Band Gap Measurements of Nano-Meter Sized Rutile Thin Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Details
2.2. Theoretical Calculations
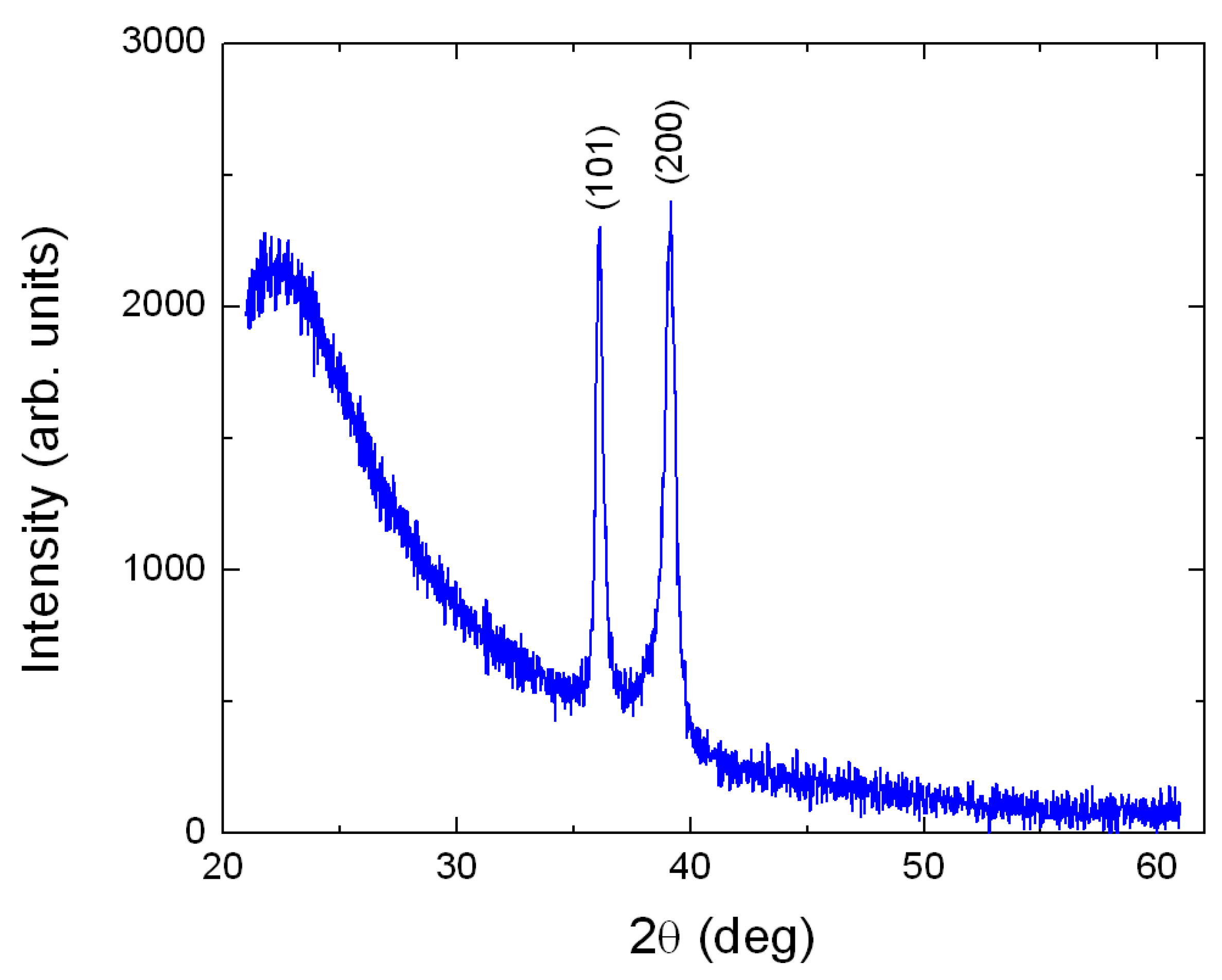
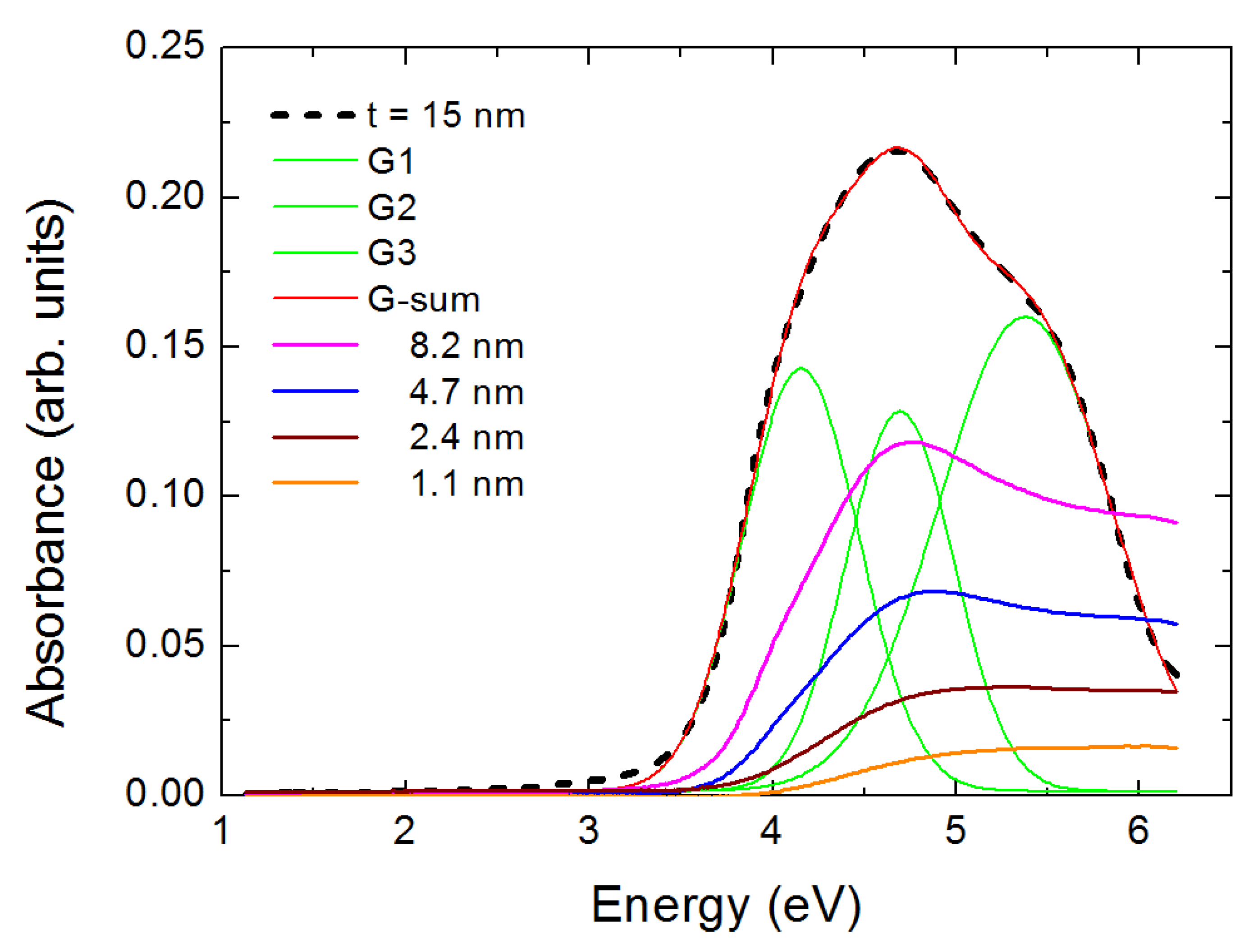
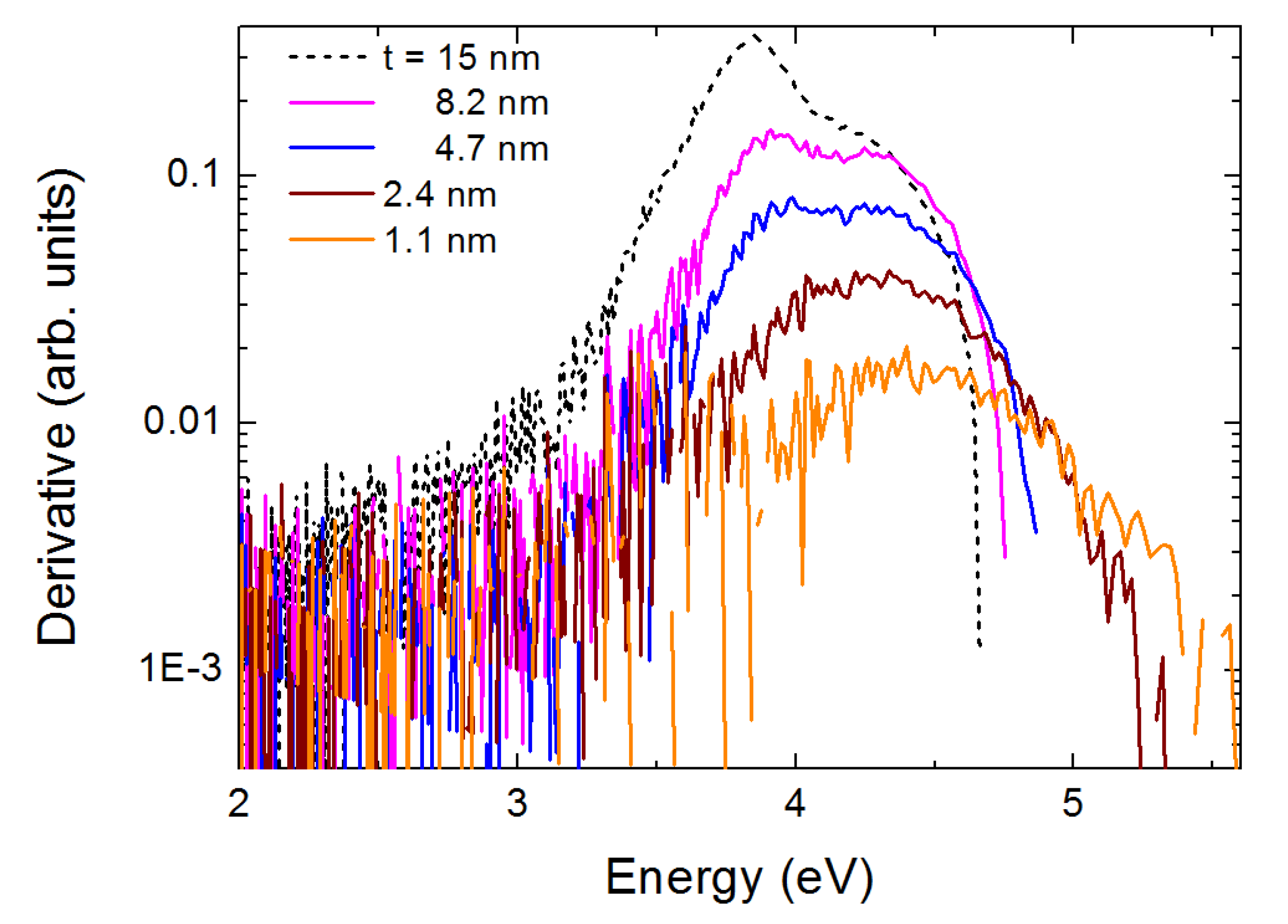
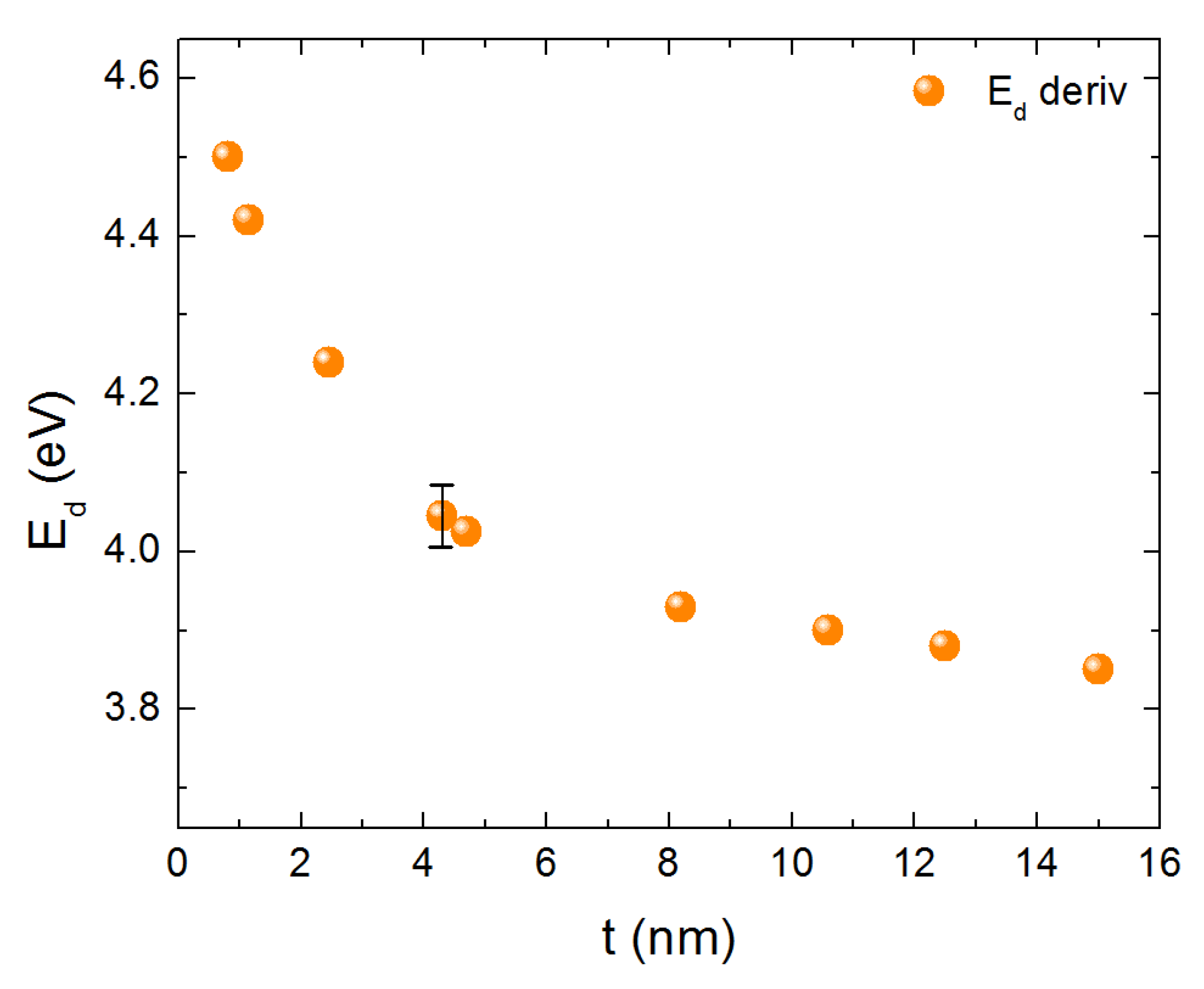
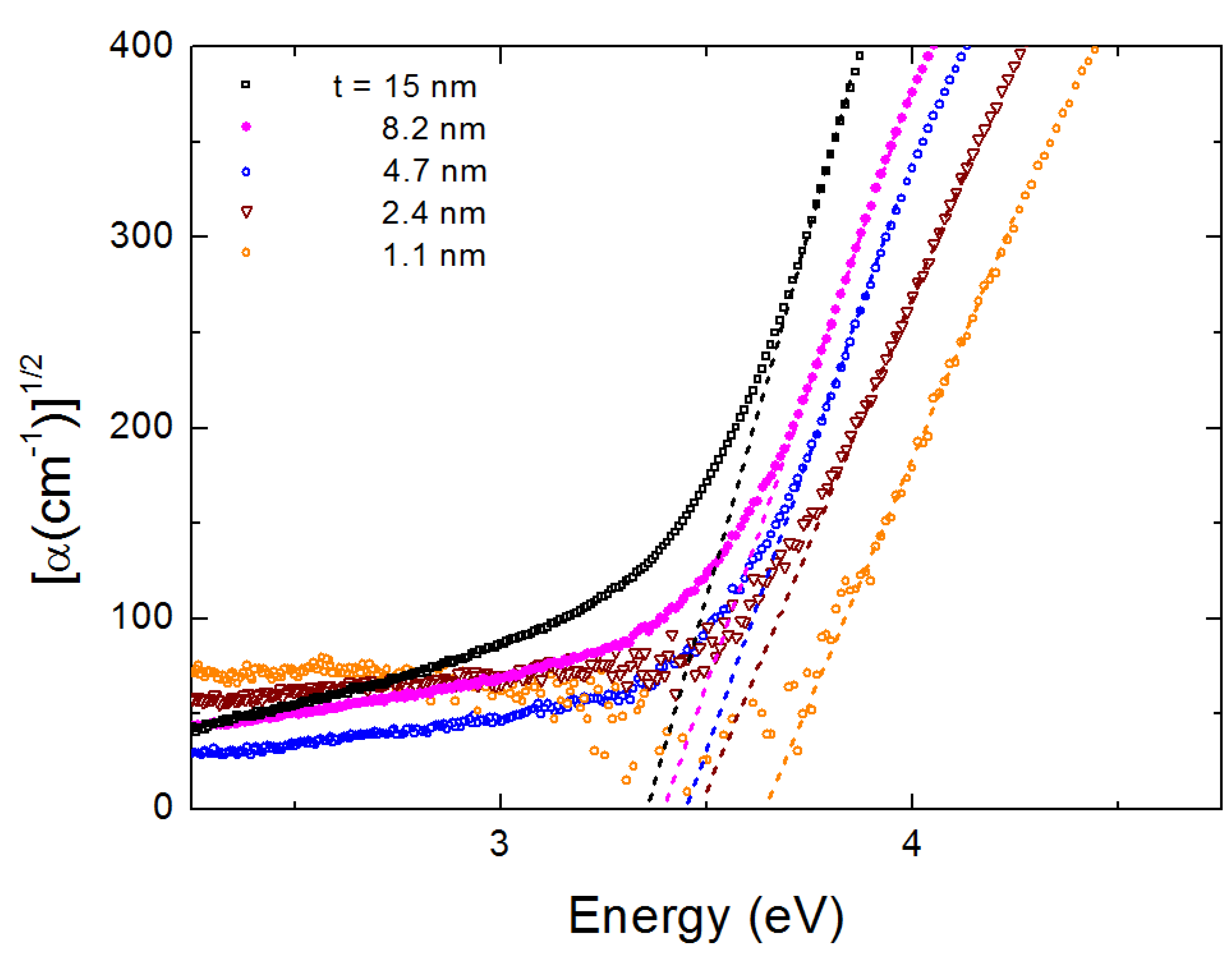
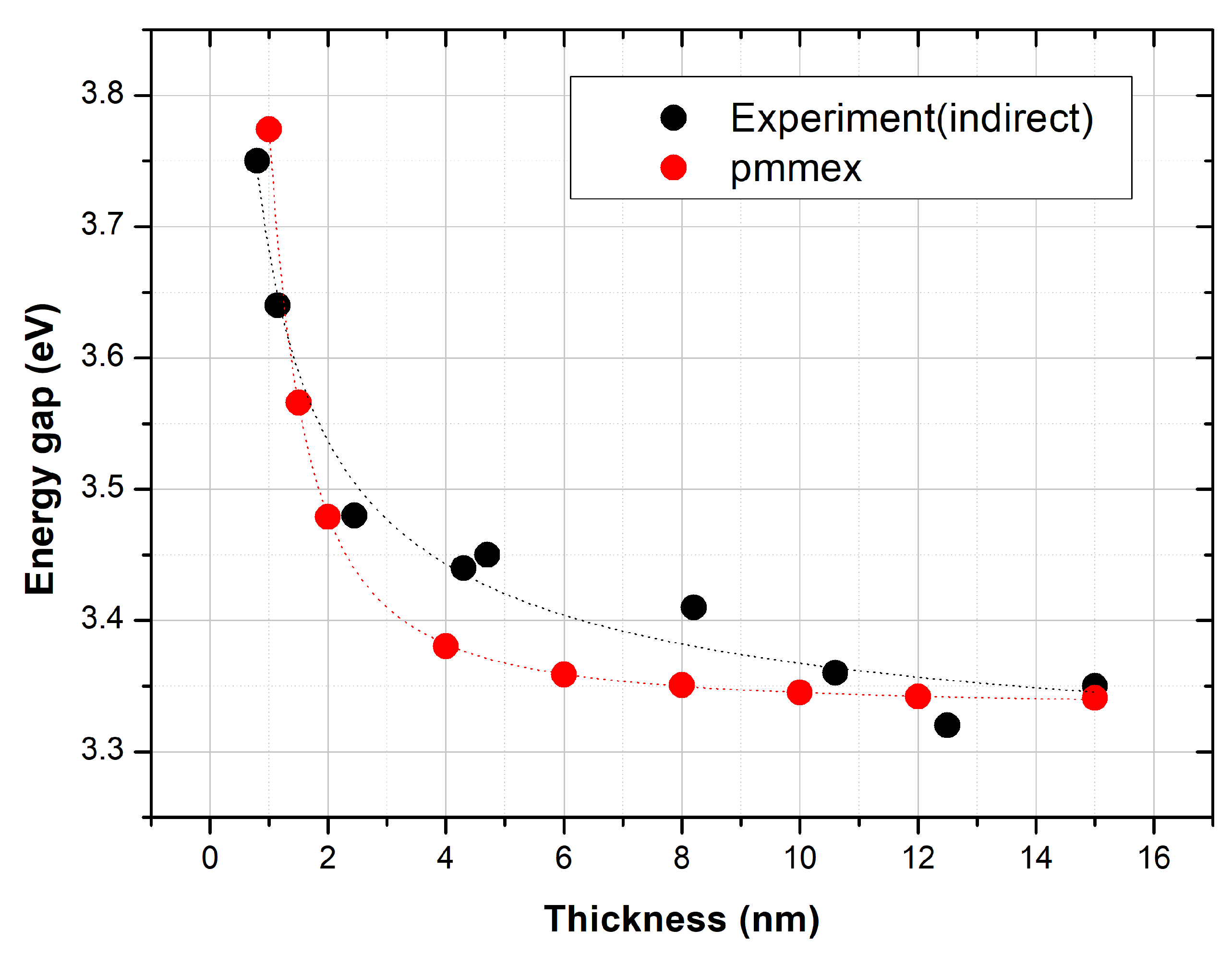
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haider, A.J.; Jameel, Z.N.; Al-Hussaini, I.H.M. Review on: Titanium Dioxide Applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Nguyen, C.C.; Nguyen, D.T.; Do, T.O. A novel route to synthesize C/Pt/TiO2 phase tunable anatase—Rutile TiO2 for efficient sunlight-driven photocatalytic applications. Appl. Catal. B Environ. 2018, 226, 46–52. [Google Scholar] [CrossRef]
- Gong, C.; Du, J.; Li, X.; Yu, Z.; Ma, J.; Qi, W.; Zhang, K.; Yang, J.; Luo, M.; Peng, H. One-Step Acidic Hydrothermal Preparation of Dendritic Rutile TiO2 Nanorods for Photocatalytic Performance. Nanomaterials 2018, 8, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; Kim, H.-I.; Moon, G.-H.; Choi, W. Photoinduced charge transfer processes in solar photocatalysis based on modified TiO2. Energy Environ. Sci. 2016, 9, 411–433. [Google Scholar] [CrossRef] [Green Version]
- Brancho, J.J.; Bartlett, B.M. Challenges in Co-Alloyed Titanium Oxynitrides, a Promising Class of Photochemically Active Materials. Chem. Mater. 2015, 27, 7207–7217. [Google Scholar] [CrossRef]
- Matos, J.; Marino, T.; Molinari, R.; García, H. Hydrogen photoproduction under visible irradiation of Au—TiO2/activated carbon. Appl. Catal. A 2012, 263–272. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, G.; Wang, X.; Yuan, X.; Lin, T.; Huang, F. Progress in Black Titania: A New Material for Advanced Photocatalysis. Adv. Energy Mater. 2016, 6, 1600452. [Google Scholar] [CrossRef]
- Nolan, M.; Iwaszuk, A.; Lucid, A.K.; Carey, J.J.; Fronzi, M. Design of novel visible light active photocatalyst materials: Surface modified TiO2. Adv. Mater. 2016, 28, 5425–5446. [Google Scholar] [CrossRef]
- Buzatu, D.L.; Ianasi, P.; Putz, M.V. Quantum Metrological Matrices for Sustainable Graphentronics. In Solar Energy Conversion in Communities; Springer Proceedings in Energy; Springer: Cham, Switzerland, 2020; pp. 315–326. [Google Scholar]
- Putz, M.V.; Ori, O. Bondonic Effects in Group-IV Honeycomb Nanoribbons with Stone-Wales Topological Defects. Molecules 2014, 19, 4157–4188. [Google Scholar] [CrossRef] [Green Version]
- Frederikse, H.P.R. Recent Studies on Rutile (TiO2). J. Appl. Phys. 1961, 32, 2211. [Google Scholar] [CrossRef]
- Grant, F.A. Properties of Rutile (Titanium Dioxide). Revs. Modern Phys. 1959, 31, 646. [Google Scholar] [CrossRef]
- Pasqual, J.; Camassel, J.; Mathieu, H. Resoved quadrupolar transition in TiO2. Phys. Rev. Lett. 1977, 39, 1490–1493. [Google Scholar] [CrossRef] [Green Version]
- Martin, N.; Rousselot, C.; Rondot, D.; Palmino, F.; Mercier, R. Microstructure modification of amorphous titanium oxide thin films during annealing treatment. Thin Solid Film. 1997, 300, 113–121. [Google Scholar] [CrossRef]
- Fox, M. Optical Properties of Solids, 2nd ed.; Oxford University Press: New York, NY, USA, 2010; ISBN 9780199573370. [Google Scholar]
- Zhou, B.; Jiang, X.; Shen, R.; Rogachev, A.V. Preparation and characterization of TiO2 thin film by thermal oxidation of sputtered Ti film. Mater. Sci. Semicond. Process. 2013, 16, 513–519. [Google Scholar] [CrossRef]
- Shi, Y.J.; Zhang, R.J.; Zheng, H.; Li, D.H.; Wei, W.; Chen, X.; Sun, Y.; Wei, Y.F.; Lu, H.L.; Dai, N.; et al. Optical Constants and Band Gap Evolution with Phase Transition in Sub-20-nm-Thick TiO2 Films Prepared by ALD. Nanoscale Res. Lett. 2017, 12, 243. [Google Scholar] [CrossRef] [Green Version]
- Tanemura, S.; Miao, L.; Wunderlich, W.; Tanemura, M.; Mori, Y.; Toh, S.; Kaneko, K. Fabrication and characterization of anatase/rutile—TiO2 thin films by magnetron sputtering: A review. Sci. Technol. Adv. Mater. 2005, 6, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Mardare, D.; Tasca, M.; Delibas, M.; Rusu, G.I. On the structural properties and optical transmittance of TiO2 r.f. sputtered thin films. Appl. Surf. Sci. 2000, 156, 200–206. [Google Scholar] [CrossRef]
- Coronado, D.R.; Gattorno, D.R.; Pesqueira, M.E.; Cab, C.; Coss, R.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef]
- Khan, A.F.; Mehmood, M.; Durrani, S.K.; Ali, M.L.; Rahim, N.A. Structural and optoelectronic properties of nanostructured TiO2 thin films with annealing. Mater. Sci. Semicon. Process. 2015, 29, 161–169. [Google Scholar] [CrossRef]
- Samokhalov, A. Visible Emission in Nanocrystalline Rutile: Free Exciton and Water as Probes for Midgap States in Adsorption/Desorption Using “Conventional” and Synchronous Luminescence Spectroscopy. J. Phys. Chem. C 2017, 121, 21985–21994. [Google Scholar] [CrossRef]
- Cardona, M.; Harbeke, G. Optical Properties and Band Structure of Wurtzite-Type Crystals and Rutile. Phys. Rev. 1965, 137, A1467–A1476. [Google Scholar] [CrossRef]
- Glassford, K.M.; Chelikowsky, J.R. Optical properties of titanium dioxide in the rutile structure. Phys. Rev. B 1992, 45, 3874. [Google Scholar] [CrossRef] [PubMed]
- Rieth, M.; Schommers, W.; Baskoutas, S. Exact numerical solution of Schrodinger’s equation for a particle in an interaction potential of general shape. Int. J. Mod. Phys. B 1992, 16, 4081. [Google Scholar] [CrossRef]
- Baskoutas, S.; Terzis, A.F. Size-dependent band gap of colloidal quantum dots. J. Appl. Phys. 2006, 99, 013708. [Google Scholar] [CrossRef]
- Baskoutas, S.; Terzis, A.F.; Schommers, W. Size-dependent exciton energy of narrow band gap colloidal quantum dots in the finite depth square-well effective mass approximation. J. Comp. Theor. Nanosci. 2006, 3, 269. [Google Scholar] [CrossRef]
- Poulopoulos, P.; Baskoutas, S.; Pappas, S.D.; Garoufalis, C.S.; Droulias, S.A.; Zamani, A.; Kapaklis, V. Intense Quantum Confinement Effects in Cu2O Thin Films. J. Phys. Chem. C 2011, 30, 115. [Google Scholar] [CrossRef]
- Garoufalis, C.S.; Poulopoulos, P.; Bouropoulos, N.; Barnasas, A.; Baskoutas, S. Growth and optical properties of Fe2O3 thin films: A study of quantum confinement effects by experiment and theory. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 89, 67. [Google Scholar] [CrossRef]
- Garoufalis, C.S.; Barnasas, A.; Stamatelatos, A.; Karoutsos, V.; Grammatikopoulos, S.; Poulopoulos, P.; Baskoutas, S. A Study of Quantum Confinement Effects in Ultrathin NiO Films Performed by Experiment and Theory. Materials 2018, 11, 949. [Google Scholar] [CrossRef] [Green Version]
- Barnasas, A.; Kanistras, N.; Ntagkas, A.; Anyfantis, D.I.; Stamatelatos, A.; Kapaklis, V.; Bouropoulos, N.; Mystiridou, E.; Poulopoulos, P.; Garoufalis, C.S.; et al. Quantum confinement effects of thin ZnO films by experiment and theory. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 120, 114072. [Google Scholar] [CrossRef]
- Baskoutas, S. Novel formulation of the Hartree—Fock approximation: Effective band gap calculation of InAs nanorods. Phys. Lett. A 2005, 341, 303. [Google Scholar] [CrossRef]
- Karoutsos, V. Scanning probe microscopy: Instrumentation and applications on thin films and magnetic multilayers. J. Nanosci. Nanotechnol. 2009, 9, 6783. [Google Scholar] [CrossRef] [PubMed]
- Kapaklis, V.; Poulopoulos, P.; Karoutsos, V.; Manouras, T.; Politis, C. Growth of thin Ag films produced by radio frequency magnetron sputtering. Thin Solid Films 2006, 510, 138–142. [Google Scholar] [CrossRef]
- Escobar, M.A.; Magana, L.F.; Valenzuela, R. Effect of the grain size distribution on the magnetization curve. J. Appl. Phys. 1985, 57, 2142–2147. [Google Scholar] [CrossRef]
- Chronis, A.G.; Stamatelatos, A.; Grammatikopoulos, S.; Sigalas, M.M.; Karoutsos, V.; Maratos, D.M.; Lysandrou, S.P.; Trachylis, D.; Politis, C.; Poulopoulos, P. Microstructure and plasmonic behavior of self-assembled silver nanoparticles and nanorings. J. Appl. Phys. 2019, 125, 023106. [Google Scholar] [CrossRef]
- Brendan Enright, E.; Donald Fitzmaurice, F. Spectroscopic Determination of Electron and Hole Effective Masses in a Nanocrystalline Semiconductor Film. J. Phys. Chem. 1996, 100, 1027–1035. [Google Scholar] [CrossRef]
- Hanken, H. Kopplung nichtrelativistischer teilchen mit einem quantisierten feld. Nuovo C 1956, 3, 1230. [Google Scholar] [CrossRef]
- Berberich, L.J.; Bell, M.E. The Dielectric Properties of the Rutile Form of TiO2. J. Appl. Phys. 1940, 11, 681. [Google Scholar] [CrossRef]
- Schöche, S.; Hofmann, T.; Korlacki, R.; Tiwald, T.E.; Schubert, M. Infrared dielectric anisotropy and phonon modes of rutile TiO2. J. Appl. Phys. 2013, 113, 164102. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diamantopoulos, N.C.; Barnasas, A.; Garoufalis, C.S.; Anyfantis, D.I.; Bouropoulos, N.; Poulopoulos, P.; Baskoutas, S. Band Gap Measurements of Nano-Meter Sized Rutile Thin Films. Nanomaterials 2020, 10, 2379. https://doi.org/10.3390/nano10122379
Diamantopoulos NC, Barnasas A, Garoufalis CS, Anyfantis DI, Bouropoulos N, Poulopoulos P, Baskoutas S. Band Gap Measurements of Nano-Meter Sized Rutile Thin Films. Nanomaterials. 2020; 10(12):2379. https://doi.org/10.3390/nano10122379
Chicago/Turabian StyleDiamantopoulos, Nikolaos C., Alexandros Barnasas, Christos. S. Garoufalis, Dimitrios I. Anyfantis, Nikolaos Bouropoulos, Panagiotis Poulopoulos, and Sotirios Baskoutas. 2020. "Band Gap Measurements of Nano-Meter Sized Rutile Thin Films" Nanomaterials 10, no. 12: 2379. https://doi.org/10.3390/nano10122379










