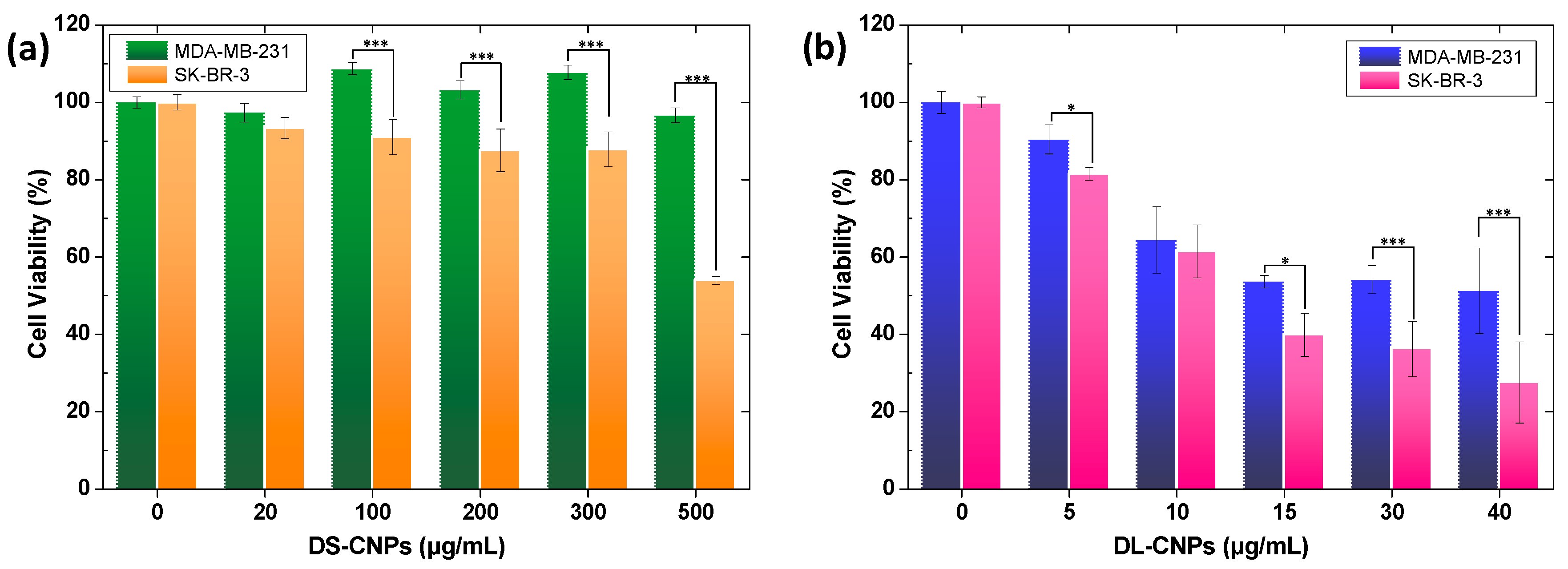
3.3. Evaluation of Active Targeting and Intracellular Accumulation In Vitro
3.4. In Vivo Antitumor Efficacy and Toxicity of DL-CNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, W.; Luo, L.; Wang, Y.; Wu, Q.; Dai, H.-B.; Li, J.-S.; Durkan, C.; Wang, N.; Wang, G.-X. Endogenous pH-responsive nanoparticles with programmable size changes for targeted tumor therapy and imaging applications. Theranostics 2018, 8, 3038–3058. [Google Scholar] [CrossRef]
- Stubelius, A.; Sheng, W.; Lee, S.; Olejniczak, J.; Guma, M.; Almutairi, A. Disease-Triggered Drug Release Effectively Prevents Acute Inflammatory Flare-Ups, Achieving Reduced Dosing. Small 2018, 14. [Google Scholar] [CrossRef]
- Li, L.; Yang, W.-W.; Xu, D.-G. Stimuli-responsive nanoscale drug delivery systems for cancer therapy. J. Drug Target. 2019, 27, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of Glutathione in Cancer Progression and Chemoresistance. J. Oxid. Med. Cell. Longev. 2013, 2013, 10. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.S.M.; Rechnitz, G.A. Determination of glutathione and glutathione reductase with a silver sulfide membrane electrode. Anal. Chem. 1982, 54, 1972–1976. [Google Scholar] [CrossRef]
- Wen, H.; Dong, C.; Dong, H.; Shen, A.; Xia, W.; Cai, X.; Song, Y.; Li, X.; Li, Y.; Shi, D. Engineered redox-responsive PEG detachment mechanism in PEGylated nano-graphene oxide for intracellular drug delivery. Small 2012, 8, 760–769. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H. Endogenous and exogenous stimuli-responsive drug delivery systems for programmed site-specific release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef] [Green Version]
- Tsarevsky, N.V.; Matyjaszewski, K. Reversible Redox Cleavage/Coupling of Polystyrene with Disulfide or Thiol Groups Prepared by Atom Transfer Radical Polymerization. Macromolecules 2002, 35, 9009–9014. [Google Scholar] [CrossRef]
- Li, C.; Madsen, J.; Armes, S.P.; Lewis, A.L. A new class of biochemically degradable, stimulus-responsive triblock copolymer gelators. Angew. Chem. Int. Ed. 2006, 45, 3510–3513. [Google Scholar] [CrossRef]
- You, Y.-Z.; Zhou, Q.-H.; Manickam, D.S.; Wan, L.; Mao, G.-Z.; Oupicky, D. Dually Responsive Multiblock Copolymers via Reversible Addition-Fragmentation Chain Transfer Polymerization: Synthesis of Temperature- and Redox-Responsive Copolymers of Poly(N-isopropylacrylamide) and Poly(2-(dimethylamino)ethyl methacrylate). Macromolecules 2007, 40, 8617–8624. [Google Scholar] [CrossRef] [Green Version]
- Talelli, M.; Rijcken, C.J.; Oliveira, S.; van der Meel, R.; van Bergen en Henegouwen, P.M.; Lammers, T.; van Nostrum, C.F.; Storm, G.; Hennink, W.E. Nanobody—Shell functionalized thermosensitive core-crosslinked polymeric micelles for active drug targeting. J. Control. Release 2011, 151, 183–192. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Mellman, I.; Fuchs, R.; Helenius, A. Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 1986, 55, 663–700. [Google Scholar] [CrossRef]
- Thomas, M.; Ge, Q.; Lu, J.J.; Chen, J.; Klibanov, A. Cross-linked small polyethylenimines: While still nontoxic, deliver DNA efficiently to mammalian cells in vitro and in vivo. Pharm. Res. 2005, 22, 373–380. [Google Scholar] [CrossRef]
- Russ, V.; Elfberg, H.; Thoma, C.; Kloeckner, J.; Ogris, M.; Wagner, E. Novel degradable oligoethylenimine acrylate ester-based pseudodendrimers for in vitro and in vivo gene transfer. Gene Ther. 2008, 15, 18. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, A.J.M.; Topp, E.M. Release from polymeric prodrugs: Linkages and their degradation. J. Pharm. Sci. 2004, 93, 1962–1979. [Google Scholar] [CrossRef]
- Knorr, V.; Russ, V.; Allmendinger, L.; Ogris, M.; Wagner, E. Acetal Linked Oligoethylenimines for Use as pH-Sensitive Gene Carriers. Bioconjug. Chem. 2008, 19, 1625–1634. [Google Scholar] [CrossRef]
- Wang, L.; Liu, G.; Wang, X.; Hu, J.; Zhang, G.; Liu, S. Acid-disintegratable polymersomes of pH-responsive amphiphilic diblock copolymers for intracellular drug delivery. Macromolecules 2015, 48, 7262–7272. [Google Scholar] [CrossRef]
- Liu, B.; Thayumanavan, S. Substituent effects on the pH sensitivity of acetals and ketals and their correlation with encapsulation stability in polymeric nanogels. J. Am. Chem. Soc. 2017, 139, 2306–2317. [Google Scholar] [CrossRef] [Green Version]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-based nanomaterials: Multifunctional materials for biomedical engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef]
- Kumar, N.; Kumbhat, S. Essentials in Nanoscience and Nanotechnology; Wiley Online Library: Hoboken, NJ, USA, 2016. [Google Scholar]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Z. Functional Graphene Oxide as a Nanocarrier for Controlled Loading and Targeted Delivery of Mixed Anticancer Drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Ko, N.R.; Nafiujjaman, M.; Lee, J.S.; Lim, H.N.; Lee, Y.k.; Kwon, I.K. Graphene quantum dot-based theranostic agents for active targeting of breast cancer. RSC Adv. 2017, 7, 11420–11427. [Google Scholar] [CrossRef] [Green Version]
- Ko, N.R.; Hong, S.H.; Nafiujjaman, M.; An, S.Y.; Revuri, V.; Lee, S.J.; Kwon, I.K.; Lee, Y.-K.; Oh, S.J. Glutathione-responsive PEGylated GQD-based nanomaterials for diagnosis and treatment of breast cancer. J. Ind. Eng. Chem. 2019, 71, 301–307. [Google Scholar] [CrossRef]
- Ross, J.S.; Slodkowska, E.A.; Symmans, W.F.; Pusztai, L.; Ravdin, P.M.; Hortobagyi, G.N. The HER-2 receptor and breast cancer: Ten years of targeted anti–HER-2 therapy and personalized medicine. Oncologist 2009, 14, 320–368. [Google Scholar] [CrossRef] [Green Version]
- Izumi, Y.; Xu, L.; di Tomaso, E.; Fukumura, D.; Jain, R.K. Tumour biology: Herceptin acts as an anti-angiogenic cocktail. Nature 2002, 416, 279–280. [Google Scholar] [CrossRef]
- Bianchini, G.; Gianni, L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 2014, 15, e58–e68. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Barry, W.T.; Dang, C.T.; Yardley, D.A.; Moy, B.; Marcom, P.K.; Albain, K.S.; Rugo, H.S.; Ellis, M.; Shapira, I.; et al. Adjuvant Paclitaxel and Trastuzumab for Node-Negative, HER2-Positive Breast Cancer. N. Engl. J. Med. 2015, 372, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Liu, H.-J.; Cheng, F.; Chen, Y. Preparation and characterization of multi stimuli-responsive photoluminescent nanocomposites of graphene quantum dots with hyperbranched polyethylenimine derivatives. Nanoscale 2014, 6, 7453–7460. [Google Scholar] [CrossRef]
- Stella, V.J.; Rao, V.M.; Zannou, E.A.; Zia, V. Mechanisms of drug release from cyclodextrin complexes. Adv. Drug Deliv. Rev. 1999, 36, 3–16. [Google Scholar] [CrossRef]
- Saha, S.; Roy, A.; Roy, K.; Roy, M.N. Study to explore the mechanism to form inclusion complexes of β-cyclodextrin with vitamin molecules. Sci. Rep. 2016, 6, 35764. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, L.l.; Fan, Y.S.; Wang, H. Host–guest supramolecular nanosystems for cancer diagnostics and therapeutics. Adv. Mater. 2013, 25, 3888–3898. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.; Claret, F.X. Trastuzumab: Updated mechanisms of action and resistance in breast cancer. Front. Oncol. 2012, 2, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Borandeh, S.; Abdolmaleki, A.; Abolmaali, S.S.; Tamaddon, A.M. Synthesis, structural and in-vitro characterization of β-cyclodextrin grafted L-phenylalanine functionalized graphene oxide nanocomposite: A versatile nanocarrier for pH-sensitive doxorubicin delivery. Carbohydr. Polym. 2018, 201, 151–161. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H. β-Cyclodextrin grafted magnetic graphene oxide applicable as cancer drug delivery agent: Synthesis and characterization. Mater. Chem. Phys. 2018, 218, 62–69. [Google Scholar] [CrossRef]
- Liang, W.; Huang, Y.; Lu, D.; Ma, X.; Gong, T.; Cui, X.; Yu, B.; Yang, C.; Dong, C.; Shuang, S. β-Cyclodextrin–Hyaluronic Acid Polymer Functionalized Magnetic Graphene Oxide Nanocomposites for Targeted Photo-Chemotherapy of Tumor Cells. Polymers 2019, 11, 133. [Google Scholar] [CrossRef] [Green Version]
- Gao, N.; Xing, C.; Wang, H.; Feng, L.; Zeng, X.; Mei, L.; Peng, Z. pH-Responsive Dual Drug-Loaded Nanocarriers Based on Poly (2-Ethyl-2-Oxazoline) Modified Black Phosphorus Nanosheets for Cancer Chemo/Photothermal Therapy. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Swierczewska, M.; Lee, K.C.; Lee, S. What is the future of PEGylated therapies? Expert Opin. Emerg. 2015, 20, 531–536. [Google Scholar] [CrossRef]
- Kurinomaru, T.; Shiraki, K. Noncovalent PEGylation of l-Asparaginase Using PEGylated Polyelectrolyte. J. Pharm. Sci. 2015, 104, 587–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichert, C.; Borchard, G. Noncovalent PEGylation, an innovative subchapter in the field of protein modification. J. Pharm. Sci. 2016, 105, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Truffi, M.; Colombo, M.; Sorrentino, L.; Pandolfi, L.; Mazzucchelli, S.; Pappalardo, F.; Pacini, C.; Allevi, R.; Bonizzi, A.; Corsi, F. Multivalent exposure of trastuzumab on iron oxide nanoparticles improves antitumor potential and reduces resistance in HER2-positive breast cancer cells. Sci. Rep. 2018, 8, 6563. [Google Scholar] [CrossRef] [PubMed]
- Dziawer, Ł.; Majkowska-Pilip, A.; Gaweł, D.; Godlewska, M.; Pruszyński, M.; Jastrzębski, J.; Wąs, B.; Bilewicz, A. Trastuzumab-modified gold nanoparticles labeled with 211At as a prospective tool for local treatment of HER2-positive breast cancer. Nanomaterials 2019, 9, 632. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Iwamoto, M.; Kimura, K.; Matsunami, N.; Morishima, H.; Yoshidome, K.; Nomura, T.; Morimoto, T.; Yamamoto, D.; Tsubota, Y. Phase II study of neoadjuvant anthracycline-based regimens combined with nanoparticle albumin-bound paclitaxel and trastuzumab for human epidermal growth factor receptor 2-positive operable breast cancer. Clin. Breast Cancer 2015, 15, 191–196. [Google Scholar] [CrossRef]
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control. Release 2014, 190, 485–499. [Google Scholar] [CrossRef] [Green Version]
- Vogel, C.L.; Cobleigh, M.A.; Tripathy, D.; Gutheil, J.C.; Harris, L.N.; Fehrenbacher, L.; Slamon, D.J.; Murphy, M.; Novotny, W.F.; Burchmore, M.; et al. First-Line Herceptin® Monotherapy in Metastatic Breast Cancer. Oncology 2001, 61, 37–42. [Google Scholar] [CrossRef]
- Osoba, D.; Slamon, D.J.; Burchmore, M.; Murphy, M. Effects on Quality of Life of Combined Trastuzumab and Chemotherapy in Women With Metastatic Breast Cancer. J. Clin. Oncol. 2002, 20, 3106–3113. [Google Scholar] [CrossRef]
- Montemurro, F.; Valabrega, G.; Aglietta, M. Trastuzumab-based combination therapy for breast cancer. Expert Opin. Pharmacother. 2004, 5, 81–96. [Google Scholar] [CrossRef]
- Rodallec, A.; Brunel, J.-M.; Giacometti, S.; Maccario, H.; Correard, F.; Mas, E.; Orneto, C.; Savina, A.; Bouquet, F.; Lacarelle, B. Docetaxel–trastuzumab stealth immunoliposome: Development and in vitro proof of concept studies in breast cancer. Int. J. Nanomed. 2018, 13, 3451. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Shao, B.; Yan, Y.; Song, G.; Liu, X.; Wang, J.; Liang, X. Efficacy and safety of trastuzumab combined with chemotherapy for first-line treatment and beyond progression of HER2-overexpressing advanced breast cancer. Chin. J. Cancer Res. 2016, 28, 330–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baselga, J. Herceptin® Alone or in Combination with Chemotherapy in the Treatment of HER2-Positive Metastatic Breast Cancer: Pivotal Trials. Oncology 2001, 61, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.; Piccart-Gebhart, M.J.; Gelber, R.D.; Procter, M.; Goldhirsch, A.; de Azambuja, E.; Castro, G.; Untch, M.; Smith, I.; Gianni, L.; et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: Final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017, 389, 1195–1205. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Singh, S.; Lillard, J.W., Jr.; Singh, R. Drug delivery approaches for breast cancer. Int. J. Nanomed. 2017, 12, 6205–6218. [Google Scholar] [CrossRef] [Green Version]
- Marty, M.; Cognetti, F.; Maraninchi, D.; Snyder, R.; Mauriac, L.; Tubiana-Hulin, M.; Chan, S.; Grimes, D.; Antón, A.; Lluch, A.; et al. Randomized Phase II Trial of the Efficacy and Safety of Trastuzumab Combined With Docetaxel in Patients With Human Epidermal Growth Factor Receptor 2–Positive Metastatic Breast Cancer Administered As First-Line Treatment: The M77001 Study Group. J. Clin. Oncol. 2005, 23, 4265–4274. [Google Scholar] [CrossRef]
- Denard, B.; Seemann, J.; Chen, Q.; Gay, A.; Huang, H.; Chen, Y.; Ye, J. The Membrane-Bound Transcription Factor CREB3L1 Is Activated in Response to Virus Infection to Inhibit Proliferation of Virus-Infected Cells. Cell Host Microbe 2011, 10, 65–74. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, N.R.; Van, S.Y.; Hong, S.H.; Kim, S.-Y.; Kim, M.; Lee, J.S.; Lee, S.J.; Lee, Y.-k.; Kwon, I.K.; Oh, S.J. Dual pH- and GSH-Responsive Degradable PEGylated Graphene Quantum Dot-Based Nanoparticles for Enhanced HER2-Positive Breast Cancer Therapy. Nanomaterials 2020, 10, 91. https://doi.org/10.3390/nano10010091
Ko NR, Van SY, Hong SH, Kim S-Y, Kim M, Lee JS, Lee SJ, Lee Y-k, Kwon IK, Oh SJ. Dual pH- and GSH-Responsive Degradable PEGylated Graphene Quantum Dot-Based Nanoparticles for Enhanced HER2-Positive Breast Cancer Therapy. Nanomaterials. 2020; 10(1):91. https://doi.org/10.3390/nano10010091
Chicago/Turabian StyleKo, Na Re, Se Young Van, Sung Hwa Hong, Seog-Young Kim, Miran Kim, Jae Seo Lee, Sang Ju Lee, Yong-kyu Lee, Il Keun Kwon, and Seung Jun Oh. 2020. "Dual pH- and GSH-Responsive Degradable PEGylated Graphene Quantum Dot-Based Nanoparticles for Enhanced HER2-Positive Breast Cancer Therapy" Nanomaterials 10, no. 1: 91. https://doi.org/10.3390/nano10010091
APA StyleKo, N. R., Van, S. Y., Hong, S. H., Kim, S.-Y., Kim, M., Lee, J. S., Lee, S. J., Lee, Y.-k., Kwon, I. K., & Oh, S. J. (2020). Dual pH- and GSH-Responsive Degradable PEGylated Graphene Quantum Dot-Based Nanoparticles for Enhanced HER2-Positive Breast Cancer Therapy. Nanomaterials, 10(1), 91. https://doi.org/10.3390/nano10010091