Substitution of Ag for Cu in Cu2ZnSn(S,Se)4: Toward Wide Band Gap Absorbers with Low Antisite Defects for Thin Film Solar Cells
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Green, M.A.; Emery, K.; Hishikawa, Y.; Warta, W.; Dunlop, E.D. Solar cell efficiency tables (Version 45). Prog. Photovolt. Res. Appl. 2015, 23, 1–9. [Google Scholar] [CrossRef]
- Mitzi, D.B.; Gunawan, O.; Todorov, T.K.; Wang, K.; Guha, S. The path towards a high-performance solution-processed kesterite solar cell. Sol. Energy Mater. Sol. Cells 2011, 95, 1421–1436. [Google Scholar] [CrossRef]
- Mitzi, D.B.; Gunawan, O.; Todorov, T.K.; Barkhouse, D.A. Prospects and performance limitations for Cu-Zn-Sn-S-Se photovoltaic technology. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2013, 371, 20110432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Feng, Y.; Cui, H.; Liu, F.; Hao, X.; Conibeer, G.; Mitzi, D.B.; Green, M. The current status and future prospects of kesterite solar cells: A brief review. Prog. Photovolt. Res. Appl. 2016, 24, 879–898. [Google Scholar] [CrossRef]
- Moushumy, N.; Das, N.; Alameh, K.; Lee, Y.T. Design and development of silver nanoparticles to reduce the reflection loss of solar cells. In Proceedings of the 8th International Conference on High-capacity Optical Networks and Emerging Technologies (IEEE), Riyadh, Saudi Arabia, 19–21 December 2011; pp. 38–41. [Google Scholar]
- Sharma, M.; Das, N.; Helwig, A.; Ahfock, T. Impact of incident light angle on the conversion efficiency of nano-structured GaAs solar cells. In Proceedings of the 2017 Australasian Universities Power Engineering Conference (AUPEC) (IEEE), Melbourne, Australia, 19–22 November 2017; pp. 1–4. [Google Scholar]
- Fadakar Masouleh, F.; Das, N.; Rozati, S.; Seyed, M. Nano-structured gratings for improved light absorption efficiency in solar cells. Energies 2016, 9, 756. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y.; Mitzi, D.B. Device characteristics of cztsse thin-film solar cells with 12.6% efficiency. Adv. Energy Mater. 2014, 4, 1301465. [Google Scholar] [CrossRef]
- Gokmen, T.; Gunawan, O.; Todorov, T.K.; Mitzi, D.B. Band tailing and efficiency limitation in kesterite solar cells. Appl. Phys. Lett. 2013, 103, 103506. [Google Scholar] [CrossRef]
- Moore, J.E.; Hages, C.J.; Agrawal, R.; Lundstrom, M.S.; Gray, J.L. The importance of band tail recombination on current collection and open-circuit voltage in CZTSSe solar cells. Appl. Phys. Lett. 2016, 109, 021102. [Google Scholar] [CrossRef]
- Werner, J.H.; Mattheis, J.; Rau, U. Efficiency limitations of polycrystalline thin film solar cells: case of Cu(In,Ga)Se2. Thin Solid Films 2005, 480, 399–409. [Google Scholar] [CrossRef]
- Rau, U.; Werner, J.H. Radiative efficiency limits of solar cells with lateral band-gap fluctuations. Appl. Phys. Lett. 2004, 84, 3735–3737. [Google Scholar] [CrossRef]
- Yuan, Z.-K.; Chen, S.; Xiang, H.; Gong, X.G.; Walsh, A.; Park, J.S.; Repins, I.; Wei, S.H. Engineering solar cell absorbers by exploring the band alignment and defect disparity: The case of Cu- and Ag-based kesterite compounds. Adv. Funct. Mater. 2015, 25, 6733–6743. [Google Scholar] [CrossRef]
- Giraldo, S.; Thersleff, T.; Larramona, G.; Neuschitzer, M.; Pistor, P.; Leifer, K.; Pérez-Rodríguez, A.; Moisan, C.; Dennler, G.; Saucedo, E. Cu2ZnSnSe4 solar cells with 10.6% efficiency through innovative absorber engineering with Ge superficial nanolayer. Prog. Photovolt. Res. Appl. 2016, 24, 1359–1367. [Google Scholar] [CrossRef]
- Su, Z.; Tan, J.M.R.; Li, X.; Zeng, X.; Batabyal, S.K.; Wong, L.H. Cation substitution of solution-processed cu2znsns4 thin film solar cell with over 9% efficiency. Adv. Energy Mater. 2015, 5, 1500682. [Google Scholar] [CrossRef]
- Cui, H.; Liu, X.; Liu, F.; Hao, X.; Song, N.; Yan, C. Boosting Cu2ZnSnS4 solar cells efficiency by a thin Ag intermediate layer between absorber and back contact. Appl. Phys. Lett. 2014, 104, 041115. [Google Scholar] [CrossRef]
- Giraldo, S.; Neuschitzer, M.; Thersleff, T.; López-Marino, S.; Sánchez, Y.; Xie, H.; Colina, M.; Placidi, M.; Pistor, P.; Izquierdo-Roca, V.; et al. Large efficiency improvement in cu2znsnse4 solar cells by introducing a superficial Ge nanolayer. Adv. Energy Mater. 2015, 5, 1501070. [Google Scholar] [CrossRef]
- Hages, C.J.; Koeper, M.J.; Agrawal, R. Optoelectronic and material properties of nanocrystal-based CZTSe absorbers with Ag-alloying. Sol. Energy Mater. Sol. Cells 2016, 145, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Liu, X.; Cui, H.; Huang, S.; Hao, X. The role of Ag in (Ag,Cu)2ZnSnS4 thin film for solar cell application. J. Alloys Compd. 2015, 625, 277–283. [Google Scholar] [CrossRef]
- Gershon, T.; Lee, Y.S.; Antunez, P.; Mankad, R.; Singh, S.; Bishop, D.; Gunawan, O.; Hopstaken, M.; Haight, R. Photovoltaic materials and devices based on the alloyed Kesterite absorber (AgxCu1−x)2ZnSnSe4. Adv. Energy Mater. 2016, 6, 1502468. [Google Scholar] [CrossRef]
- Guchhait, A.; Su, Z.H.; Tay, Y.F.; Shukla, S.; Li, W.J. Enhancement of open-circuit voltage of solution-processed Cu2ZnSnS4 solar cells with 7.2% efficiency by incorporation of silver. ACS Energy Lett. 2016, 1, 1256–1261. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Zhou, W.H.; Pei, Y.L.; Tian, Q.W.; Kou, D.X.; Zhou, Z.J.; Meng, Y.N.; Wu, S.X. High efficiency CZTSSe thin film solar cells from pure element solution: A study of additional Sn complement. Sol. Energy Mater. Sol. Cells 2016, 155, 209–215. [Google Scholar] [CrossRef]
- Berg, D.M.; Arasimowicz, M.; Djemour, R.; Gütay, L.; Siebentritt, S.; Schorr, S.; Fontané, X.; Izquierdo-Roca, V.; Pérez-Rodriguez, A.; Dale, P.J. Discrimination and detection limits of secondary phases in Cu2ZnSnS4 using X-ray diffraction and Raman spectroscopy. Thin Solid Films 2014, 569, 113–123. [Google Scholar] [CrossRef]
- Lin, X.; Kavalakkatt, J.; Kornhuber, K.; Levcenko, S.; Lux-Steiner, M.C.; Ennaoui, A. Structural and optical properties of Cu2ZnSnS4 thin film absorbers from ZnS and Cu3SnS4 nanoparticle precursors. Thin Solid Films 2013, 535, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.; Tabata, T.; Takei, K.; Morihama, M.; Maeda, T.; Wada, T. Crystallographic and optical properties of (Cu,Ag)2ZnSnS4 and (Cu,Ag)2ZnSnSe4 solid solutions. Phys. Status Solidi C 2015, 12, 700–703. [Google Scholar] [CrossRef]
- Khare, A.; Himmetoglu, B.; Johnson, M.; Norris, D.J.; Cococcioni, M.; Aydil, E.S. Calculation of the lattice dynamics and Raman spectra of copper zinc tin chalcogenides and comparison to experiments. J. Appl. Phys. 2012, 111, 083707. [Google Scholar] [CrossRef]
- Chen, H.; Yu, S.M.; Shin, D.W.; Yoo, J.B. Solvothermal synthesis and characterization of chalcopyrite CuInSe2 nanoparticles. Nano. Res. Lett. 2009, 5, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danilson, M.; Altosaar, M.; Kauk, M.; Katerski, A.; Krustok, J.; Raudoja, J. XPS study of CZTSSe monograin powders. Thin Solid Films 2011, 519, 7407–7411. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, D.; Sui, Y.; Wu, Y.; Wang, Z.; Yang, L.; Wang, F.; Lv, S.; Yao, B. Synthesis and investigation of environmental protection and earth-abundant kesterite Cu2MgxZn1-xSn(S,Se)4 thin films for solar cells. Ceram. Int. 2018, 44, 15249–15255. [Google Scholar] [CrossRef]
- Tsega, M.; Dejene, F.B.; Kuo, D.H. Morphological evolution and structural properties of Cu2ZnSn(S,Se)4 thin films deposited from single ceramic target by a one-step sputtering process and selenization without H2Se. J. Alloys Compd. 2015, 642, 140–147. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Sui, Y.; Wang, Z.; Lv, S.; Wei, M.; Sun, Y.; Yao, B.; Liu, X.; Yang, L. Bandgap engineering of Cu2InxZn1−xSn(S,Se)4 alloy films for photovoltaic applications. Ceram. Int. 2018, 44, 1942–1950. [Google Scholar] [CrossRef]
- Chen, S.; Gong, X.G.; Walsh, A.; Wei, S.H. Defect physics of the kesterite thin-film solar cell absorber Cu2ZnSnS4. Appl. Phys. Lett. 2010, 96, 021902. [Google Scholar] [CrossRef]
- Sutter-Fella, C.M.; Stückelberger, J.A.; Hagendorfer, H.; La Mattina, F.; Kranz, L.; Nishiwaki, S.; Uhl, A.R.; Romanyuk, Y.E.; Tiwari, A.N. Sodium assisted sintering of chalcogenides and its application to solution processed Cu2ZnSn(S,Se)4 thin film solar cells. Chem. Mater. 2014, 26, 1420–1425. [Google Scholar] [CrossRef]
- Maeda, T.; Kawabata, A.; Wada, T. First-principles study on alkali-metal effect of Li, Na, and K in Cu2ZnSnS4 and Cu2ZnSnSe4. Phys. Status Solidi C 2015, 12, 631–637. [Google Scholar] [CrossRef]
- Hsieh, Y.-T.; Han, Q.; Jiang, C.; Song, T.B.; Chen, H.; Meng, L.; Zhou, H.; Yang, Y. Efficiency enhancement of Cu2ZnSn(S,Se)4 solar cells via alkali metals doping. Adv. Energy Mater. 2016, 6, 1502386. [Google Scholar] [CrossRef]
- Chen, S.; Walsh, A.; Luo, Y.; Yang, J.H.; Gong, X.G.; Wei, S.-H. Wurtzite-derived polytypes of kesterite and stannite quaternary chalcogenide semiconductors. Phys. Rev. B 2010, 82, 195203. [Google Scholar] [CrossRef] [Green Version]
- Walsh, A.; Chen, S.; Wei, S.-H.; Gong, X.G. Kesterite thin-film solar cells: advances in materials modelling of Cu2ZnSnS4. Adv. Energy Mater. 2012, 2, 400–409. [Google Scholar] [CrossRef]
- Chen, S.; Gong, X.G.; Walsh, A.; Wei, S.H. Electronic structure and stability of quaternary chalcogenide semiconductors derived from cation cross-substitution of II-VI and I-III-VI2 compounds. Phy. Rev. B 2009, 79, 165211. [Google Scholar] [CrossRef]
- Fu, J.; Tian, Q.; Zhou, Z.; Kou, D.; Meng, Y.; Zhou, W.; Wu, S. Improving the performance of solution-processed Cu2ZnSn(S,Se)4 photovoltaic materials by Cd2+ substitution. Chem. Mater. 2016, 28, 5821–5828. [Google Scholar] [CrossRef]
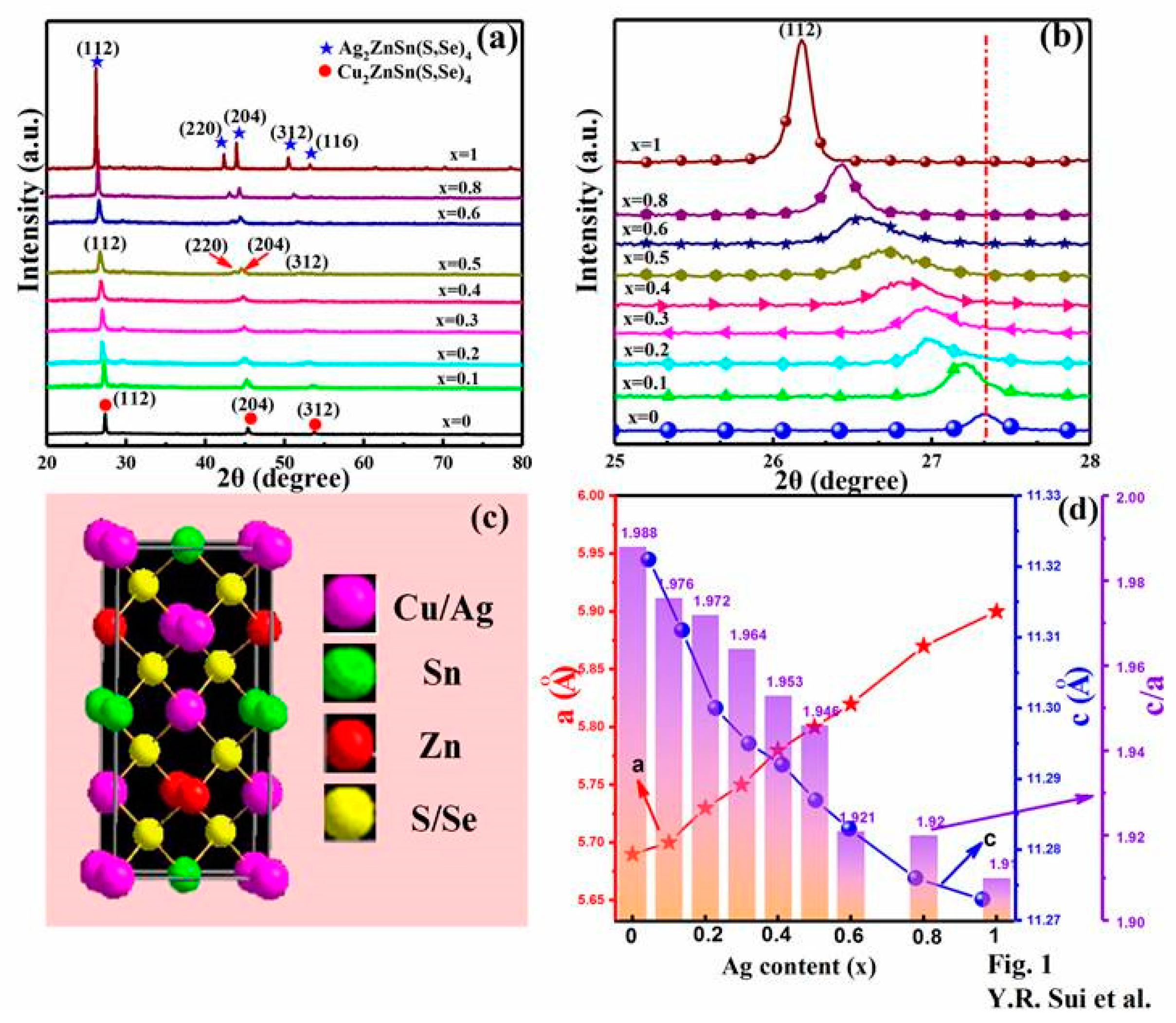



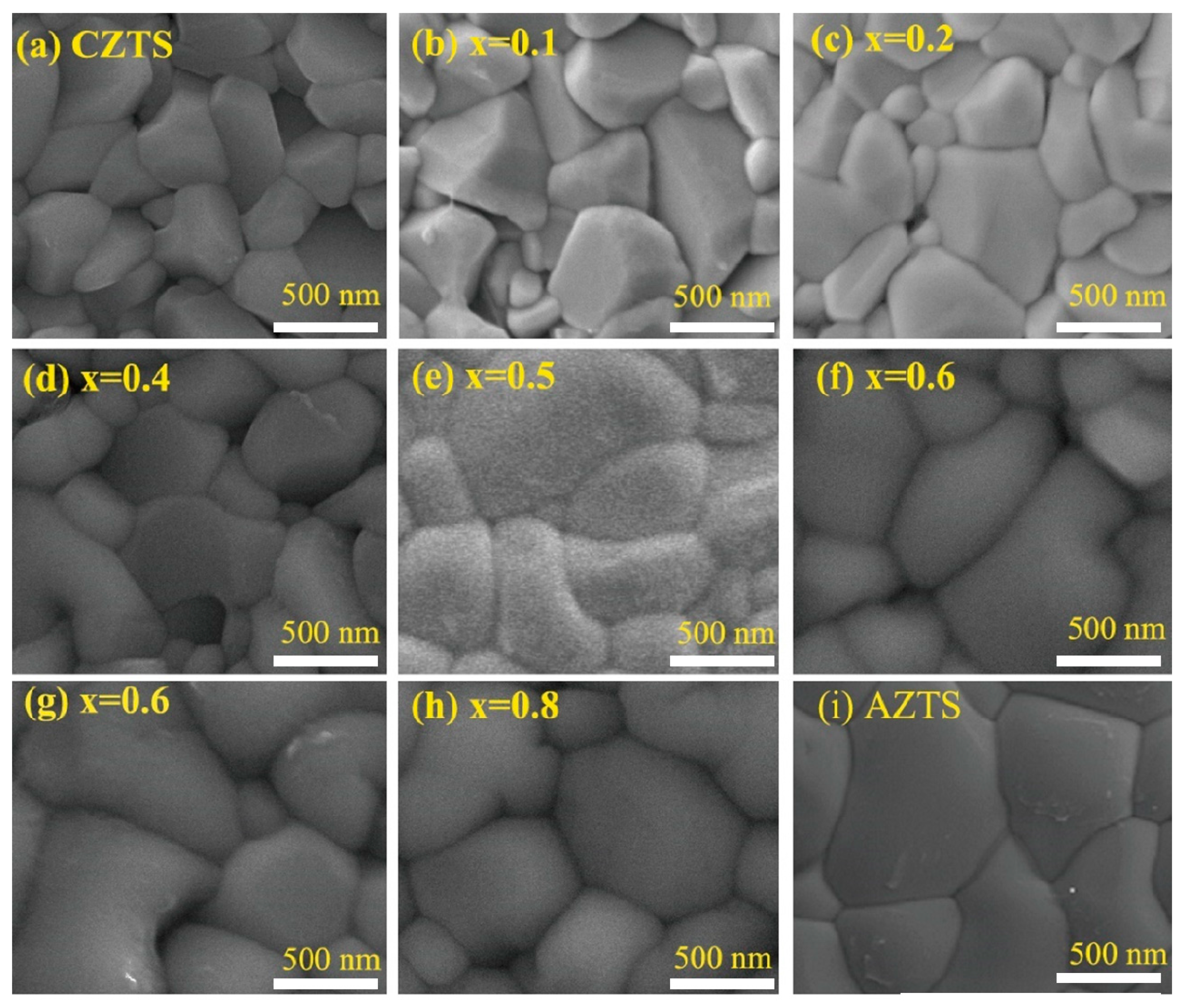


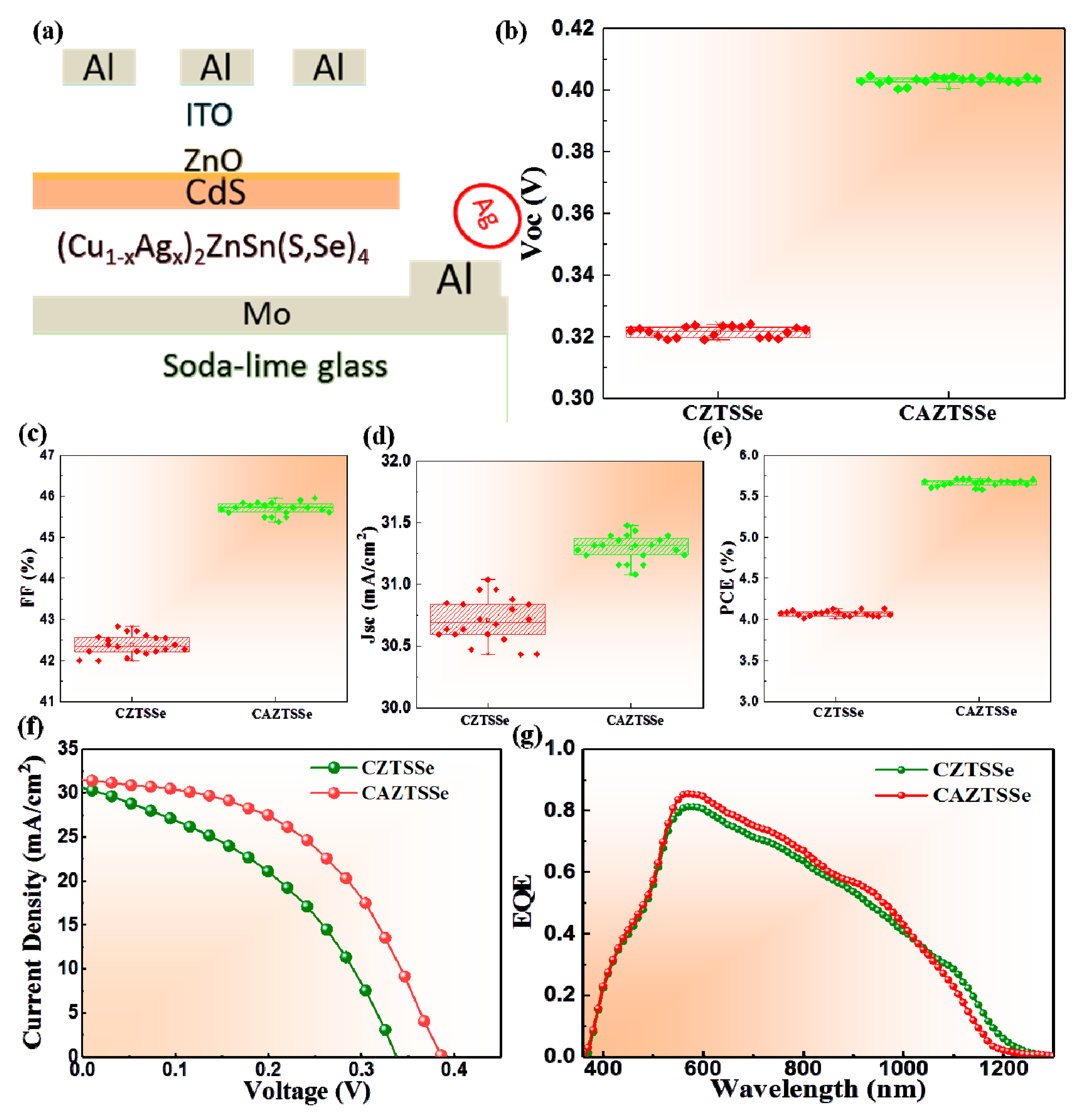
| Sample | Cu (at%) | Zn (at%) | Sn (at%) | Ag (at%) | Se (at%) | S (at%) | (Ag+Cu)/Zn+Sn | Ag/(Cu+Ag) |
|---|---|---|---|---|---|---|---|---|
| x = 0.0 | 21.01 | 12.51 | 12.21 | 0.00 | 50.57 | 5.52 | 0.85 | 0.00 |
| x = 0.1 | 18.91 | 12.81 | 12.12 | 2.02 | 50.23 | 4.93 | 0.84 | 0.09 |
| x = 0.2 | 16.44 | 12.16 | 12.52 | 4.22 | 49.60 | 5.80 | 0.84 | 0.20 |
| x = 0.3 | 14.73 | 12.25 | 12.52 | 6.25 | 50.56 | 4.64 | 084 | 0.29 |
| x = 0.4 | 13.54 | 13.52 | 10.59 | 8.09 | 54.47 | 4.70 | 0.89 | 0.37 |
| x = 0.5 | 12.42 | 12.03 | 12.38 | 9.30 | 50.77 | 5.22 | 0.88 | 0.42 |
| x = 0.6 | 9.24 | 12.08 | 12.18 | 11.76 | 50.27 | 5.62 | 0.87 | 0.56 |
| x = 0.8 | 5.46 | 12.09 | 12.46 | 15.54 | 50.29 | 5.76 | 0.85 | 0.74 |
| x = 1 | 0.00 | 12.13 | 12.47 | 20.16 | 50.07 | 5.93 | 0.86 | 1.00 |
| Device | Active Area | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) | Rs (Ω cm2) | Rsh (Ω cm2) |
|---|---|---|---|---|---|---|---|
| CZTSSe | 0.19 cm2 | 0.33 | 30.28 | 43 | 4.24 | 2.5 | 456.5 |
| CAZTSSe (x = 0.1) | 0.19 cm2 | 0.49 | 31.41 | 46 | 5.95 | 1.2 | 675.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Sui, Y.; He, W.; Zeng, F.; Wang, Z.; Wang, F.; Yao, B.; Yang, L. Substitution of Ag for Cu in Cu2ZnSn(S,Se)4: Toward Wide Band Gap Absorbers with Low Antisite Defects for Thin Film Solar Cells. Nanomaterials 2020, 10, 96. https://doi.org/10.3390/nano10010096
Wu Y, Sui Y, He W, Zeng F, Wang Z, Wang F, Yao B, Yang L. Substitution of Ag for Cu in Cu2ZnSn(S,Se)4: Toward Wide Band Gap Absorbers with Low Antisite Defects for Thin Film Solar Cells. Nanomaterials. 2020; 10(1):96. https://doi.org/10.3390/nano10010096
Chicago/Turabian StyleWu, Yanjie, Yingrui Sui, Wenjie He, Fancong Zeng, Zhanwu Wang, Fengyou Wang, Bin Yao, and Lili Yang. 2020. "Substitution of Ag for Cu in Cu2ZnSn(S,Se)4: Toward Wide Band Gap Absorbers with Low Antisite Defects for Thin Film Solar Cells" Nanomaterials 10, no. 1: 96. https://doi.org/10.3390/nano10010096
APA StyleWu, Y., Sui, Y., He, W., Zeng, F., Wang, Z., Wang, F., Yao, B., & Yang, L. (2020). Substitution of Ag for Cu in Cu2ZnSn(S,Se)4: Toward Wide Band Gap Absorbers with Low Antisite Defects for Thin Film Solar Cells. Nanomaterials, 10(1), 96. https://doi.org/10.3390/nano10010096




