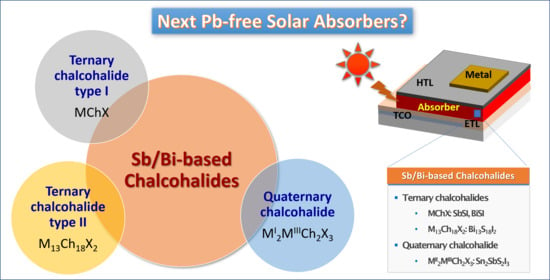
Recent Progress in Fabrication of Antimony/Bismuth Chalcohalides for Lead-Free Solar Cell Applications
Abstract
:1. Introduction
2. Crystal and Energy Band Structures of Sb/Bi Chalcohalides
3. Theoretical Insights on Sb/Bi Chalcohalides as Solar Absorbers
4. Sb/Bi Chalcohalide Solar Cells Fabrication
4.1. One-Step Deposition
4.2. Two-Step Deposition Method
4.3. Other Methods
5. Sb/Bi Chalcohalides as Interfacial Layer
6. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.C.; Lee, S.W.; Jo, H.J.; Kim, D.-H.; Sung, S.-J. Controlled growth of organic-inorganic hybrid CH3NH3PbI3 perovskite thin films from phase-controlled crystalline powders. RSC Adv. 2016, 6, 104359–104365. [Google Scholar] [CrossRef]
- Choi, Y.C.; Lee, S.W.; Kim, D.-H. Antisolvent-assisted powder engineering for controlled growth of hybrid CH3NH3PbI3 perovskite thin films. APL Mater. 2017, 5, 026101. [Google Scholar] [CrossRef] [Green Version]
- Jung, E.H.; Jeon, N.J.; Park, E.Y.; Moon, C.S.; Shin, T.J.; Yang, T.-Y.; Noh, J.H.; Seo, J. Efficient, stable and scalable perovskite solar cells using poly (3-hexylthiophene). Nature 2019, 567, 511. [Google Scholar] [CrossRef] [PubMed]
- Seok, S.I.; Grätzel, M.; Park, N.G. Methodologies toward Highly Efficient Perovskite Solar Cells. Small 2018, 14, 1704177. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide perovskite photovoltaics: Background, status, and future prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Seok, S.I. Molecular aspects of organic cations affecting the humidity stability of perovskites. Energy Environ. Sci. 2020, 13, 805–820. [Google Scholar] [CrossRef]
- Green, M.A.; Dunlop, E.D.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar cell efficiency tables (version 56). Prog. Photovolt. 2020, 28, 629–638. [Google Scholar] [CrossRef]
- Granas, O.; Vinichenko, D.; Kaxiras, E. Establishing the limits of efficiency of perovskite solar cells from first principles modeling. Sci. Rep. 2016, 6, 36108. [Google Scholar] [CrossRef]
- Nasti, G.; Abate, A. Tin Halide Perovskite (ASnX3) Solar Cells: A Comprehensive Guide toward the Highest Power Conversion Efficiency. Adv. Energy Mater. 2020, 10, 1902467. [Google Scholar] [CrossRef]
- Hasan, S.A.U.; Lee, D.S.; Im, S.H.; Hong, K.-H. Present Status and Research Prospects of Tin-based Perovskite Solar Cells. Solar RRL 2019, 4, 1900310. [Google Scholar] [CrossRef]
- Kung, P.-K.; Li, M.-H.; Lin, P.-Y.; Jhang, J.-Y.; Pantaler, M.; Lupascu, D.C.; Grancini, G.; Chen, P. Lead-Free Double Perovskites for Perovskite Solar Cells. Solar RRL 2019, 4, 1900306. [Google Scholar] [CrossRef]
- Miyasaka, T.; Kulkarni, A.; Kim, G.M.; Öz, S.; Jena, A.K. Perovskite Solar Cells: Can We Go Organic-Free, Lead-Free, and Dopant-Free? Adv. Energy Mater. 2020, 10, 1902500. [Google Scholar] [CrossRef]
- Nie, R.; Sumukam, R.R.; Reddy, S.H.; Banavoth, M.; Seok, S.I. Lead-free perovskite solar cells enabled by hetero-valent substitutes. Energy Environ. Sci. 2020, 13, 2363–2385. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, Z.; Xiu, J.; Song, H.; Gatti, T.; He, Z. A critical review on bismuth and antimony halide based perovskites and their derivatives for photovoltaic applications: Recent advances and challenges. J. Mater. Chem. A 2020, 8, 16166–16188. [Google Scholar] [CrossRef]
- Uddin, A.; Upama, M.; Yi, H.; Duan, L. Encapsulation of Organic and Perovskite Solar Cells: A Review. Coatings 2019, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Mujahid, M.; Duan, Y.; Wang, Z.K.; Xue, J.; Yang, Y. A Review of Perovskites Solar Cell Stability. Adv. Funct. Mater. 2019, 29, 1808843. [Google Scholar] [CrossRef]
- Wang, C.; Gu, F.; Zhao, Z.; Rao, H.; Qiu, Y.; Cai, Z.; Zhan, G.; Li, X.; Sun, B.; Yu, X.; et al. Self-Repairing Tin-Based Perovskite Solar Cells with a Breakthrough Efficiency Over 11%. Adv. Mater. 2020, 32, 1907623. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Liu, P.; Xiang, H.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Simultaneous Power Conversion Efficiency and Stability Enhancement of Cs2AgBiBr6 Lead-Free Inorganic Perovskite Solar Cell through Adopting a Multifunctional Dye Interlayer. Adv. Funct. Mater. 2020, 30, 2001557. [Google Scholar] [CrossRef]
- Chen, M.; Ju, M.-G.; Carl, A.D.; Zong, Y.; Grimm, R.L.; Gu, J.; Zeng, X.C.; Zhou, Y.; Padture, N.P. Cesium Titanium(IV) Bromide Thin Films Based Stable Lead-free Perovskite Solar Cells. Joule 2018, 2, 558–570. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, C.; Cai, M.; Liao, Y.; Ding, Y.; Ma, S.; Liu, X.; Guli, M.; Dai, S.; Nazeeruddin, M.K. Dimension-Controlled Growth of Antimony-Based Perovskite-like Halides for Lead-Free and Semitransparent Photovoltaics. ACS Appl. Mater. Interfaces 2020, 12, 17062–17069. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, R.; Jiang, C.; Lian, W.; Ju, H.; Jiang, G.; Li, Z.; Zhu, C.; Chen, T. Manipulating the Electrical Properties of Sb2(S,Se)3 Film for High-Efficiency Solar Cell. Adv. Energy Mater. 2020, 10, 2002341. [Google Scholar] [CrossRef]
- Nie, R.; Im, J.; Seok, S.I. Efficient Solar Cells Employing Light-Harvesting Sb0.67Bi0.33SI. Adv. Mater. 2019, 31, 1808344. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.; Lee, K.S.; Hu, M.; Paik, M.J.; Seok, S.I. Heteroleptic Tin-Antimony Sulfoiodide for Stable and Lead-free Solar Cells. Matter 2020, 3, 1701–1713. [Google Scholar] [CrossRef]
- Chen, C.; Tang, J. Open-Circuit Voltage Loss of Antimony Chalcogenide Solar Cells: Status, Origin, and Possible Solutions. ACS Energy Lett. 2020, 5, 2294–2304. [Google Scholar] [CrossRef]
- Lei, H.; Chen, J.; Tan, Z.; Fang, G. Review of Recent Progress in Antimony Chalcogenide-Based Solar Cells: Materials and Devices. Solar RRL 2019, 3, 1900026. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Chen, S.; Qin, S.; Liu, X.; Chen, J.; Xue, D.-J.; Luo, M.; Cao, Y.; Cheng, Y.; et al. Thin-film Sb2Se3 photovoltaics with oriented one-dimensional ribbons and benign grain boundaries. Nat. Photonics 2015, 9, 409–415. [Google Scholar] [CrossRef]
- Choi, Y.C.; Lee, D.U.; Noh, J.H.; Kim, E.K.; Seok, S.I. Highly Improved Sb2S3 Sensitized-Inorganic-Organic Heterojunction Solar Cells and Quantification of Traps by Deep-Level Transient Spectroscopy. Adv. Funct. Mater. 2014, 24, 3587–3592. [Google Scholar] [CrossRef]
- Choi, Y.C.; Mandal, T.N.; Yang, W.S.; Lee, Y.H.; Im, S.H.; Noh, J.H.; Seok, S.I. Sb2Se3-Sensitized Inorganic-Organic Heterojunction Solar Cells Fabricated Using a Single-Source Precursor. Angew. Chem. Int. Ed. 2014, 53, 1329–1333. [Google Scholar] [CrossRef]
- Choi, Y.C.; Lee, Y.H.; Im, S.H.; Noh, J.H.; Mandal, T.N.; Yang, W.S.; Seok, S.I. Efficient Inorganic-Organic Heterojunction Solar Cells Employing Sb2(Sx/Se1−x)3 Graded-Composition Sensitizers. Adv. Energy Mater. 2014, 4, 1301680. [Google Scholar] [CrossRef]
- Choi, Y.C.; Seok, S.I. Efficient Sb2S3-Sensitized Solar Cells Via Single-Step Deposition of Sb2S3 Using S/Sb-Ratio-Controlled SbCl3-Thiourea Complex Solution. Adv. Funct. Mater. 2015, 25, 2892–2898. [Google Scholar] [CrossRef]
- Choi, Y.C.; Yeom, E.J.; Ahn, T.K.; Seok, S.I. CuSbS2-Sensitized Inorganic-Organic Heterojunction Solar Cells Fabricated Using a Metal-Thiourea Complex Solution. Angew. Chem. Int. Ed. 2015, 54, 4005–4009. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, K.; Chen, S.; Wang, L.; Lu, S.; Liu, Y.; Li, D.; Song, H.; Tang, J. Efficiency Improvement of Sb2Se3 Solar Cells via Grain Boundary Inversion. ACS Energy Lett. 2018, 3, 2335–2341. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, L.; Ding, H.; Ju, H.; Jin, X.; Wang, X.; Zhu, C.; Chen, T. Direct solution deposition of device quality Sb2S3−xSex films for high efficiency solar cells. Sol. Energy Mater. Sol. Cells 2018, 183, 52–58. [Google Scholar] [CrossRef]
- Tang, R.; Wang, X.; Jiang, C.; Li, S.; Jiang, G.; Yang, S.; Zhu, C.; Chen, T. Vacuum assisted solution processing for highly efficient Sb2S3 solar cells. J. Mater. Chem. A 2018, 6, 16322–16327. [Google Scholar] [CrossRef]
- Li, Z.; Liang, X.; Li, G.; Liu, H.; Zhang, H.; Guo, J.; Chen, J.; Shen, K.; San, X.; Yu, W.; et al. 9.2%-efficient core-shell structured antimony selenide nanorod array solar cells. Nat. Commun. 2019, 10, 125. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.; Wang, X.; Lian, W.; Huang, J.; Wei, Q.; Huang, M.; Yin, Y.; Jiang, C.; Yang, S.; Xing, G.; et al. Hydrothermal deposition of antimony selenosulfide thin films enables solar cells with 10% efficiency. Nat. Energy 2020, 5, 587–595. [Google Scholar] [CrossRef]
- Nie, R.; Yun, H.-S.; Paik, M.-J.; Mehta, A.; Park, B.-W.; Choi, Y.C.; Seok, S.I. Efficient Solar Cells Based on Light-Harvesting Antimony Sulfoiodide. Adv. Energy Mater. 2017, 8, 1701901. [Google Scholar] [CrossRef]
- Shi, H.; Ming, W.; Du, M.-H. Bismuth chalcohalides and oxyhalides as optoelectronic materials. Phys. Rev. B 2016, 93, 104108. [Google Scholar] [CrossRef] [Green Version]
- Hahn, N.T.; Rettie, A.J.E.; Beal, S.K.; Fullon, R.R.; Mullins, C.B. n-BiSI Thin Films: Selenium Doping and Solar Cell Behavior. J. Phys. Chem. C 2012, 116, 24878–24886. [Google Scholar] [CrossRef]
- Kunioku, H.; Higashi, M.; Abe, R. Low-Temperature Synthesis of Bismuth Chalcohalides: Candidate Photovoltaic Materials with Easily, Continuously Controllable Band gap. Sci. Rep. 2016, 6, 32664. [Google Scholar] [CrossRef] [PubMed]
- Butler, K.T.; McKechnie, S.; Azarhoosh, P.; van Schilfgaarde, M.; Scanlon, D.O.; Walsh, A. Quasi-particle electronic band structure and alignment of the V-VI-VII semiconductors SbSI, SbSBr, and SbSeI for solar cells. Appl. Phys. Lett. 2016, 108, 112103. [Google Scholar] [CrossRef] [Green Version]
- Ganose, A.M.; Butler, K.T.; Walsh, A.; Scanlon, D.O. Relativistic electronic structure and band alignment of BiSI and BiSeI: Candidate photovoltaic materials. J. Mater. Chem. A 2016, 4, 2060–2068. [Google Scholar] [CrossRef] [Green Version]
- Ganose, A.M.; Matsumoto, S.; Buckeridge, J.; Scanlon, D.O. Defect Engineering of Earth-Abundant Solar Absorbers BiSI and BiSeI. Chem. Mater. 2018, 30, 3827–3835. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.C.; Hwang, E.; Kim, D.-H. Controlled growth of SbSI thin films from amorphous Sb2S3 for low-temperature solution processed chalcohalide solar cells. APL Mater. 2018, 6, 121108. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, D.; Cardoso-Delgado, F.; Alibhai, D.; Mombrú, M.; Fermín, D.J. Photovoltaic Performance of Phase-Pure Orthorhombic BiSI Thin-Films. ACS Appl. Energy Mater. 2019, 2, 3878–3885. [Google Scholar] [CrossRef]
- Choi, Y.C.; Hwang, E. Controlled Growth of BiSI Nanorod-Based Films Through a Two-Step Solution Process for Solar Cell Applications. Nanomaterials 2019, 9, 1650. [Google Scholar] [CrossRef] [Green Version]
- Pathak, A.K.; Prasad, M.D.; Batabyal, S.K. One-dimensional SbSI crystals from Sb, S, and I mixtures in ethylene glycol for solar energy harvesting. Appl. Phys. A 2019, 125, 213. [Google Scholar] [CrossRef]
- Xiong, J.; You, Z.; Lei, S.; Zhao, K.; Bian, Q.; Xiao, Y.; Cheng, B. Solution Growth of BiSI Nanorod Arrays on a Tungsten Substrate for Solar Cell Application. ACS Sustain. Chem. Eng. 2020, 8, 13488–13496. [Google Scholar] [CrossRef]
- Mistewicz, K.; Matysiak, W.; Jesionek, M.; Jarka, P.; Kępińska, M.; Nowak, M.; Tański, T.; Stróż, D.; Szade, J.; Balin, K.; et al. A simple route for manufacture of photovoltaic devices based on chalcohalide nanowires. Appl. Surf. Sci. 2020, 517, 146138. [Google Scholar] [CrossRef]
- Nishikubo, R.; Kanda, H.; García-Benito, I.; Molina-Ontoria, A.; Pozzi, G.; Asiri, A.M.; Nazeeruddin, M.K.; Saeki, A. Optoelectronic and Energy Level Exploration of Bismuth and Antimony-Based Materials for Lead-Free Solar Cells. Chem. Mater. 2020, 32, 6416–6424. [Google Scholar] [CrossRef]
- Murtaza, S.Z.M.; Vaqueiro, P. Rapid synthesis of chalcohalides by ball milling: Preparation and characterisation of BiSI and BiSeI. J. Solid State Chem. 2020, 291, 121625. [Google Scholar] [CrossRef]
- Li, S.; Xu, L.; Kong, X.; Kusunose, T.; Tsurumachi, N.; Feng, Q. Bismuth chalcogenide iodides Bi13S18I2 and BiSI: Solvothermal synthesis, photoelectric behavior, and photovoltaic performance. J. Mater. Chem. C 2020, 8, 3821–3829. [Google Scholar] [CrossRef]
- Nie, R.; Kim, B.; Hong, S.-T.; Seok, S.I. Nanostructured Heterojunction Solar Cells Based on Pb2SbS2I3: Linking Lead Halide Perovskites and Metal Chalcogenides. ACS Energy Lett. 2018, 3, 2376–2382. [Google Scholar] [CrossRef]
- Butler, K.T.; Frost, J.M.; Walsh, A. Ferroelectric materials for solar energy conversion: Photoferroics revisited. Energy Environ. Sci. 2015, 8, 838–848. [Google Scholar] [CrossRef] [Green Version]
- Nie, R.; Mehta, A.; Park, B.W.; Kwon, H.W.; Im, J.; Seok, S.I. Mixed Sulfur and Iodide-Based Lead-Free Perovskite Solar Cells. J. Am. Chem. Soc. 2018, 140, 872–875. [Google Scholar] [CrossRef]
- Savytskii, D.; Sanders, M.; Golovchak, R.; Knorr, B.; Dierolf, V.; Jain, H.; Heo, J. Crystallization of Stoichiometric SbSI Glass. J. Am. Ceram. Soc. 2014, 97, 198–205. [Google Scholar] [CrossRef]
- Groom, R.; Jacobs, A.; Cepeda, M.; Drummey, R.; Latturner, S.E. Bi13S18I2: (Re)discovery of a Subvalent Bismuth Compound Featuring [Bi2]4+ Dimers Grown in Sulfur/Iodine Flux Mixtures. Chem. Mater. 2017, 29, 3314–3323. [Google Scholar] [CrossRef]
- Doussier, C.; Moëlo, Y.; Léone, P.; Meerschaut, A.; Evain, M. Crystal structure of Pb2SbS2I3, and re-examination of the crystal chemistry within the group of (Pb/Sn/Sb) chalcogeno-iodides. Solid State Sci. 2007, 9, 792–803. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed Balance Limit of Efficiency of p-n Junction Solar Cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Ganose, A.M.; Savory, C.N.; Scanlon, D.O. Beyond methylammonium lead iodide: Prospects for the emergent field of ns(2) containing solar absorbers. Chem. Commun. 2016, 53, 20–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, R.E.; Stevanović, V.; Ginley, D.S.; Buonassisi, T. Identifying defect-tolerant semiconductors with high minority-carrier lifetimes: Beyond hybrid lead halide perovskites. MRS Commun. 2015, 5, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Ran, Z.; Wang, X.; Li, Y.; Yang, D.; Zhao, X.-G.; Biswas, K.; Singh, D.J.; Zhang, L. Bismuth and antimony-based oxyhalides and chalcohalides as potential optoelectronic materials. NPJ Comput. Mater. 2018, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Peng, B.; Xu, K.; Zhang, H.; Ning, Z.; Shao, H.; Ni, G.; Li, J.; Zhu, Y.; Zhu, H.; Soukoulis, C.M. 1D SbSeI, SbSI, and SbSBr With High Stability and Novel Properties for Microelectronic, Optoelectronic, and Thermoelectric Applications. Adv. Theory Simul. 2018, 1, 1700005. [Google Scholar] [CrossRef] [Green Version]
- Nie, R.; Seok, S.I. Efficient Antimony-Based Solar Cells by Enhanced Charge Transfer. Small Methods 2019, 4, 1900698. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, D.; Ma, Y.; Lu, Z.; Chen, Z.; Wang, S.; Xiao, L.; Gong, Q. Morphology control of the perovskite films for efficient solar cells. Dalton Trans. 2015, 44, 10582–10593. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.; Ding, D.; Marin-Beloqui, J.M.; Lanzetta, L.; Bu, X.; Rath, T.; Haque, S.A. Improved Charge Separation and Photovoltaic Performance of BiI3 Absorber Layers by Use of an In Situ Formed BiSI Interlayer. ACS Appl. Energy Mater. 2019, 2, 7056–7061. [Google Scholar] [CrossRef]
- Kang, A.K.; Zandi, M.H.; Gorji, N.E. Fabrication and Degradation Anaylsis of Perovskite Solar Cells with Graphene Reduced Oxide as Hole Trasnporting Layer. J. Electron. Mater. 2020, 49, 2289–2295. [Google Scholar] [CrossRef]







| Metal (M) Ions | Chemical Compound | Record Device Performance | |||
|---|---|---|---|---|---|
| PCE | Material | Ref. | |||
| Perovskites | Sn2+, Ge2+ | Perovskite/AMX3 | 11.4% | FASnI3 | [18] |
| Ag+, Bi3+ | Double perovskite/A2MIMIIIX6 | 2.84% | Cs2AgBiBr6 | [19] | |
| Sn4+ | Vacancy-ordered double perovskite/A2MIVX6 | 3.28% | Cs2TiBr6 | [20] | |
| Sb3+, Bi3+ | 2D perovskite/ | 3.34% | MA3Sb2I9−xClx | [21] | |
| Sb/Bi-based non-perovskites | Sb3+ | Sb chalcogenides/M2Ch3, CuMCh2 | 10.5% | Sb2(S,Se)3 | [22] |
| Sb3+, Bi3+ | Ternary chalcohalides/MChX, M13Ch18X2 | 4.07% | Sb0.67Bi0.33SI | [23] | |
| Sn2+, Pb2+, Sb3+, Bi3+ | Quaternary chalcohalides/ | 4.04% | Sn2SbS2I3 | [24] | |
| Chemical Formula | Structure/Space Group | Typical Materials | Ref. | |
|---|---|---|---|---|
| Ternary chalcohalides | MChX | Orthorhombic/Pnma | SbSI, BiSI | [23,38,39,40,41,42,43,44,45,46,47,49,58] |
| M13Ch18X2 | Hexagonal/P63 | Bi13S18I2 | [53,58] | |
| Quaternary chalcohalide | Orthorhombic/Cmcm | Pb2SbS2I3, Sn2SbS2I3 | [24,54,59] |
| MChX Compounds | mh* | me* | Static Dielectric Constant | Absorption Coefficient 1 | References |
|---|---|---|---|---|---|
| Pb-perovskite 2 | 0.10 | 0.16 | 20.07 | >1 × 105 cm−1 | [61,62] |
| BiSI | 0.61–4.79 | 0.53–2.33 | 14.26–71.32 | >1 × 105 cm−1 | [39,44,62,63] |
| BiSeI | 0.81–5.89 | 0.25–1.61 | 14.78–62.82 | >1 × 105 cm−1 | [39,44,62,63] |
| SbSI | 0.27–2.06 | 0.21–1.25 | 10.56–69.38 | - | [42,55,62,63,64] |
| SbSeI | 0.57–4.37 | 0.35–1.83 | 14.70–57.18 | - | [42,55,62,63,64] |
| SbSBr | 0.24–3.55 | 0.51, 0.52 | 13.81–105.15 | - | [42,55,63,64] |
| Method | Chalcohalide | Device Structure | PCE (%)/JSC 1 (mA·cm−2)/VOC 2 (V)/FF 3 | Ref. |
|---|---|---|---|---|
| One-step deposition | Bi(S,Se)I | FTO/Pt/CuSCN/Bi(S,Se)I/FTO | 0.01/0.07/0.39/0.4 | [40] |
| BiSI | Au/F8/BiSI/SnO2/FTO | 1.32/8.44/0.45/0.35 | [46] | |
| SbSI | Au/PEDOT:PSS 4/PCPDTBT/Sb2S3-SbSI/mp-TiO2/TiO2-BL/FTO | 2.91/12.0/0.47/0.52 | [51] | |
| Sn2SbS2I3 | Au/PCPDTBT/Sn2SbS2I3/mp-TiO2/TiO2-BL/FTO | 4.04/16.1/0.44/0.57 | [24] | |
| Two-step deposition | SbSI | Au/PCPDTBT/SbSI/mp-TiO2/TiO2-BL/FTO | 3.05/9.11/0.58/0.58 | [38] |
| Sb0.67Bi0.33SI | Au/PEDOT:PSS/PCPDTBT/Sb0.67Bi0.33SI/mp-TiO2/TiO2-BL/FTO | 4.07/14.54/0.53/0.53 | [23] | |
| SbSI | Au/PCPDTBT/SbSI/mp-TiO2/TiO2-BL/FTO | 3.62/9.26/0.6 /0.65 | [65] | |
| SbSI | Au/P3HT/SbSI/TiO2-BL/FTO | 0.93/5.45/0.55/0.31 | [45] | |
| BiSI | Au/P3HT/BiSI/TiO2-BL/FTO | - | [47] | |
| BiSI | ITO/CuSCN/BiSI/W | 0.66/2.73/0.46/0.53 | [49] | |
| Pb2SbS2I3 | Au/PCPDTBT/Pb2SbS2I3/mp-TiO2/TiO2-BL/FTO | 3.12/8.79/0.61/0.58 | [54] | |
| Oxyhalides conversion | Bi(S,Se)(I,Br) | No device | - | [41] |
| Mixed sonication-heating | SbSI | Carbon/ZrO2/SbSI/mp-TiO2/TiO2-BL/FTO | 0.04/0.05/0.29/0.31 | [48] |
| Sonochemical method | SbSI | Au/P3HT/SbSI-PAN/TiO2 NP/ITO | - | [50] |
| Solvothermal method | Bi13S18I2 | Pt/Electrolyte/Bi13S18I2/mp-TiO2/TiO2-BL/FTO | 0.85/3.82/0.58/0.38 | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.C.; Jung, K.-W. Recent Progress in Fabrication of Antimony/Bismuth Chalcohalides for Lead-Free Solar Cell Applications. Nanomaterials 2020, 10, 2284. https://doi.org/10.3390/nano10112284
Choi YC, Jung K-W. Recent Progress in Fabrication of Antimony/Bismuth Chalcohalides for Lead-Free Solar Cell Applications. Nanomaterials. 2020; 10(11):2284. https://doi.org/10.3390/nano10112284
Chicago/Turabian StyleChoi, Yong Chan, and Kang-Won Jung. 2020. "Recent Progress in Fabrication of Antimony/Bismuth Chalcohalides for Lead-Free Solar Cell Applications" Nanomaterials 10, no. 11: 2284. https://doi.org/10.3390/nano10112284