Molecular Dynamics Studies of Poly(Lactic Acid) Nanoparticles and Their Interactions with Vitamin E and TLR Agonists Pam1CSK4 and Pam3CSK4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pam3CSK4 Encapsulation into PLA NPs
2.2. Vitamin E Encapsulation into PLA NPs
2.3. NP Physicochemical Characterization
2.4. Quantification of Vitamin E Loading
2.5. Molecular Dynamics
3. Results
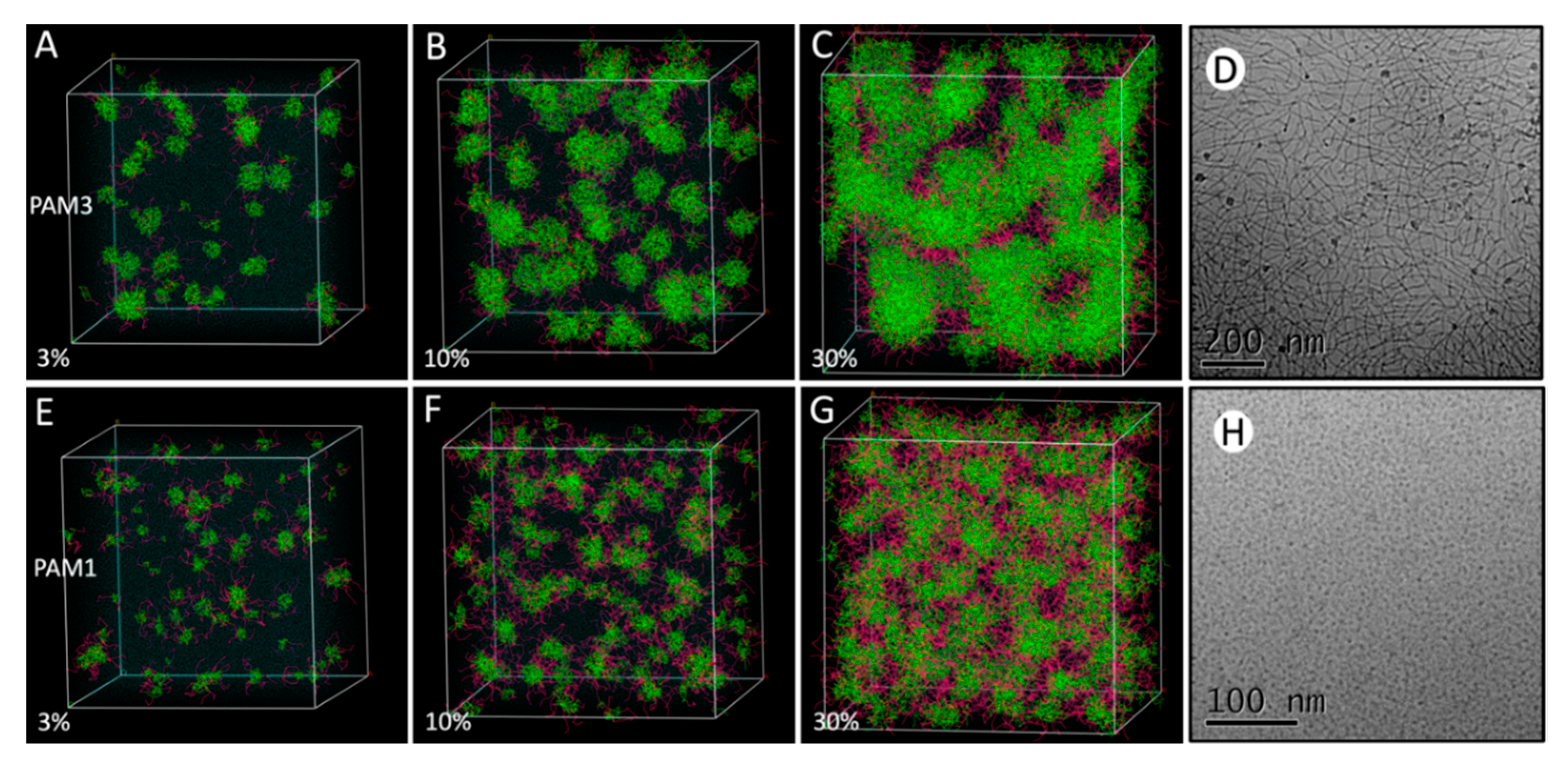
3.1. Behavior of Pam1CSK4 and Pam3CSK4 at Increasing Concentrations
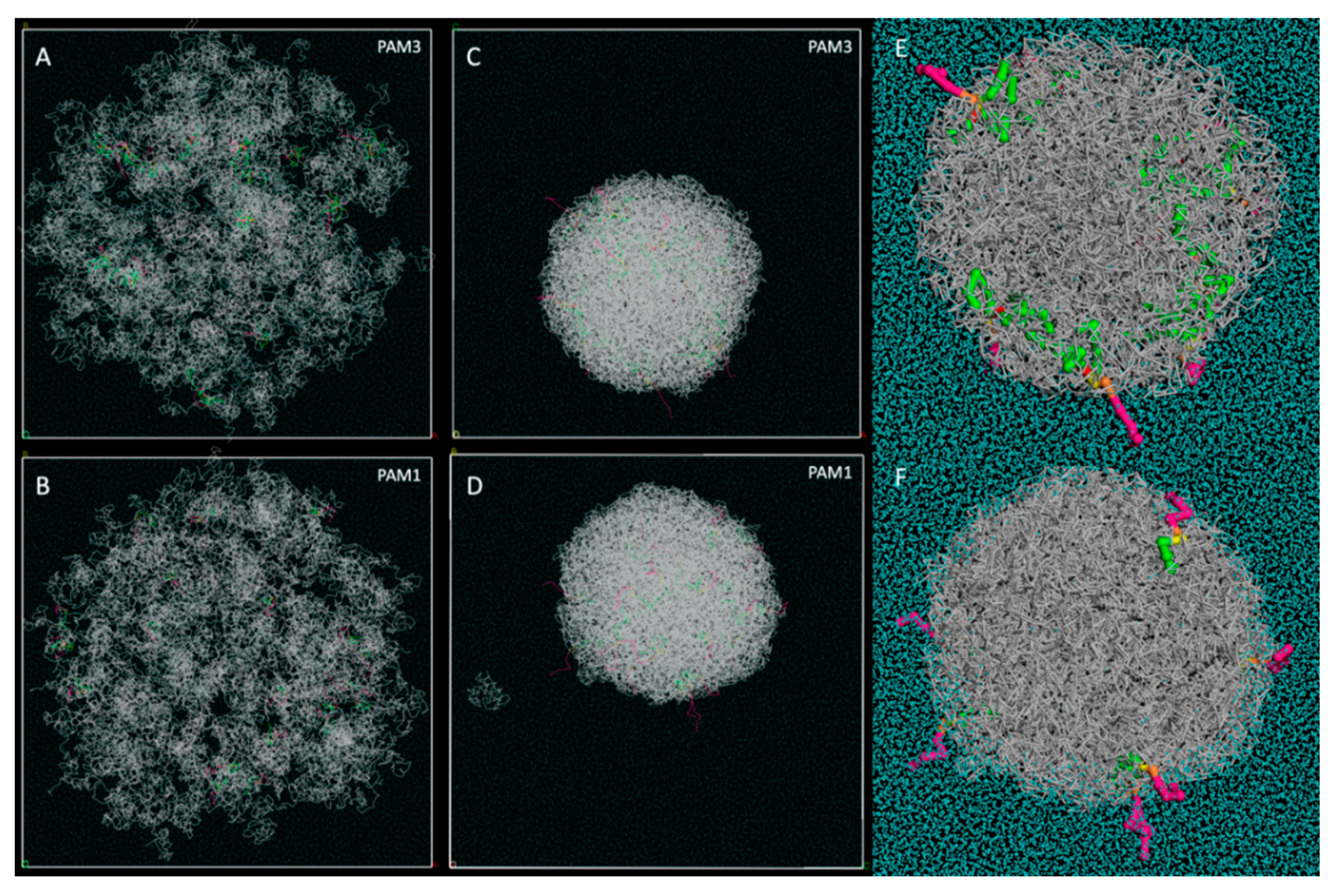
3.2. Pam3CSK4 and Pam1CSK4 Particle Formation
3.3. Pam3CSK4/PLA Saturation Studies
3.4. Vitamin E Encapsulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peng, Q.; Mu, H. The potential of protein–nanomaterial interaction for advanced drug delivery. J. Control. Release 2016, 225, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Dufort, S.; Sancey, L.; Coll, J.-L. Physico-chemical parameters that govern nanoparticles fate also dictate rules for their molecular evolution. Adv. Drug Deliv. Rev. 2012, 64, 179–189. [Google Scholar] [CrossRef]
- Rawat, P.; Manglani, K.; Gupta, S.; Kalam, A.; Vohora, D.; Ahmad, F.J.; Talegaonkar, S. Design and Development of Bioceramic Based Functionalized PLGA Nanoparticles of Risedronate for Bone Targeting: In-vitro Characterization and Pharmacodynamic Evaluation. Pharm. Res. 2015, 32, 3149–3158. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zheng, Y.; Jehng, J.-M.; Tang, Y.; Wachs, I.E.; Podkolzin, S.G. Identification of molybdenum oxide nanostructures on zeolites for natural gas conversion. Science 2015, 348, 686–690. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggi, L.; Bruni, G.; Maietta, M.; Canobbio, A.; Cardini, A.; Conte, U.I. Technological approaches to improve the dissolution behavior of nateglinide, a lipophilic insoluble drug: Nanoparticles and co-mixing. Int. J. Pharm. 2013, 454, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Petschauer, J.S.; Madden, A.J.; Kirschbrown, W.P.; Song, G.; Zamboni, W.C. The effects of nanoparticle drug loading on the pharmacokinetics of anticancer agents. Nanomedicine 2015, 10, 447–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abouelmagd, S.A.; Sun, B.; Chang, A.C.; Ku, Y.J.; Yeo, Y. Release Kinetics Study of Poorly Water-Soluble Drugs from Nanoparticles: Are We Doing It Right? Mol. Pharm. 2015, 12, 997–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the plasma exposure of (−)-epigallocatechin gallate in mice through an enhancement in intestinal stability. Eur. J. Pharm. Sci. 2011, 44, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Shi, C.; Sun, Y.; Zhu, C.; Sun, C.C.; Mao, S. Designing Micellar Nanocarriers with Improved Drug Loading and Stability Based on Solubility Parameter. Mol. Pharm. 2015, 12, 816–825. [Google Scholar] [CrossRef]
- Morales, J.O.; Valdés, K.; Morales, J.; Oyarzun-Ampuero, F. Lipid nanoparticles for the topical delivery of retinoids and derivatives. Nanomedicine 2015, 10, 253–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Q.; Zhang, Z.-R.; Gong, T.; Chen, G.-Q.; Sun, X. A rapid-acting, long-acting insulin formulation based on a phospholipid complex loaded PHBHHx nanoparticles. Biomaterials 2012, 33, 1583–1588. [Google Scholar] [CrossRef]
- Peng, Q.; Sun, X.; Gong, T.; Wu, C.-Y.; Zhang, T.; Tan, J.; Zhang, Z.-R. Injectable and biodegradable thermosensitive hydrogels loaded with PHBHHx nanoparticles for the sustained and controlled release of insulin. Acta Biomater. 2013, 9, 5063–5069. [Google Scholar] [CrossRef]
- Mauro, P.P.D.; Borrós, S. Development of High Drug Loaded and Customizing Novel Nanoparticles for Modulated and Controlled Release of Paclitaxel. Pharm. Res. 2014, 31, 3461–3477. [Google Scholar] [CrossRef]
- Varga, N.; Benkő, M.; Sebők, D.; Dékány, I. BSA/polyelectrolyte core–shell nanoparticles for controlled release of encapsulated ibuprofen. Colloids Surf. B Biointerfaces 2014, 123, 616–622. [Google Scholar] [CrossRef]
- Joshi, G.; Kumar, A.; Sawant, K. Enhanced bioavailability and intestinal uptake of Gemcitabine HCl loaded PLGA nanoparticles after oral delivery. Eur. J. Pharm. Sci. 2014, 60, 80–89. [Google Scholar] [CrossRef]
- Wei, Y.; Li, L.; Xi, Y.; Qian, S.; Gao, Y.; Zhang, J. Sustained release and enhanced bioavailability of injectable scutellarin-loaded bovine serum albumin nanoparticles. Int. J. Pharm. 2014, 476, 142–148. [Google Scholar] [CrossRef]
- Borkar, N.; Xia, D.; Holm, R.; Gan, Y.; Müllertz, A.; Yang, M.; Mu, H. Investigating the correlation between in vivo absorption and in vitro release of fenofibrate from lipid matrix particles in biorelevant medium. Eur. J. Pharm. Sci. 2014, 51, 204–210. [Google Scholar] [CrossRef]
- Gutjahr, A.; Phelip, C.; Coolen, A.-L.; Monge, C.; Boisgard, A.-S.; Paul, S.; Verrier, B. Biodegradable Polymeric Nanoparticles-Based Vaccine Adjuvants for Lymph Nodes Targeting. Vaccines 2016, 4, 34. [Google Scholar] [CrossRef]
- Ce, A.; Cm, S. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289. [Google Scholar]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed In-Vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.-X.; Mitter, N.; Yu, C.; Middelberg, A.P.J. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, V.B.; Geary, S.M.; Salem, A.K. Biodegradable Particles as Vaccine Delivery Systems: Size Matters. AAPS J. 2013, 15, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Primard, C.; Rochereau, N.; Luciani, E.; Genin, C.; Delair, T.; Paul, S.; Verrier, B. Traffic of poly(lactic acid) nanoparticulate vaccine vehicle from intestinal mucus to sub-epithelial immune competent cells. Biomaterials 2010, 31, 6060–6068. [Google Scholar] [CrossRef]
- Lamrayah, M.; Charriaud, F.; Hu, S.; Megy, S.; Terreux, R.; Verrier, B. Molecular modelling of TLR agonist Pam3CSK4 entrapment in PLA nanoparticles as a tool to explain loading efficiency and functionality. Int. J. Pharm. 2019, 568, 118569. [Google Scholar] [CrossRef]
- Westwood, A.; Elvin, S.J.; Healey, G.D.; Williamson, E.D.; Eyles, J.E. Immunological responses after immunisation of mice with microparticles containing antigen and single stranded RNA (polyuridylic acid). Vaccine 2006, 24, 1736–1743. [Google Scholar] [CrossRef]
- Kanchan, V.; Panda, A.K. Interactions of antigen-loaded polylactide particles with macrophages and their correlation with the immune response. Biomaterials 2007, 28, 5344–5357. [Google Scholar] [CrossRef]
- Hajleh, M.N.A.; AL-Samydai, A.; Al-Dujaili, E.A.S. Nano, micro particulate and cosmetic delivery systems of polylactic acid: A mini review. J. Cosmet. Dermatol. 2020. [Google Scholar] [CrossRef]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-I.; Lee, W.-J.; Young, T.-F.; Ju, S.-P.; Chang, C.-W.; Chen, H.-L.; Chang, J.-G. Adsorption mechanism of water molecules surrounding Au nanoparticles of different sizes. J. Chem. Phys. 2008, 128, 154703. [Google Scholar] [CrossRef]
- Ju, S.-P. A molecular dynamics simulation of the adsorption of water molecules surrounding an Au nanoparticle. J. Chem. Phys. 2005, 122, 094718. [Google Scholar] [CrossRef]
- Weiss, D.R.; Raschke, T.M.; Levitt, M. How hydrophobic buckminsterfullerene affects surrounding water structure. J. Phys. Chem. B 2008, 112, 2981–2990. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.; Moore, P.B.; Shinoda, W.; Nielsen, S.O. Size-dependent hydrophobic to hydrophilic transition for nanoparticles: A molecular dynamics study. J. Chem. Phys. 2009, 131, 244706. [Google Scholar] [CrossRef]
- Li, L.; Bedrov, D.; Smith, G.D. Water-Induced Interactions between Carbon Nanoparticles. J. Phys. Chem. B 2006, 110, 10509–10513. [Google Scholar] [CrossRef] [PubMed]
- Brancolini, G.; Kokh, D.B.; Calzolai, L.; Wade, R.C.; Corni, S. Docking of Ubiquitin to Gold Nanoparticles. ACS Nano 2012, 6, 9863–9878. [Google Scholar] [CrossRef] [PubMed]
- Verde, A.V.; Acres, J.M.; Maranas, J.K. Investigating the Specificity of Peptide Adsorption on Gold Using Molecular Dynamics Simulations. Biomacromolecules 2009, 10, 2118–2128. [Google Scholar] [CrossRef] [Green Version]
- Ding, F.; Radic, S.; Chen, R.; Chen, P.; Geitner, N.K.; Brown, J.M.; Ke, P.C. Direct observation of a single nanoparticle–ubiquitin corona formation. Nanoscale 2013, 5, 9162–9169. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Feng, Z.; Zhang, L.; Hou, T.; Li, Y. The Selective Interaction between Silica Nanoparticles and Enzymes from Molecular Dynamics Simulations. PLoS ONE 2014, 9, e107696. [Google Scholar] [CrossRef]
- Meunier, M.; Goupil, A.; Lienard, P. Predicting drug loading in PLA-PEG nanoparticles. Int. J. Pharm. 2017, 526, 157–166. [Google Scholar] [CrossRef]
- Hoogerbrugge, P.J.; Koelman, J.M.V.A. Simulating Microscopic Hydrodynamic Phenomena with Dissipative Particle Dynamics. EPL 1992, 19, 155–160. [Google Scholar] [CrossRef]
- Koelman, J.M.V.A.; Hoogerbrugge, P.J. Dynamic Simulations of Hard-Sphere Suspensions under Steady Shear. EPL 1993, 21, 363. [Google Scholar] [CrossRef]
- Kong, Y.; Manke, C.W.; Madden, W.G.; Schlijper, A.G. Simulation of a confined polymer in solution using the dissipative particle dynamics method. Int. J. 1994, 15, 1093–1101. [Google Scholar] [CrossRef]
- Schlijper, A.G.; Hoogerbrugge, P.J.; Manke, C.W. Computer simulation of dilute polymer solutions with the dissipative particle dynamics method. J. Rheol. 1995, 39, 567–579. [Google Scholar] [CrossRef] [Green Version]
- Español, P.; Warren, P. Statistical Mechanics of Dissipative Particle Dynamics. EPL 1995, 30, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Groot, R.D.; Warren, P.B. Dissipative particle dynamics: Bridging the gap between atomistic and mesoscopic simulation. J. Chem. Phys. 1997, 107, 4423–4435. [Google Scholar] [CrossRef]
- Groot, R.D.; Rabone, K.L. Mesoscopic Simulation of Cell Membrane Damage, Morphology Change and Rupture by Nonionic Surfactants. Biophys. J. 2001, 81, 725–736. [Google Scholar] [CrossRef] [Green Version]
- Hamley, I.W.; Kirkham, S.; Dehsorkhi, A.; Castelletto, V.; Reza, M.; Ruokolainen, J. Toll-like receptor agonist lipopeptides self-assemble into distinct nanostructures. Chem. Commun. 2014, 50, 15948–15951. [Google Scholar] [CrossRef] [Green Version]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Vert, M.; Coudane, J.; Schwach, G.; Huet, O.J. Catalyseur et Composition Catalytique Pour la Fabrication d’un Polymere Biocompatible Resorbable, et Procedes les Mettant en Œuvre. FR2745005A1, 11 November 1996. [Google Scholar]
- Sun, H. Compass: An ab Initio Force-Field Optimized for Condensed-Phase ApplicationsOverview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Sun, H.; Jin, Z.; Yang, C.; Akkermans, R.L.C.; Robertson, S.H.; Spenley, N.A.; Miller, S.; Todd, S.M. COMPASS II: Extended coverage for polymer and drug-like molecule databases. J. Mol. Model 2016, 22, 1–10. [Google Scholar] [CrossRef]
- Zhao, L.; Tu, Y.; Fang, H.; Hamley, I.W.; Wang, Z. Self-Assembled Micellar Structures of Lipopeptides with Variable Number of Attached Lipid Chains Revealed by Atomistic Molecular Dynamics Simulations. J. Phys. Chem. B 2018, 122, 9605–9961. [Google Scholar] [CrossRef]




| G | W | F | C | S | K | LA | |
|---|---|---|---|---|---|---|---|
| G | 78 | ||||||
| W | 99.4 | 78 | |||||
| F | 26.1 | 91.3 | 78 | ||||
| C | 26.0 | 82.6 | 28.5 | 78 | |||
| S | 28.9 | 61.4 | 31.8 | 26.1 | 78 | ||
| K | 161.3 | 26.7 | 157.3 | 134.6 | 101.3 | 78 | |
| LA | 25.4 | 91.3 | 25.1 | 27.2 | 30.3 | 154.3 | 78 |
| W | CHR | C5 | LA | |
|---|---|---|---|---|
| W | 78 | |||
| CHR | 169.6 | 78 | ||
| C5 | 115.0 | 25.9 | 78 | |
| LA | 91.3 | 25.0 | 25.3 | 78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Megy, S.; Aguero, S.; Da Costa, D.; Lamrayah, M.; Berthet, M.; Primard, C.; Verrier, B.; Terreux, R. Molecular Dynamics Studies of Poly(Lactic Acid) Nanoparticles and Their Interactions with Vitamin E and TLR Agonists Pam1CSK4 and Pam3CSK4. Nanomaterials 2020, 10, 2209. https://doi.org/10.3390/nano10112209
Megy S, Aguero S, Da Costa D, Lamrayah M, Berthet M, Primard C, Verrier B, Terreux R. Molecular Dynamics Studies of Poly(Lactic Acid) Nanoparticles and Their Interactions with Vitamin E and TLR Agonists Pam1CSK4 and Pam3CSK4. Nanomaterials. 2020; 10(11):2209. https://doi.org/10.3390/nano10112209
Chicago/Turabian StyleMegy, Simon, Stephanie Aguero, David Da Costa, Myriam Lamrayah, Morgane Berthet, Charlotte Primard, Bernard Verrier, and Raphael Terreux. 2020. "Molecular Dynamics Studies of Poly(Lactic Acid) Nanoparticles and Their Interactions with Vitamin E and TLR Agonists Pam1CSK4 and Pam3CSK4" Nanomaterials 10, no. 11: 2209. https://doi.org/10.3390/nano10112209







