Simulations of Graphene Nanoribbon Field Effect Transistor for the Detection of Propane and Butane Gases: A First Principles Study
Abstract
:1. Introduction
2. Materials and Methods
Graphene Armchair and Zigzag Nanoribbons
3. Results and Discussion
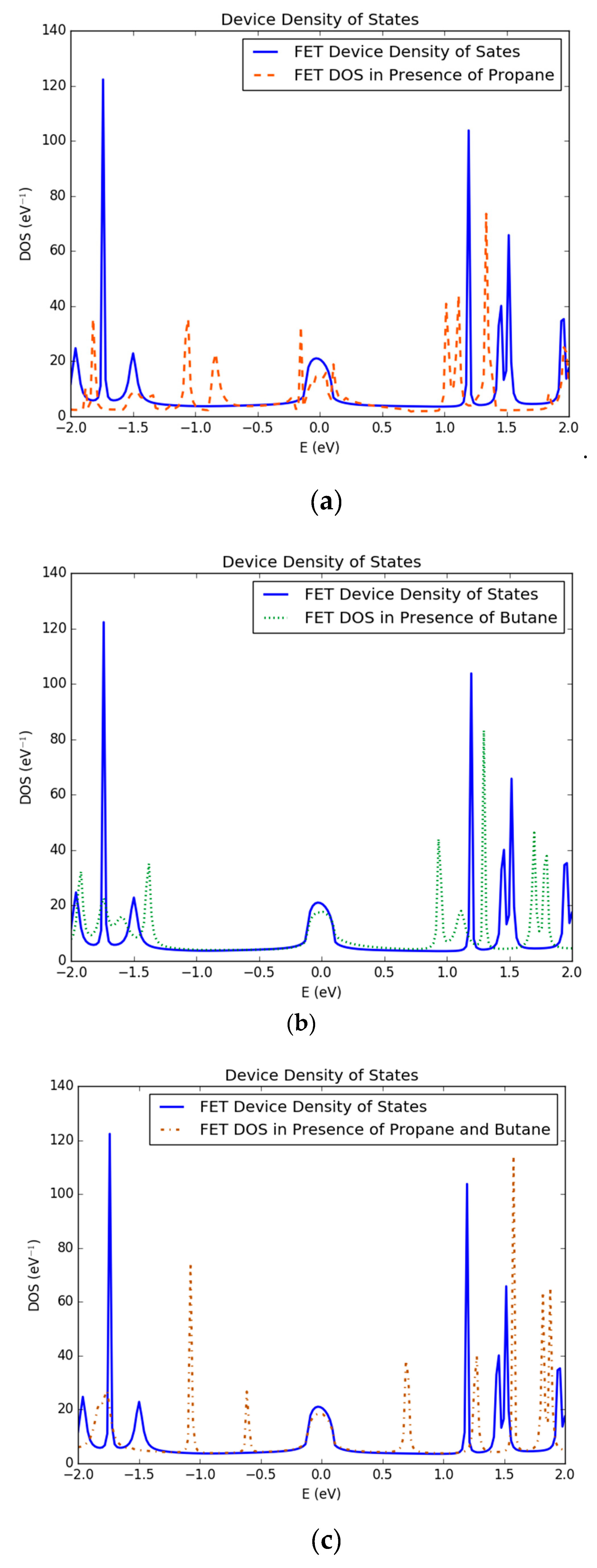
3.1. Density of States of Simulated Graphene Nanoribbon Field Effect Transistor Device
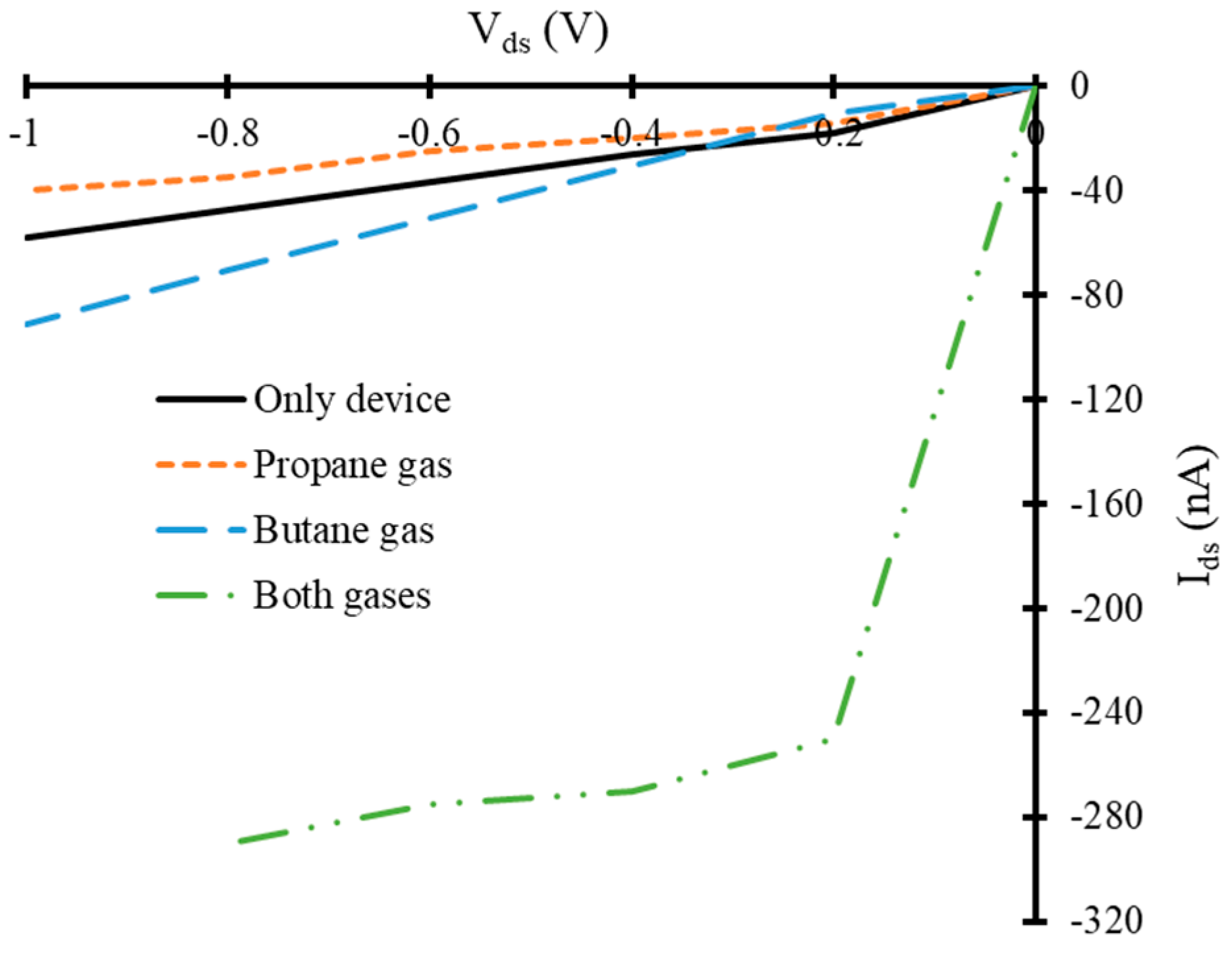
3.2. Current-Voltage Characteristics of Simulated Graphene Nanoribbon Field Effect Transistor Device in Presence of Only Propane and Butane Molecules
3.3. Influence of Oxygen and Water Molecules on the Current-voltage Characteristics of Simulated Graphene Nanoribbon Field Effect Transistor
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joshi, S.; Bhatt, V.D.; Wu, H.; Becherer, M.; Lugli, P. Flexible lactate and glucose sensors using electrolyte-gated carbon nanotube field effect transistor for non-invasive real-time monitoring. IEEE Sens. J. 2017, 17, 4315–4321. [Google Scholar] [CrossRef]
- Hosseini-Golgoo, S.M.; Salimi, F.; Saberkari, A.; Rahbarpour, S. Comparison of information content of temporal response of chemoresistive gas sensor under three different temperature modulation regimes for gas detection of different feature reduction methods. J. Phys. 2017, 939, 012005. [Google Scholar] [CrossRef]
- Moseley, P.T. Solid state gas sensors. Meas. Sci. Technol. 1997, 8, 223–237. [Google Scholar] [CrossRef]
- Simonetta, C.; Forleo, A.; Francioso, L.; Rella, R.; Siciliano, P.; Spadavecchia, J.; Presicce, D.S.; Taurino, A.M.; Forleo, D.A. Solid state gas sensors: State of the art and future activities. J. Optoelectron. Adv. Mater. 2003, 5, 1335–1348. [Google Scholar]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Horn, P.M. Low-frequency fluctuations in solids: 1/f noise. Rev. Mod. Phys. 1981, 53, 497–516. [Google Scholar] [CrossRef] [Green Version]
- Ramamoorthy, R.; Dutta, P.K.; Akbar, S.A. Oxygen sensors: Materials, methods, designs and applications. J. Mater. Sci. 2003, 38, 4271–4282. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance Spectroscopy: Emphasizing Solid Materials and Systems; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Park, C.O.; Akbar, S.A.; Weppner, W. Ceramic electrolytes and electrochemical sensors. J. Mater. Sci. 2003, 38, 4639–4660. [Google Scholar] [CrossRef]
- Liu, Y.; Parisi, J.; Sun, X.; Lei, Y. Solid-state gas sensors for high temperature applications-a review. J. Mater. Chem. A 2014, 2, 9919–9943. [Google Scholar] [CrossRef]
- Korotcenkov, G. Gas response control through structural and chemical modification of metal oxide films: State of the art and approaches. Sens. Actuators B Chem. 2005, 107, 209–232. [Google Scholar] [CrossRef]
- Jiménez-Cadena, G.; Riu, J.; Rius, F.X. Gas sensors based on nanostructured materials. Analyst 2007, 132, 1083–1099. [Google Scholar]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I. Dtection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Perkins, F.K.; Snow, E.S.; Wei, Z.; Sheehan, P.E. Reduced Graphene Oxide Molecular Sensors. Nano Lett. 2008, 8, 3137–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Goldsmith, B.R.; Kybert, N.J.; Johnson, A.T.C. DNA Decorated Graphene Chemical Sensors. Appl. Phys. Lett. 2010, 97, 83107. [Google Scholar] [CrossRef]
- Pearce, R.; Iakimov, T.; Andersson, M.; Hultman, L.; Spetz, A.L.; Yakimova, R. Epitaxially grown graphene based gas sensors for ultra sensitive NO2 detection. Sens. Actuators B Chem. 2011, 155, 451–455. [Google Scholar] [CrossRef] [Green Version]
- Ohno, K.; Maehashi, K.; Matsumoto, K. Chemical and biological sensing applications based on graphene field-effect transistors. Biosens. Bioelectron. 2010, 26, 1727–1730. [Google Scholar] [CrossRef]
- Syu, Y.-C.; Hsu, W.-E.; Lin, C.-T. Review—Field effect transistor biosensing: Devices and clinical application. ECS J. Solid State Sci. Technol. 2018, 7, Q3196–Q3207. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Im, J.P.; Lim, S.Y.; Kwon, J.Y.; Lee, S.M.; Moon, S.E. MEMS-based NO2 gas sensor using ZnO nano-rods for low-power IoT application. J. Korean Phys. Soc. 2017, 70, 924–928. [Google Scholar] [CrossRef]
- Bogue, R. Recent developments in MEMS sensors: A review of applications, markets and technologies. Sens. Rev. 2013, 33, 300–304. [Google Scholar] [CrossRef]
- Morales-Naraez, E.; Merkoci, A. Graphene oxide as an optical biosensing platform. Adv. Mater. 2012, 24, 3298–3308. [Google Scholar] [CrossRef]
- Varghese, S.S.; Lonkar, S.; Singh, K.K.; Swaminathan, S.; Abdala, A. Recent Advances in graphene based gas sensors. Sens. Actuators B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Yuan, W.; Shi, G. Graphene-based gas sensors. J. Mater. Chem. A 2013, 1, 10078–10091. [Google Scholar] [CrossRef]
- Latif, U.; Dickert, F.L. Graphene hybrid materials in gas sensing applications. Sensors 2015, 15, 30504–30524. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice. Mater. Sci. Eng. B 2007, 139, 1–13. [Google Scholar] [CrossRef]
- Rumyantsev, S.; Liu, G.; Potyrailo, R.A.; Balandin, A.A.; Shur, M.S. Selective sensing of individual gases using graphene devices. IEEE Sens. J. 2013, 13, 2818–2822. [Google Scholar] [CrossRef]
- Natgaz. Propane vs. Butane. Available online: http://www.natgaz.com.lb/all-about-gaz/propane-vs-butane (accessed on 10 September 2019).
- National Research Council USA. Butane: Acute exposure guide levels. In Acute Exposure Guideline Levels for Selected Airborne Chemicals; National Academies Press (US): Washington, DC, USA, 2012; Volume 12. Available online: https://www.ncbi.nlm.nih.gov/books/NBK201460/olume12 (accessed on 10 September 2019).
- Yan, Q.; Huang, B.; Yu, J.; Zheng, F.; Zang, J.; Wu, J.; Gu, B.; Liu, F.; Duan, W. Intrinsic current-voltage characteristics of graphene nanoribbon transistors and effect of edge doping. Nano Lett. 2007, 7, 1469–1473. [Google Scholar] [CrossRef]
- Erol, O.; Uyan, I.; Hatip, M.; Yilmaz, C.; Tekinay, A.B.; Guler, M.O. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2433–2454. [Google Scholar] [CrossRef] [Green Version]
- Rashid, M.H.; Koel, A.; Rang, T. Simulations of propane and butane gas sensor based on pristine armchair graphene nanoribbon. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bucharest, Romania, 7–9 March 2018; Volume 362. [Google Scholar]
- Narendar, V.; Gupta, S.K.; Saxena, S. First principle study of doped graphene for FET applications. Silicon 2019, 11, 277–286. [Google Scholar] [CrossRef]
- Siddique, S.; Mukhtar, H. Probing the performance of glucose oxidase treated graphene-based field effect transistors. J. Nanosci. Nanotechnol. 2019, 19, 7442–7446. [Google Scholar] [CrossRef]
- Hasan, N.; Hou, B.; Radadia, A.D. Ion sensing with solution-gated graphene field-effect sensors in the frequency domain. IEEE Sens. J. 2019, 19, 8758–8766. [Google Scholar] [CrossRef]
- Tsang, D.K.H.; Lieberthal, T.J.; Watts, C.; Dunlop, E.L.; Ramadan, S.; Hernandez, A.E.D.R.; Klien, N. Chemically functionalised graphene FET biosensor for the label-free sensing of exosomes. Sci. Rep. 2019, 9, 1–10. [Google Scholar]
- QuantumWise User Manual, ATK-DFT Calculator. Available online: https://docs.quantumwise.com/v2017/manuals/ATKDFT.html (accessed on 14 October 2019).
- High Performance Computing. Available online: https://www.ttu.ee/support-structure/it-services/services-4/high-performance-computing-2/ (accessed on 15 October 2019).
- Poklonski, N.A.; Kislyakov, E.F.; Vyrko, S.A.; Bubel’, O.N.; Ratkevich, S.V. Electronic band structure and magnetic states of zigzag graphene nanoribbons: Quantum chemical calculations. J. Nanophotonics 2012, 6, 61712. [Google Scholar] [CrossRef]
- Owens, F.J. Electronic and magnetic properties of armchair and zigzag graphene nanoribbons. J. Chem. Phys. 2008, 128, 194701. [Google Scholar] [CrossRef] [PubMed]
- Quantumwise, Building a Graphene Nanoribbon Transistor. Available online: https://docs.quantumwise.com/v2017/tutorials/vnl_graphene_transistor/vnl_graphene_transistor.html (accessed on 16 October 2019).
- Yavari, F.; Koratkar, N. Graphene-based chemical sensors. J. Phys. Chem. Lett. 2012, 3, 1746–1753. [Google Scholar] [CrossRef]
- Bekduz, B.; Kampermann, L.; Mertin, W.; Punckt, C.; Aksay, I.A.; Bacher, G. Influence of the atmospheric species on the electrical properties of functionalized graphene sheets. RCS Adv. 2018, 8, 42073–42079. [Google Scholar] [CrossRef] [Green Version]
- Melios, C.; Centeno, A.; Zurutuza, A.; Panchal, V.; Giusca, C.E.; Spencer, S.; Silva, S.R.P.; Kazakova, O. Effects of humidity on the electronic properties of graphene prepared by chemical vapour deposition. Carbon 2016, 103, 273–280. [Google Scholar] [CrossRef]
- What Effect Does Humidity Have on Graphene Sensors? Available online: https://www.azosensors.com/article.aspx?ArticleID=1323 (accessed on 3 December 2019).
- Yao, Y.; Chen, X.; Zhu, J.; Zeng, B.; Wu, Z.; Li, X. The effect of ambient humidity on the electrical properties of graphene oxide films. Nanoscale Res. Lett. 2012, 7, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.D.; Elgammal, K.; Fan, X.; Lemme, M.C.; Delin, A.; Rasander, M.; Bergqvit, L.; Schroder, S.; Fischer, A.C.; Niklaus, F.; et al. Graphene-based CO2 sensing and its cross-sensitivity with humidity. RSC Adv. 2017, 7, 22329–22339. [Google Scholar] [CrossRef] [Green Version]
- Eugster, W.; Kling, G.W. Performance of low-cost methane sensor for ambient concentration measurements in preliminary studies. Atmos. Meas. Tech. 2012, 5, 1925–1934. [Google Scholar] [CrossRef] [Green Version]
- Tao, C.; Li, X.; Yang, J.; Shi, Y. Optical fiber sensing element based on luminescence quenching of silica nanowires modified with cryptophane-A for the detection of methane. Sens. Actuators B Chem. 2011, 156, 553–558. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Zhu, S.; Zhou, Z.; Yao, Y.; Quan, W.; Liu, B. Room Temperature Methane Sensor Based on Graphene Nanosheets/Polyaniline Nanocomposite Thin Film. IEEE Sens. 2013, 13, 777–782. [Google Scholar] [CrossRef]
- Roy, S.; Sarkar, C.K.; Bhattacharyya, P. A highly sensitive methane sensor with nickel alloy microheater on micromachined Si substrate. Solid State Electron. 2012, 76, 84–90. [Google Scholar] [CrossRef]
- Haridas, D.; Gupta, V. Enhanced response characteristics of SnO2 thin film based sensors loaded with Pd clusters for methane detection. Sens. Actuators B Chem. 2012, 166, 156–164. [Google Scholar] [CrossRef]
- Prasad, A.K.; Amirthapandian, S.; Dhara, S.; Dash, S.; Murali, N.; Tyagi, A.K. Novel single phase vanadium dioxide nanostructured films for methane sensing near room temperature. Sens. Actuators B Chem. 2014, 191, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Vuong, N.M.; Hieu, N.M.; Hieu, H.N.; Yi, H.; Kim, D.; Han, Y.S.; Kim, M. Ni2O3-decorated SnO2 particulate films for methane gas sensors. Sens. Actuators B Chem. 2014, 192, 327–333. [Google Scholar] [CrossRef]
- Gong, G.; Zhu, H. Development of highly sensitive sensor system for methane utilizing cataluminescence. Luminescence 2015, 31, 183–189. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Zhao, Y.; Wang, Q. Measurement of methane concentration with cryptophane E infiltrated photonic crystal microcavity. Sens. Actuators B Chem. 2015, 209, 431–437. [Google Scholar] [CrossRef]
- Yang, D.; Yang, N.; Ni, J.; Xiao, J.; Jiang, J.; Liang, Q.; Ren, T.; Chen, X. First-principles approach to design and evaluation of graphene as methane sensors. Mater. Des. 2017, 119, 401. [Google Scholar] [CrossRef]
- Humayun, M.T.; Divan, R.; Stan, L.; Rosenmann, D.; Gosztola, D.; Paul, L.G.; Solomon, A.; Paprotny, I. Ubiquitous low-cost functionalized multi-walled carbon nanotube sensors for distributed methane leak detection. IEEE Sens. J. 2016, 16, 8692–8699. [Google Scholar] [CrossRef]
- Humayun, M.T.; Divan, R.; Liu, Y.; Gundel, L.; Solomon, P.; Paprotny, I. Novel chemoresistive CH4 sensor with 10 ppm sensitivity based on multiwalled carbon nanotubes functionalized with SnO2 nanocrystals. J. Vac. Sci. Technol. A Vac. Surf. Films 2016, 34, 01A131. [Google Scholar] [CrossRef]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, M.H.; Koel, A.; Rang, T. Simulations of Graphene Nanoribbon Field Effect Transistor for the Detection of Propane and Butane Gases: A First Principles Study. Nanomaterials 2020, 10, 98. https://doi.org/10.3390/nano10010098
Rashid MH, Koel A, Rang T. Simulations of Graphene Nanoribbon Field Effect Transistor for the Detection of Propane and Butane Gases: A First Principles Study. Nanomaterials. 2020; 10(1):98. https://doi.org/10.3390/nano10010098
Chicago/Turabian StyleRashid, Muhammad Haroon, Ants Koel, and Toomas Rang. 2020. "Simulations of Graphene Nanoribbon Field Effect Transistor for the Detection of Propane and Butane Gases: A First Principles Study" Nanomaterials 10, no. 1: 98. https://doi.org/10.3390/nano10010098
APA StyleRashid, M. H., Koel, A., & Rang, T. (2020). Simulations of Graphene Nanoribbon Field Effect Transistor for the Detection of Propane and Butane Gases: A First Principles Study. Nanomaterials, 10(1), 98. https://doi.org/10.3390/nano10010098




