Manipulation of Molecular Spin State on Surfaces Studied by Scanning Tunneling Microscopy
Abstract
:1. Introduction
2. Spin State Manipulation of Molecules Composed of Main Group Elements
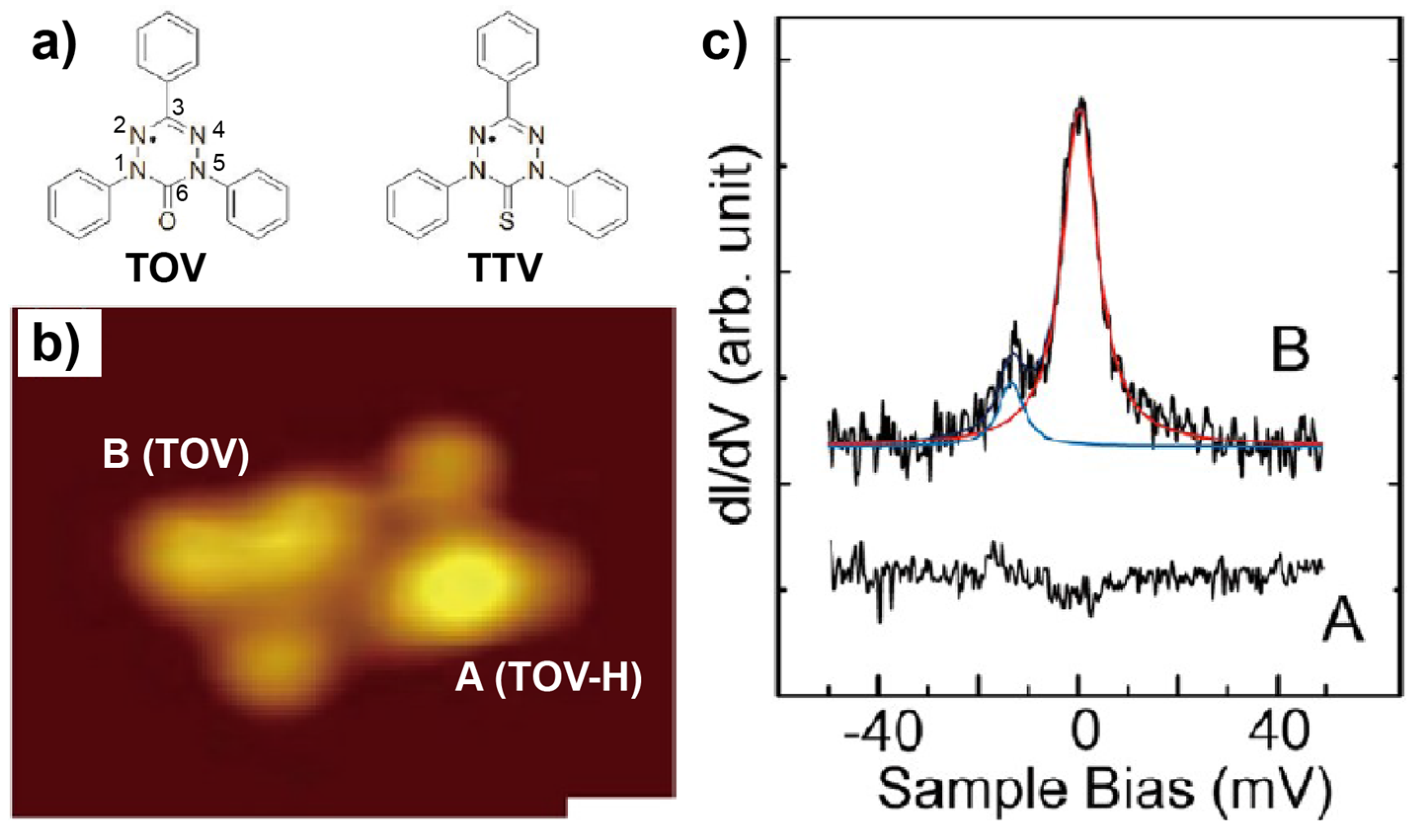
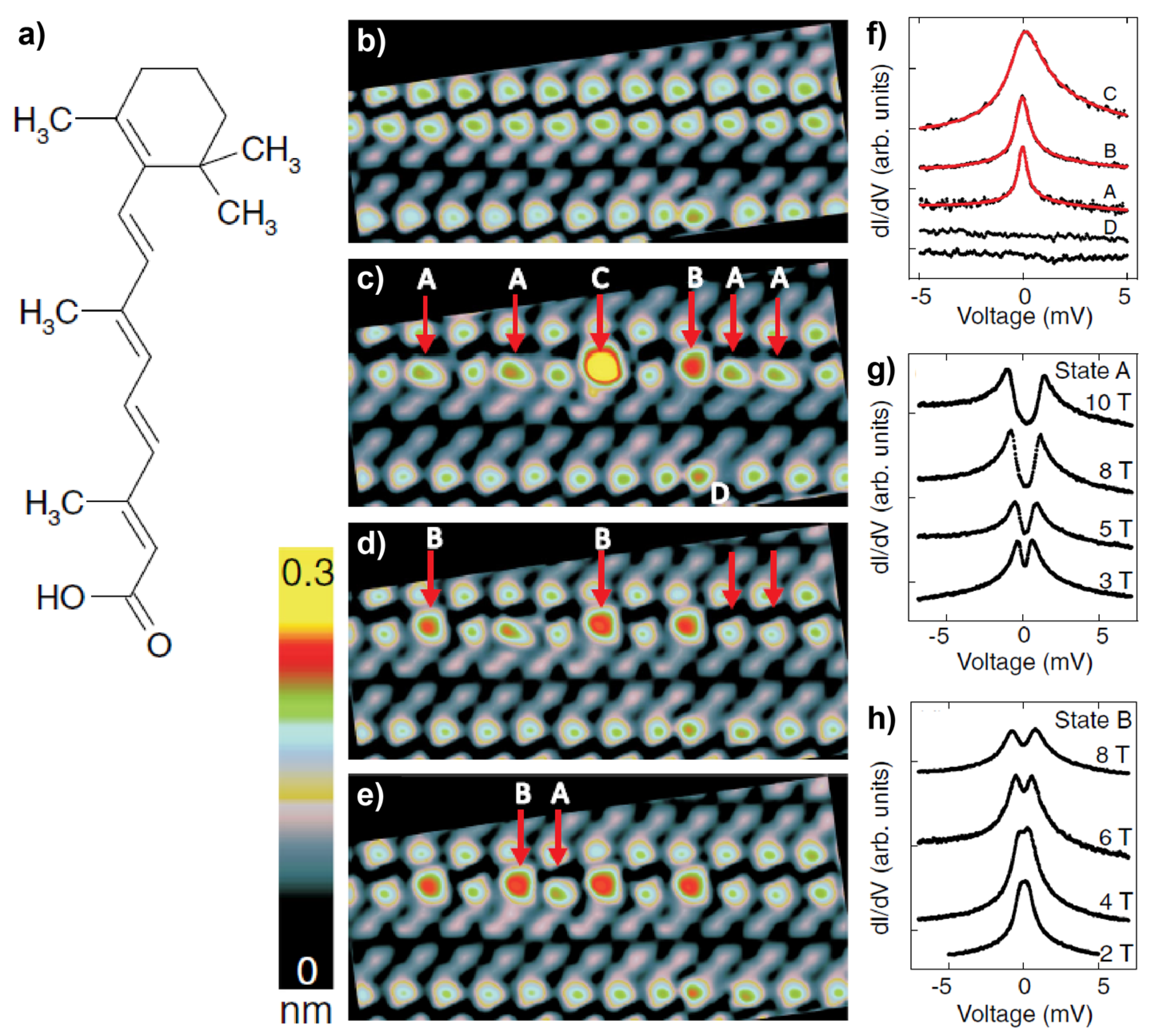
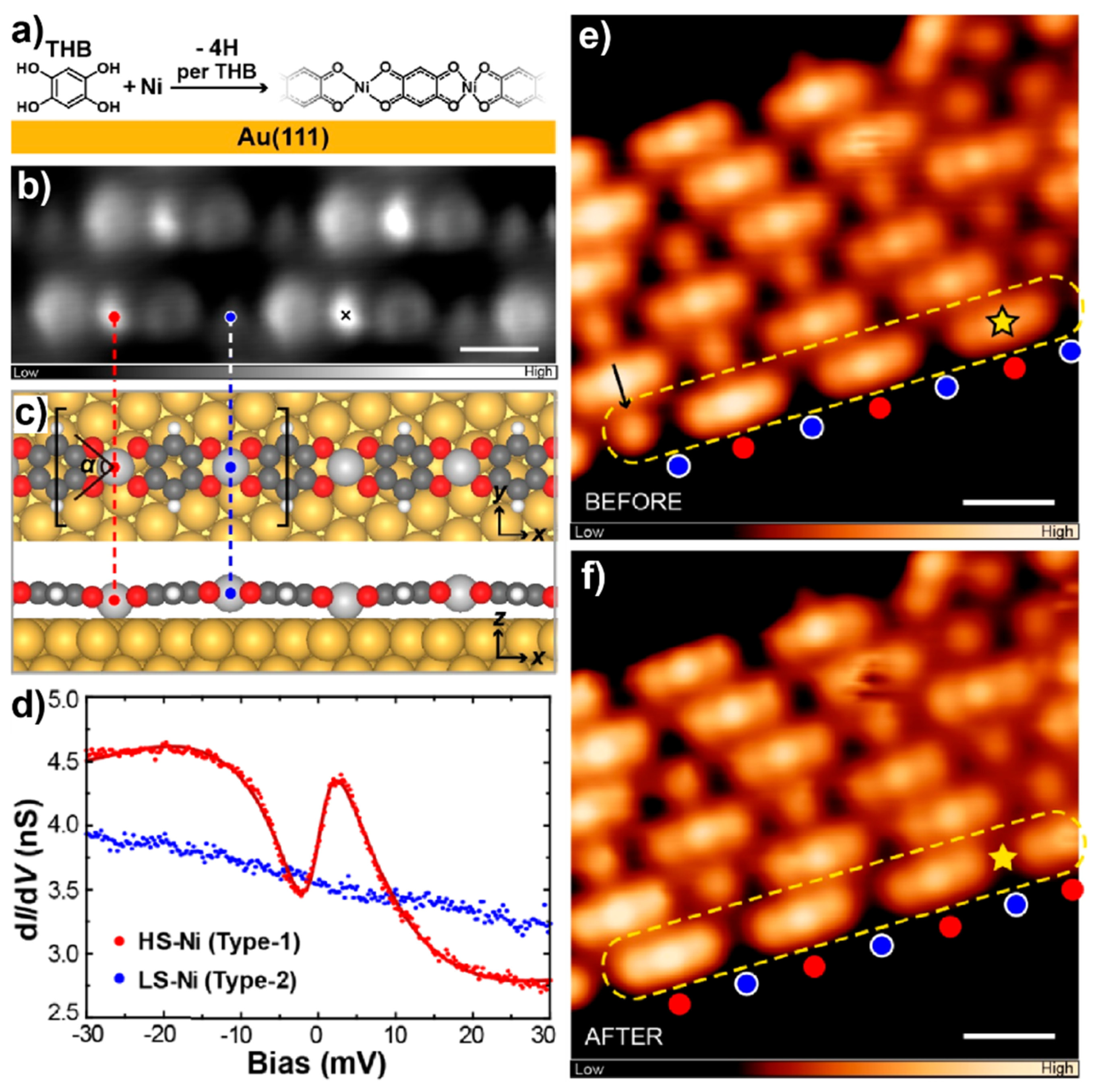
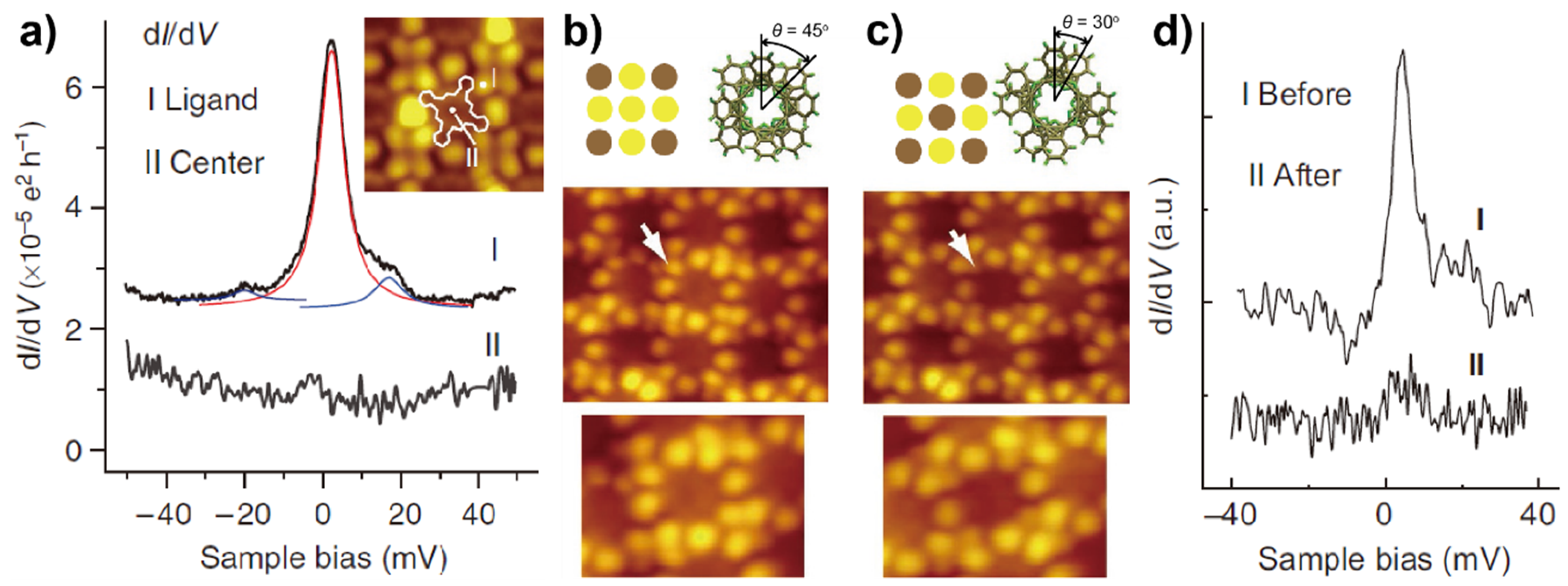
2.1. Metal-Free Molecules
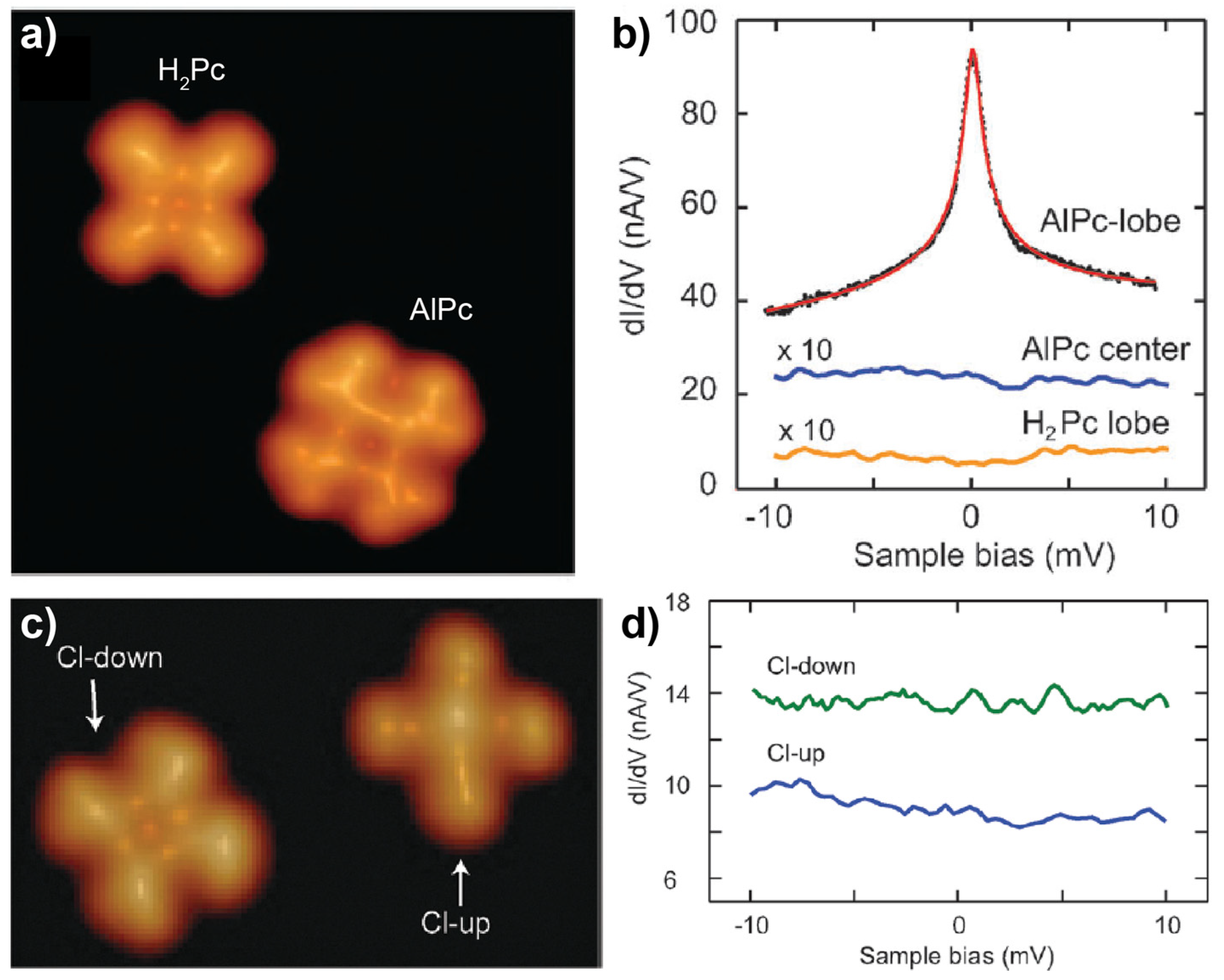
2.2. Main-Group-Metal Phthalocyanines
3. Spin State Manipulation of Molecules Comprising 3d-Metals
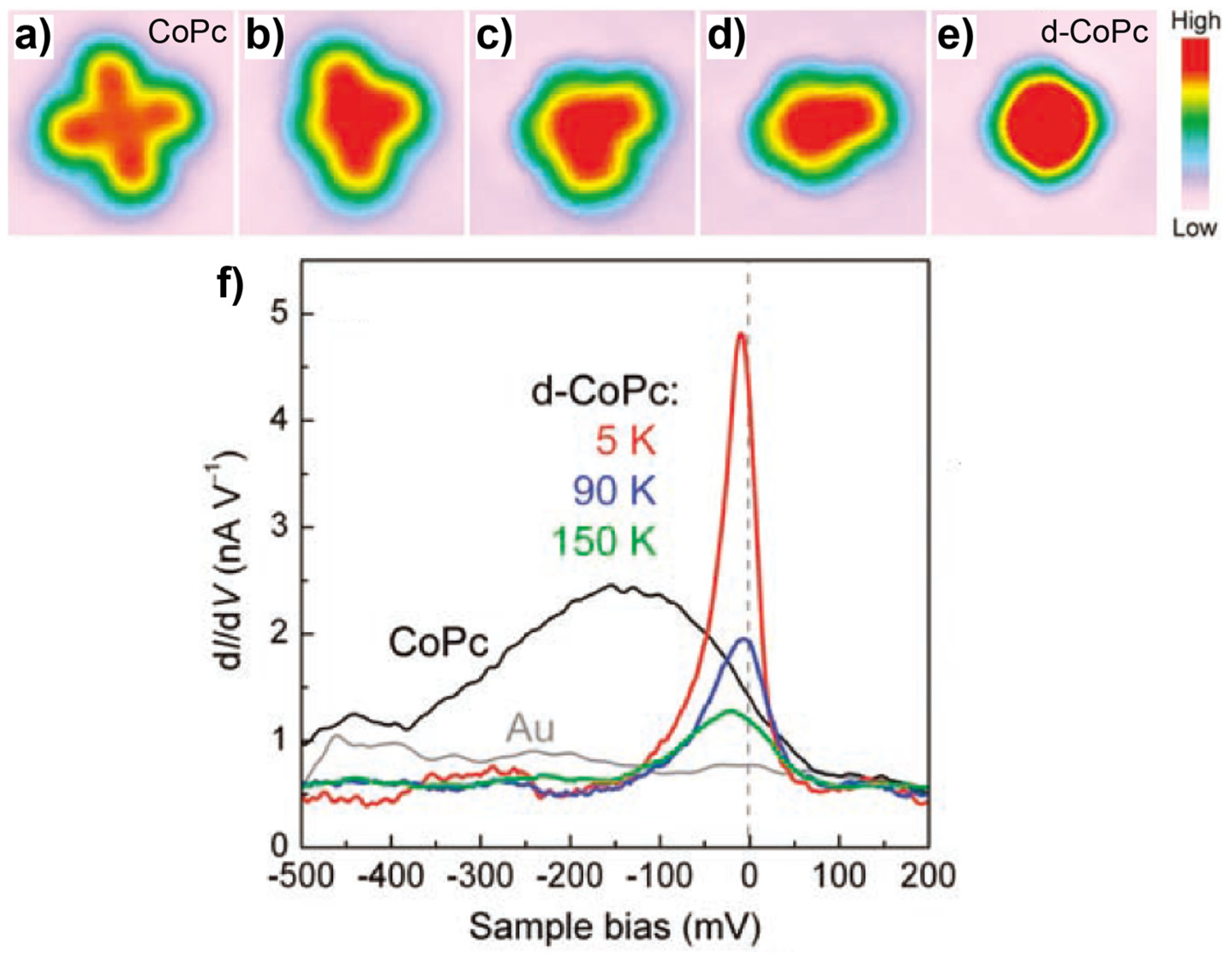
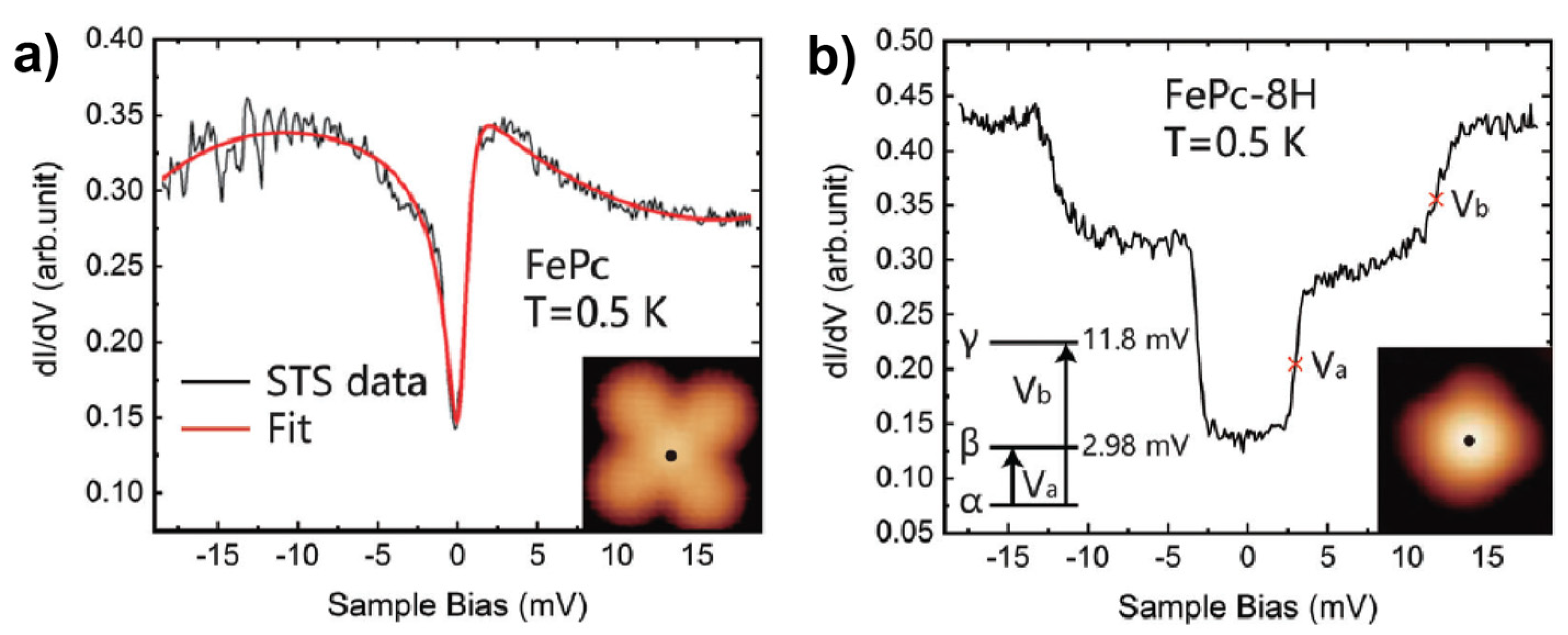
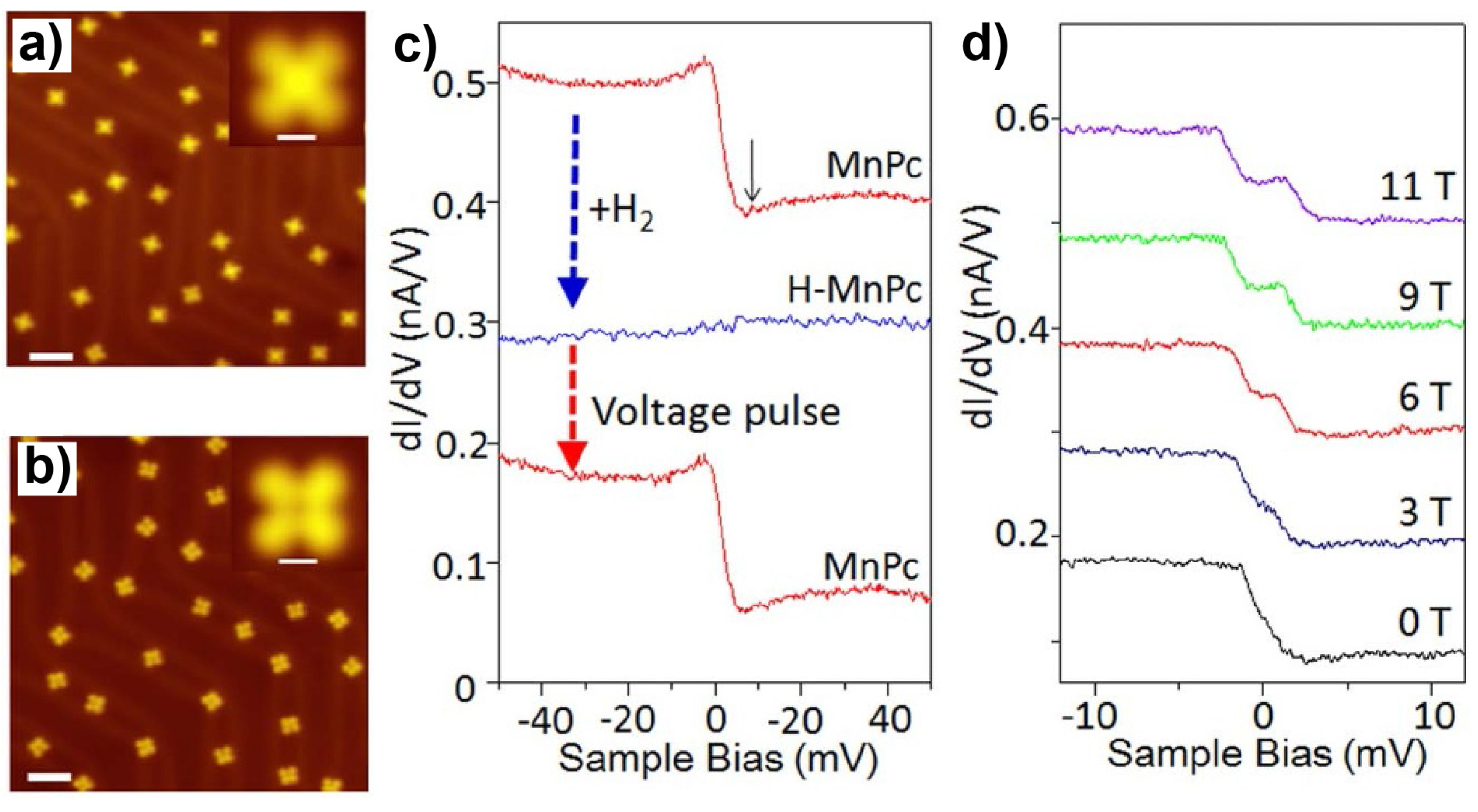
3.1. 3d-Metal Phthalocyanines
3.2. Spin Crossover (SCO) Complexes
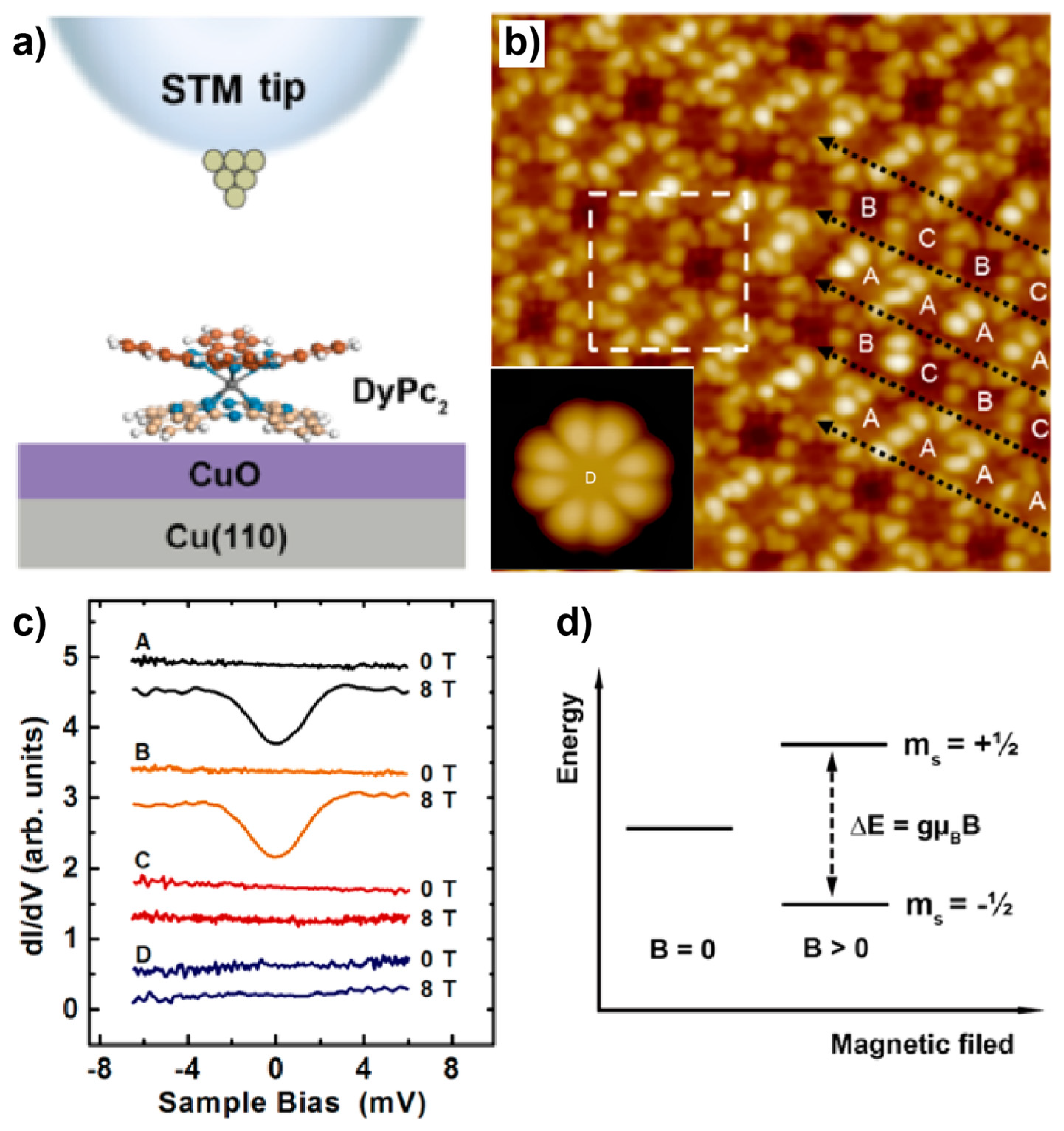
4. Spin State Manipulation of Molecules Comprising 4f-Metals
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chappert, C.; Fert, A.; Dau, F.N.V. The emergence of spin electronics in data storage. Nat. Mater. 2007, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Gatteschi, D.; Bogani, L.; Cornia, A.; Mannini, M.; Sorace, L.; Sessoli, R. Molecular magnetism, status and perspectives. Solid State Sci. 2008, 10, 1701–1709. [Google Scholar] [CrossRef]
- Robertsona, N.; Yee, G.T. Molecular magnetic materials. In Molecular Materials; Bruce, D.W., O’Hare, D., Walton, R.I., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Coronado, E. Molecular magnetism: From chemical design to spin control in molecules, materials and devices. Nat. Rev. Mater. 2019, 5, 87–104. [Google Scholar] [CrossRef]
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef]
- Sanvito, S. Molecular spintronics. Chem. Soc. Rev. 2011, 40, 3336–3355. [Google Scholar] [CrossRef]
- Guo, L.; Gu, X.; Zhu, X.; Sun, X. Recent advances in molecular spintronics: Multifunctional spintronic devices. Adv. Mater. 2019, 31, e1805355. [Google Scholar] [CrossRef] [PubMed]
- Gaita-Arino, A.; Luis, F.; Hill, S.; Coronado, E. Molecular spins for quantum computation. Nat. Chem. 2019, 11, 301–309. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaita-Arino, A.; Coronadoc, E.; Loss, D. Quantum computing with molecular spin systems. J. Mater. Chem. 2009, 19, 1672–1677. [Google Scholar] [CrossRef]
- Camarero, J.; Coronado, E. Molecular vs. inorganic spintronics: The role of molecular materials and single molecules. J. Mater. Chem. 2009, 19, 1678–1684. [Google Scholar] [CrossRef]
- Wang, F.; Vardeny, Z.V. Organic spin valves: The first organic spintronics devices. J. Mater. Chem. 2009, 19, 1685–1690. [Google Scholar] [CrossRef]
- Affronte, M. Molecular nanomagnets for information technologies. J. Mater. Chem. 2009, 19, 1731–1737. [Google Scholar] [CrossRef]
- Timco, G.A.; Faust, T.B.; Tunaa, F.; Winpenny, R.E.P. Linking heterometallic rings for quantum information processing and amusement. Chem. Soc. Rev. 2011, 40, 3067–3075. [Google Scholar] [CrossRef] [PubMed]
- Troiania, F.; Affronte, M. Molecular spins for quantum information technologies. Chem. Soc. Rev. 2011, 40, 3119–3129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hla, S.-W.; Rieder, K.-H. STM control of chemical reactions: Single-molecule synthesis. Annu. Rev. Phys. Chem. 2003, 54, 307–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Feyter, S.; De Schryver, F.C. Two-dimensional supramolecular self-assembly probed by scanning tunneling microscopy. Chem. Soc. Rev. 2003, 32, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Barth, J.V. Molecular architectonic on metal surfaces. Annu. Rev. Phys. Chem. 2007, 58, 375–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.L.; Zhong, J.Q.; Lin, J.D.; Hu, W.P.; Wu, K.; Xu, G.Q.; Wee, A.T.; Chen, W. Towards single molecule switches. Chem. Soc. Rev. 2015, 44, 2998–3022. [Google Scholar] [CrossRef] [Green Version]
- Ko, W.; Ma, C.; Nguyen, G.D.; Kolmer, M.; Li, A.P. Atomic-scale manipulation and in situ characterization with scanning tunneling microscopy. Adv. Funct. Mater. 2019, 29, 1903770. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Fu, Y.S.; Ji, S.H.; Zhang, T.; Cheng, P.; Ma, X.C.; Zou, X.L.; Duan, W.H.; Jia, J.F.; Xue, Q.K. Probing superexchange interaction in molecular magnets by spin-flip spectroscopy and microscopy. Phys. Rev. Lett. 2008, 101, 197208. [Google Scholar] [CrossRef]
- Tsukahara, N.; Noto, K.; Ohara, M.; Shiraki, S.; Takagi, N.; Takata, Y.; Miyawaki, J.; Taguchi, M.; Chainani, A.; Shin, S.; et al. Adsorption-induced switching of magnetic anisotropy in a single iron(II) phthalocyanine molecule on an oxidized Cu(110) surface. Phys. Rev. Lett. 2009, 102, 167203. [Google Scholar] [CrossRef]
- Wegner, D.; Yamachika, R.; Zhang, X.; Wang, Y.; Baruah, T.; Pederson, M.R.; Bartlett, B.M.; Long, J.R.; Crommie, M.F. Tuning molecule-mediated spin coupling in bottom-up-fabricated vanadium-tetracyanoethylene nanostructures. Phys. Rev. Lett. 2009, 103, 087205. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, N.; Shiraki, S.; Itou, S.; Ohta, N.; Takagi, N.; Kawai, M. Evolution of Kondo resonance from a single impurity molecule to the two-dimensional lattice. Phys. Rev. Lett. 2011, 106, 187201. [Google Scholar] [CrossRef] [PubMed]
- Dilullo, A.; Chang, S.H.; Baadji, N.; Clark, K.; Klockner, J.P.; Prosenc, M.H.; Sanvito, S.; Wiesendanger, R.; Hoffmann, G.; Hla, S.W. Molecular Kondo chain. Nano Lett. 2012, 12, 3174–3179. [Google Scholar] [CrossRef] [PubMed]
- Kahle, S.; Deng, Z.; Malinowski, N.; Tonnoir, C.; Forment-Aliaga, A.; Thontasen, N.; Rinke, G.; Le, D.; Turkowski, V.; Rahman, T.S.; et al. The quantum magnetism of individual manganese-12-acetate molecular magnets anchored at surfaces. Nano Lett. 2012, 12, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Minamitani, E.; Tsukahara, N.; Matsunaka, D.; Kim, Y.; Takagi, N.; Kawai, M. Symmetry-driven novel Kondo effect in a molecule. Phys. Rev. Lett. 2012, 109, 086602. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.A.; Malavolti, L.; Lanzilotto, V.; Mannini, M.; Yan, S.; Ninova, S.; Totti, F.; Rolf-Pissarczyk, S.; Cornia, A.; Sessoli, R.; et al. Magnetic fingerprint of individual Fe4 molecular magnets under compression by a scanning tunnelling microscope. Nat. Commun. 2015, 6, 8216. [Google Scholar] [CrossRef] [Green Version]
- Hiraoka, R.; Minamitani, E.; Arafune, R.; Tsukahara, N.; Watanabe, S.; Kawai, M.; Takagi, N. Single-molecule quantum dot as a Kondo simulator. Nat. Commun. 2017, 8, 16012. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Zhang, Y.; He, Y.; Song, H.; Yin, C.; Wu, K. A chemist’s overview of surface electron spins. Chem. Soc. Rev. 2017, 46, 1955–1976. [Google Scholar] [CrossRef]
- Choi, T.; Badal, M.; Loth, S.; Yoo, J.-W.; Lutz, C.P.; Heinrich, A.J.; Epstein, A.J.; Stroud, D.G.; Gupta, J.A. Magnetism in single metalloorganic complexes formed by atom manipulation. Nano Lett. 2014, 14, 1196–1201. [Google Scholar] [CrossRef]
- Ternes, M.; Heinrich, A.J.; Schneider, W.D. Spectroscopic manifestations of the Kondo effect on single adatoms. J. Phys. Condens. Matter. 2009, 21, 053001. [Google Scholar] [CrossRef]
- Heinrich, A.J.; Gupta, J.A.; Lutz, C.P.; Eigler, D.M. Single-atom spin-flip spectroscopy. Science 2004, 306, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Ternes, M. Spin excitations and correlations in scanning tunneling spectroscopy. New J. Phys. 2015, 17, 063016. [Google Scholar] [CrossRef]
- Requist, R.; Modesti, S.; Pier Paolo Baruselli, P.P.; Smogunov, A.; Fabrizio, M.; Tosatti, E. Kondo conductance across the smallest spin 1/2 radical molecule. Proc. Natl. Acad. Sci. USA 2014, 111, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Lohr, T.G.; Pignedoli, C.A.; Liu, J.; Berger, R.; Urgel, J.I.; Müllen, K.; Feng, X.; Ruffieux, P.; Fasel, R. Tailoring bond topologies in open-shell graphene nanostructures. ACS Nano 2018, 12, 11917–11927. [Google Scholar] [CrossRef]
- Liu, J.; Mishra, S.; Pignedoli, C.A.; Daniele Passerone, D.; Urgel, J.I.; Fabrizio, A.; Lohr, T.G.; Ma, J.; Komber, H.; Baumgarten, M.; et al. Open-shell nonbenzenoid nanographenes containing two pairs of pentagonal and heptagonal rings. J. Am. Chem. Soc. 2019, 141, 12011–12020. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Lodi, A.; Ma, J.; Liu, J.; Slota, M.; Narita, A.; Myers, W.K.; Müllen, K.; Feng, X.; Bogani, L. Quantum units from the topological engineering of molecular graphenoids. Science 2019, 366, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Beyer, D.; Eimre, K.; Liu, J.; Berger, R.; Groning, O.; Pignedoli, C.A.; Mullen, K.; Fasel, R.; Feng, X.; et al. Synthesis and characterization of π-extended triangulene. J. Am. Chem. Soc. 2019, 141, 10621–10625. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Beyer, D.; Eimre, K.; Kezilebieke, S.; Berger, R.; Gröning, O.; Pignedoli, C.A.; Müllen, K.; Peter Liljeroth, P.; Ruffieux, P.; et al. Topological frustration induces unconventional magnetism in a nanographene. Nat. Nanotechnol. 2020, 15, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yao, X.; Gröning, O.; Eimre, K.; Pignedoli, C.A.; Müllen, K.; Narita, A.; Fasel, R.; Ruffieux, P. Coupled spin states in armchair graphene nanoribbons with asymmetric zigzag edge extensions. Nano Lett. 2020, 20, 6429–6436. [Google Scholar] [CrossRef]
- Mishra, S.; Beyer, D.; Eimre, K.; Ortiz, R.; Fernandez-Rossier, J.; Berger, R.; Groning, O.; Pignedoli, C.A.; Fasel, R.; Feng, X. Collective all-carbon magnetism in triangulene dimers. Angew. Chem. Int. Ed. 2020, 59, 12041–12047. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Mateo, L.M.; Robles, R.; Ruffieux, P.; Lorente, N.; Bottari, G.; Torres, T.; Fasel, R. Inducing open-shell character in porphyrins through surface-assisted phenalenyl π-extension. J. Am. Chem. Soc. 2020, 142, 18109–18117. [Google Scholar] [CrossRef]
- Mishra, S.; Beyer, D.; Berger, R.; Liu, J.; Groning, O.; Urgel, J.I.; Mullen, K.; Ruffieux, P.; Feng, X.; Fasel, R. Topological defect-induced magnetism in a nanographene. J. Am. Chem. Soc. 2020, 142, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Isshiki, H.; Katoh, K.; Morita, T.; Breedlove, B.K.; Yamashita, M.; Komeda, T. First observation of a Kondo resonance for a stable neutral pure organic radical, 1,3,5-triphenyl-6-oxoverdazyl, adsorbed on the Au(111) surface. J. Am. Chem. Soc. 2013, 135, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Mullegger, S.; Rashidi, M.; Fattinger, M.; Koch, R. Surface-supported hydrocarbon pi radicals show Kondo behavior. J. Phys. Chem. C 2013, 117, 5718–5721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Kahle, S.; Herden, T.; Stroh, C.; Mayor, M.; Schlickum, U.; Ternes, M.; Wahl, P.; Kern, K. Temperature and magnetic field dependence of a Kondo system in the weak coupling regime. Nat. Commun. 2013, 4, 2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garnica, M.; Stradi, D.; Barja, S.; Calleja, F.; Díaz, C.; Alcamí, M.; Martín, N.; Vázquez de Parga, A.L.; Martín, F.; Miranda, R. Long-range magnetic order in a purely organic 2D layer adsorbed on epitaxial graphene. Nat. Phys. 2013, 9, 368–374. [Google Scholar] [CrossRef]
- Fernandez-Torrente, I.; Franke, K.J.; Pascual, J.I. Vibrational Kondo effect in pure organic charge-transfer assemblies. Phys. Rev. Lett. 2008, 101, 217203. [Google Scholar] [CrossRef]
- Simao, C.; Mas-Torrent, M.; Crivillers, N.; Lloveras, V.; Artes, J.M.; Gorostiza, P.; Veciana, J.; Rovira, C. A robust molecular platform for non-volatile memory devices with optical and magnetic responses. Nat. Chem. 2011, 3, 359–364. [Google Scholar] [CrossRef]
- Frisenda, R.; Gaudenzi, R.; Franco, C.; Mas-Torrent, M.; Rovira, C.; Veciana, J.; Alcon, I.; Bromley, S.T.; Burzurí, E.; van der Zant, H.S.J. Kondo effect in a neutral and stable all organic radical single molecule break junction. Nano Lett. 2015, 15, 3109–3114. [Google Scholar] [CrossRef] [Green Version]
- Lloveras, V.; Badetti, E.; Veciana, J.; Vidal-Gancedo, J. Dynamics of intramolecular spin exchange interaction of a nitronyl nitroxide diradical in solution and on surfaces. Nanoscale 2016, 8, 5049–5058. [Google Scholar] [CrossRef] [Green Version]
- Gaudenzi, R.; Burzurí, E.; Reta, D.; Moreira, I.D.P.R.; Bromley, S.T.; Rovira, C.; Veciana, J.; van der Zant, H.S.J. Exchange coupling inversion in a high-spin organic triradical molecule. Nano Lett. 2016, 16, 2066–2071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karan, S.; Berndt, R. Generation of spin in single cholesterol molecules on gold. Phys. Chem. Chem. Phys. 2016, 18, 9334–9337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karan, S.; Li, N.; Zhang, Y.; He, Y.; Hong, I.P.; Song, H.; Lu, J.T.; Wang, Y.; Peng, L.; Wu, K.; et al. Spin manipulation by creation of single-molecule radical cations. Phys. Rev. Lett. 2016, 116, 027201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, N.; Zhang, Y.; Berndt, R.; Wang, Y. 13-cis-retinoic acid on coinage metals: Hierarchical self-assembly and spin generation. Phys. Chem. Chem. Phys. 2017, 19, 14919–14923. [Google Scholar] [CrossRef]
- Bocquet, M.-L.; Lorente, N.; Berndt, R.; Gruber, M. Spin in a closed-shell organic molecule on a metal substrate generated by a Sigmatropic reaction. Angew. Chem. Int. Ed. 2019, 58, 821–824. [Google Scholar] [CrossRef]
- Hong, I.P.; Li, N.; Zhang, Y.J.; Wang, H.; Song, H.J.; Bai, M.L.; Zhou, X.; Li, J.L.; Gu, G.C.; Zhang, X.; et al. Vacuum synthesis of magnetic aluminum phthalocyanine on Au(111). Chem. Commun. 2016, 52, 10338–10341. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Yang, J.; Hou, J. Single-molecule chemistry of metal phthalocyanine on noble metal surfaces. Acc. Chem. Res. 2010, 43, 954–962. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, K.; Kröger, J.; Berndt, R. Structures of phthalocyanine molecules on surfaces studied by STM. AIP Adv. 2012, 2, 041402. [Google Scholar] [CrossRef]
- Malavolti, L.; Briganti, M.; Hanze, M.; Serrano, G.; Cimatti, I.; McMurtrie, G.; Otero, E.; Ohresser, P.; Totti, F.; Mannini, M.; et al. Tunable spin−superconductor coupling of spin 1/2 vanadyl phthalocyanine molecules. Nano Lett. 2018, 18, 7955–7961. [Google Scholar] [CrossRef]
- Cimatti, I.; Bondi, L.; Serrano, G.; Malavolti, L.; Cortigiani, B.; Velez-Fort, E.; Betto, D.; Ouerghi, A.; Brookes, N.B.; Loth, S.; et al. Vanadyl phthalocyanines on graphene/SiC(0001): Toward a hybrid architecture for molecular spin qubits. Nanoscale Horiz. 2019, 4, 1202–1210. [Google Scholar] [CrossRef] [Green Version]
- Franke, K.J.; Schulze, G.; Pascual, J.I. Competition of superconducting phenomena and Kondo screening at the nanoscale. Science 2011, 332, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.S.; Ji, S.H.; Chen, X.; Ma, X.C.; Wu, R.; Wang, C.C.; Duan, W.H.; Qiu, X.H.; Sun, B.; Zhang, P.; et al. Manipulating the Kondo resonance through quantum size effects. Phys. Rev. Lett. 2007, 99, 256601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Yang, K.; Jiang, Y.; Song, B.; Xiao, W.; Li, L.; Zhou, H.; Wang, Y.; Du, S.; Ouyang, M.; et al. Reversible single spin control of individual magnetic molecule by hydrogen atom adsorption. Sci. Rep. 2013, 3, 1210. [Google Scholar] [CrossRef] [PubMed]
- Strozecka, A.; Soriano, M.; Pascual, J.I.; Palacios, J.J. Reversible change of the spin state in a manganese phthalocyanine by coordination of CO molecule. Phys. Rev. Lett. 2012, 109, 147202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Ji, W.; Hu, Y.B.; Cheng, Z.H.; Deng, Z.T.; Liu, Q.; Jiang, N.; Lin, X.; Guo, W.; Du, S.X.; et al. Site-specific Kondo effect at ambient temperatures in iron-based molecules. Phys. Rev. Lett. 2007, 99, 106402. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, N.; Wang, H.; Weismann, A.; Zhang, Y.; Hou, S.; Wu, K.; Wang, Y. Tuning the spin-related transport properties of FePc on Au(111) through single-molecule chemistry. Chem. Commun. 2018, 54, 9135–9138. [Google Scholar] [CrossRef]
- Zhao, A.; Li, Q.; Chen, L.; Xiang, H.; Wang, W.; Pan, S.; Wang, B.; Xiao, X.; Yang, J.; Hou, J.G.; et al. Controlling the Kondo effect of an adsorbed magnetic ion through its chemical bonding. Science 2005, 309, 1542–1544. [Google Scholar] [CrossRef]
- Mugarza, A.; Krull, C.; Robles, R.; Stepanow, S.; Ceballos, G.; Gambardella, P. Spin coupling and relaxation inside molecule-metal contacts. Nat. Commun. 2011, 2, 490. [Google Scholar] [CrossRef] [Green Version]
- Gutlich, P.; Hauser, A.; Spiering, H. Thermal and optical switching of iron(II) complexes. Angew. Chem. Int. Ed. Engl. 1994, 33, 2024–2054. [Google Scholar] [CrossRef]
- Marchivie, M.; Guionneau, P.; Howard, J.A.K.; Chastanet, G.; Létard, J.-F.; Goeta, A.E.; Chasseau, D. Structural characterization of a photoinduced molecular switch. J. Am. Chem. Soc. 2002, 124, 194–195. [Google Scholar] [CrossRef]
- Baadji, N.; Piacenza, M.; Tugsuz, T.; Della Sala, F.; Maruccio, G.; Sanvito, S. Electrostatic spin crossover effect in polar magnetic molecules. Nat. Mater. 2009, 8, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Z.; Wen, T.; Zhou, Y.; Li, N.; Han, F.; Xiao, Y.; Chow, P.; Sun, J.; Pravica, M.; et al. Pressure-driven cooperative spin-crossover, large-volume collapse, and semiconductor-to-metal transition in manganese(II) honeycomb lattices. J. Am. Chem. Soc. 2016, 138, 15751–15757. [Google Scholar] [CrossRef] [PubMed]
- Kobke, A.; Gutzeit, F.; Rohricht, F.; Schlimm, A.; Grunwald, J.; Tuczek, F.; Studniarek, M.; Longo, D.; Choueikani, F.; Otero, E.; et al. Reversible coordination-induced spin-state switching in complexes on metal surfaces. Nat. Nanotechnol. 2020, 15, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Ruben, M. Sublimable spin crossover complexes: From spin-state switching to molecular devices. Angew. Chem. Int. Ed. 2020, 59, 2–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paez-Espejo, M.; Sy, M.; Boukheddaden, K. Elastic frustration causing two-step and multistep transitions in spin-crossover solids: Emergence of complex antiferroelastic structures. J. Am. Chem. Soc. 2016, 138, 3202–3210. [Google Scholar] [CrossRef]
- Bairagi, K.; Iasco, O.; Bellec, A.; Kartsev, A.; Li, D.; Lagoute, J.; Chacon, C.; Girard, Y.; Rousset, S.; Miserque, F.; et al. Molecular-scale dynamics of light-induced spin cross-over in a two-dimensional layer. Nat. Commun. 2016, 7, 12212. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Wang, T.; Xue, Q.; Hua, M.; Wang, Y.; Huang, L.; Lin, N. Collective spin manipulation in antiferroelastic spin-crossover metallo-supramolecular chains. ACS Nano 2020, 14, 11283–11293. [Google Scholar] [CrossRef]
- Woodruff, D.N.; Winpenny, R.E.; Layfield, R.A. Lanthanide single-molecule magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef]
- Gomez-Segura, J.; Diez-Perez, I.; Ishikawa, N.; Nakano, M.; Veciana, J.; Ruiz-Molina, D. 2-D self-assembly of the bis(phthalocyaninato)terbium(III) single-molecule magnet studied by scanning tunnelling microscopy. Chem. Commun. 2006, 2866–2868. [Google Scholar] [CrossRef]
- Vitali, L.; Fabris, S.; Conte, A.M.; Brink, S.; Ruben, M.; Baroni, S.; Kern, K. Electronic structure of surface-supported bis(phthalocyaninato) terbium(III) single molecular magnets. Nano Lett. 2008, 8, 3364–3368. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Yoshida, Y.; Yamashita, M.; Miyasaka, H.; Breedlove, B.K.; Kajiwara, T.; Takaishi, S.; Ishikawa, N.; Isshiki, H.; Zhang, Y.F.; et al. Direct observation of lanthanide(III)-phthalocyanine molecules on Au(111) by using scanning tunneling microscopy and scanning tunneling spectroscopy and thin-film field-effect transistor properties of Tb(III)- and Dy(III)-phthalocyanine molecules. J. Am. Chem. Soc. 2009, 131, 9967–9976. [Google Scholar] [CrossRef] [PubMed]
- Komeda, T.; Isshiki, H.; Liu, J.; Zhang, Y.F.; Lorente, N.; Katoh, K.; Breedlove, B.K.; Yamashita, M. Observation and electric current control of a local spin in a single-molecule magnet. Nat. Commun. 2011, 2, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.S.; Schwobel, J.; Hla, S.W.; Dilullo, A.; Hoffmann, G.; Klyatskaya, S.; Ruben, M.; Wiesendanger, R. Reversible chiral switching of bis(phthalocyaninato) terbium(III) on a metal surface. Nano Lett. 2012, 12, 3931–3935. [Google Scholar] [CrossRef]
- Schwobel, J.; Fu, Y.; Brede, J.; Dilullo, A.; Hoffmann, G.; Klyatskaya, S.; Ruben, M.; Wiesendanger, R. Real-space observation of spin-split molecular orbitals of adsorbed single-molecule magnets. Nat. Commun. 2012, 3, 953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahrendorf, S.; Atodiresei, N.; Besson, C.; Caciuc, V.; Matthes, F.; Blugel, S.; Kogerler, P.; Burgler, D.E.; Schneider, C.M. Accessing 4f-states in single-molecule spintronics. Nat. Commun. 2013, 4, 2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Zhang, Y.; Hong, I.P.; Cheng, F.; Zhou, X.; Shen, Q.; Li, J.; Wang, Y.; Jiang, J.; Wu, K. Low-temperature scanning tunneling microscopy study of double-decker DyPc2 on Pb surface. Nanoscale 2014, 6, 10779–10783. [Google Scholar] [CrossRef] [PubMed]
- Komeda, T.; Isshiki, H.; Liu, J.; Katoh, K.; Yamashita, M. Variation of Kondo temperature induced by molecule-substrate decoupling in film formation of bis(phthalocyaninato)terbium(III) molecules on Au(111). ACS Nano 2014, 8, 4866–4875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liao, P.; Kan, J.; Yin, C.; Li, N.; Liu, J.; Chen, Q.; Wang, Y.; Chen, W.; Xu, G.Q.; et al. Low-temperature scanning tunneling microscopy study on the electronic properties of a double-decker DyPc2 molecule at the surface. Phys. Chem. Chem. Phys. 2015, 17, 27019–27026. [Google Scholar] [CrossRef]
- Serrano, G.; Wiespointner-Baumgarthuber, S.; Tebi, S.; Klyatskaya, S.; Ruben, M.; Koch, R.; Mullegger, S. Bilayer of terbium double-decker single-molecule magnets. J. Phys. Chem. C 2016, 120, 13581–13586. [Google Scholar] [CrossRef] [Green Version]
- Warner, B.; El Hallak, F.; Atodiresei, N.; Seibt, P.; Pruser, H.; Caciuc, V.; Waters, M.; Fisher, A.J.; Blugel, S.; van Slageren, J.; et al. Sub-molecular modulation of a 4f driven Kondo resonance by surface-induced asymmetry. Nat. Commun. 2016, 7, 12785. [Google Scholar] [CrossRef] [Green Version]
- Amokrane, A.; Klyatskaya, S.; Boero, M.; Ruben, M.; Bucher, J.P. Role of pi-radicals in the spin connectivity of clusters and networks of Tb double-decker single molecule magnets. ACS Nano 2017, 11, 10750–10760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Liao, P.; Wang, K.; Huang, Z.; Liu, J.; Chen, Q.; Jiang, J.; Wu, K. Detection and manipulation of charge states for double-decker DyPc2 molecules on ultrathin CuO films. ACS Nano 2018, 12, 2991–2997. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, R.; Amokrane, A.; Klyatskay, S.; Boero, M.; Ruben, M.; Bucher, J.-R. Screening the 4f-electron spin of TbPc2 singlemolecule magnets on metal substrates by ligand channeling. Nanoscale 2019, 11, 21167–21179. [Google Scholar] [CrossRef] [PubMed]
- Cervetti, C.; Rettori, A.; Pini, M.G.; Cornia, A.; Ana Repollés, A.; Luis, F.; Dressel, M.; Rauschenbach, S.; Kern, K.; Burghard, M.; et al. The classical and quantum dynamics of molecular spins on graphene. Nat. Mater. 2016, 15, 164–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gragnaniello, L.; Paschke, F.; Erler, P.; Schmitt, P.; Barth, N.; Simon, S.; Brune, H.; Rusponi, S.; Fonin, M. Uniaxial 2D superlattice of Fe4 molecular magnets on graphene. Nano Lett. 2017, 17, 7177–7182. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, L.; Zhang, Z.; Jiang, J.; Wang, K.; Peng, L.-M.; Yu, G. Sensitivity enhancement of graphene Hall sensors modified by single-molecule magnets at room temperature. RSC Adv. 2017, 7, 1776–1781. [Google Scholar] [CrossRef] [Green Version]
- Serrano, G.; Velez-Fort, E.; Cimatti, I.; Cortigiani, B.; Malavolti, L.; Betto, D.; Ouerghi, A.; Brookes, N.B.; Mannini, M.; Sessoli, R. Magnetic bistability of a TbPc2 submonolayer on a graphene/SiC(0001) conductive electrode. Nanoscale 2018, 10, 2715. [Google Scholar] [CrossRef]
- Paschke, F.; Erler, P.; Enenkel, V.; Gragnaniello, L.; Fonin, M. Bulk-like magnetic signature of individual. Fe4H molecular magnets on graphene. ACS Nano 2019, 13, 780–785. [Google Scholar] [CrossRef]
- Gajarushi, A.S.; Wasim, M.; Nabi, R.; Kancharlapalli, S.; Rao, V.R.; Rajaraman, G.; Subramaniam, C.; Shanmugam, M. Lanthanide complexes as molecular dopants for realizing air-stable n-type graphene logic inverters with symmetric transconductance. Mater. Horiz. 2019, 6, 743–750. [Google Scholar] [CrossRef]
- Sakurai, M.; Koley, P.; Aono, M. Tunable magnetism of organometallic nanoclusters by graphene oxide on-surface chemistry. Sci. Rep. 2019, 9, 14509. [Google Scholar] [CrossRef] [Green Version]
- Bogani, L.; Danieli, C.; Biavardi, E.; Bendiab, N.; Barra, A.-L.; Dalcanale, E.; Wernsdorfer, W.; Cornia, A. Single-molecule-magnet carbon-nanotube hybrids. Angew. Chem. Int. Ed. 2009, 48, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, R.; Satoh, J.; Katoh, K.; Zhang, H.; Breedlove, B.K.; Nishijima, M.; Nakanishi, Y.; Omachi, H.; Shinohara, H.; Yamashita, M. DySc2N@C80 single-molecule magnetic metallofullerene encapsulated in a single-walled carbon nanotube. J. Am. Chem. Soc. 2018, 140, 10955–10959. [Google Scholar] [CrossRef] [PubMed]
- Giusti, A.; Charron, G.; Mazerat, S.; Compain, J.-D.; Mialane, P.; Dolbecq, A.; Riviere, E.; Wernsdorfer, W.; Biboum, R.N.; Keita, B.; et al. Magnetic bistability of individual single-molecule magnets grafted on single-wall carbon nanotubes. Angew. Chem. Int. Ed. 2009, 48, 4949–4952. [Google Scholar] [CrossRef] [PubMed]
- Kyatskaya, S.; Mascaros, J.R.G.; Bogani, L.; Hennrich, F.; Kappes, M.; Wernsdorfer, W.; Ruben, M. Anchoring of rare-earth-based single-molecule magnets on single-walled carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 15143–15151. [Google Scholar] [CrossRef] [PubMed]
- Ganzhorn, M.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W. Strong spin–phonon coupling between a single-molecule magnet and a carbon nanotube nanoelectromechanical system. Nat. Nanotechnol. 2013, 8, 165–169. [Google Scholar] [CrossRef]
- Ganzhorn, M.; Klyatskaya, S.; Ruben, M.; Wernsdorfer†, W. Carbon nanotube nanoelectromechanical systems as magnetometers for single-molecule magnets. ACS Nano 2013, 7, 6225–6236. [Google Scholar] [CrossRef]
- Urdampilleta, M.; Klyatskaya, S.; Cleuziou, J.-P.; Ruben, M.; Wernsdorfer, W. Supramolecular spin valves. Nat. Mater. 2011, 10, 502–506. [Google Scholar] [CrossRef]
- Cervetti, C.; Heintze, E.; Bogani, L. Interweaving spins with their environment: Novel inorganic nanohybrids with controllable magnetic properties. Dalton Trans. 2014, 43, 4220–4232. [Google Scholar] [CrossRef] [Green Version]
- Pineda, E.M.; Komeda, T.; Katoh, K.; Yamashita, M.; Ruben, M. Surface confinement of TbPc2-SMMs: Structural, electronic and magnetic properties. Dalton Trans. 2016, 45, 18417–18433. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Liu, J.; Hou, S.; Wang, Y. Manipulation of Molecular Spin State on Surfaces Studied by Scanning Tunneling Microscopy. Nanomaterials 2020, 10, 2393. https://doi.org/10.3390/nano10122393
Xu Z, Liu J, Hou S, Wang Y. Manipulation of Molecular Spin State on Surfaces Studied by Scanning Tunneling Microscopy. Nanomaterials. 2020; 10(12):2393. https://doi.org/10.3390/nano10122393
Chicago/Turabian StyleXu, Zhen, Jing Liu, Shimin Hou, and Yongfeng Wang. 2020. "Manipulation of Molecular Spin State on Surfaces Studied by Scanning Tunneling Microscopy" Nanomaterials 10, no. 12: 2393. https://doi.org/10.3390/nano10122393




