Repeated vs. Acute Exposure of RAW264.7 Mouse Macrophages to Silica Nanoparticles: A Bioaccumulation and Functional Change Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles Characterization with Dynamic Light Scattering (DLS)
2.2. Bacteria Stimulation
2.3. Cell Culture of RAW264.7 Cells and Viability Assay
2.4. Phagocytosis Activity Measurement
2.5. Nitric Oxide Production
2.6. Cytokine Dosage in RAW264.7
2.7. Immunofluorescence of RAW264.7 Cells in Microscopy
2.8. Numerical Analyses
3. Results
3.1. Nanoparticle Characterization
3.2. Silica Accumulation in Various Exposure Modes
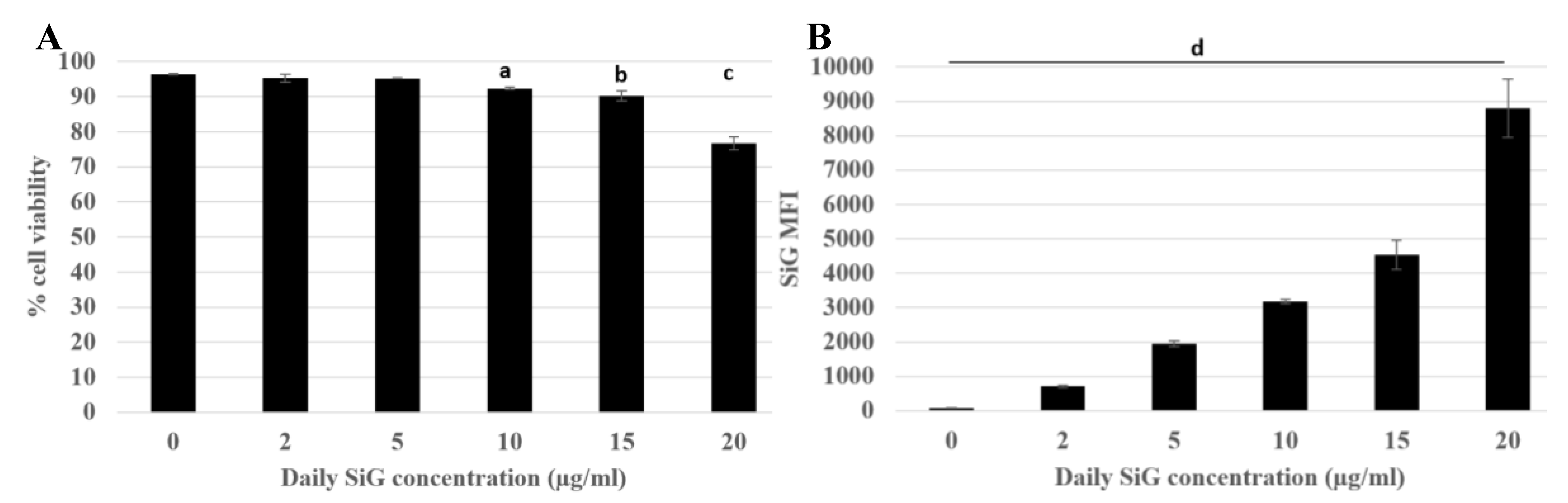
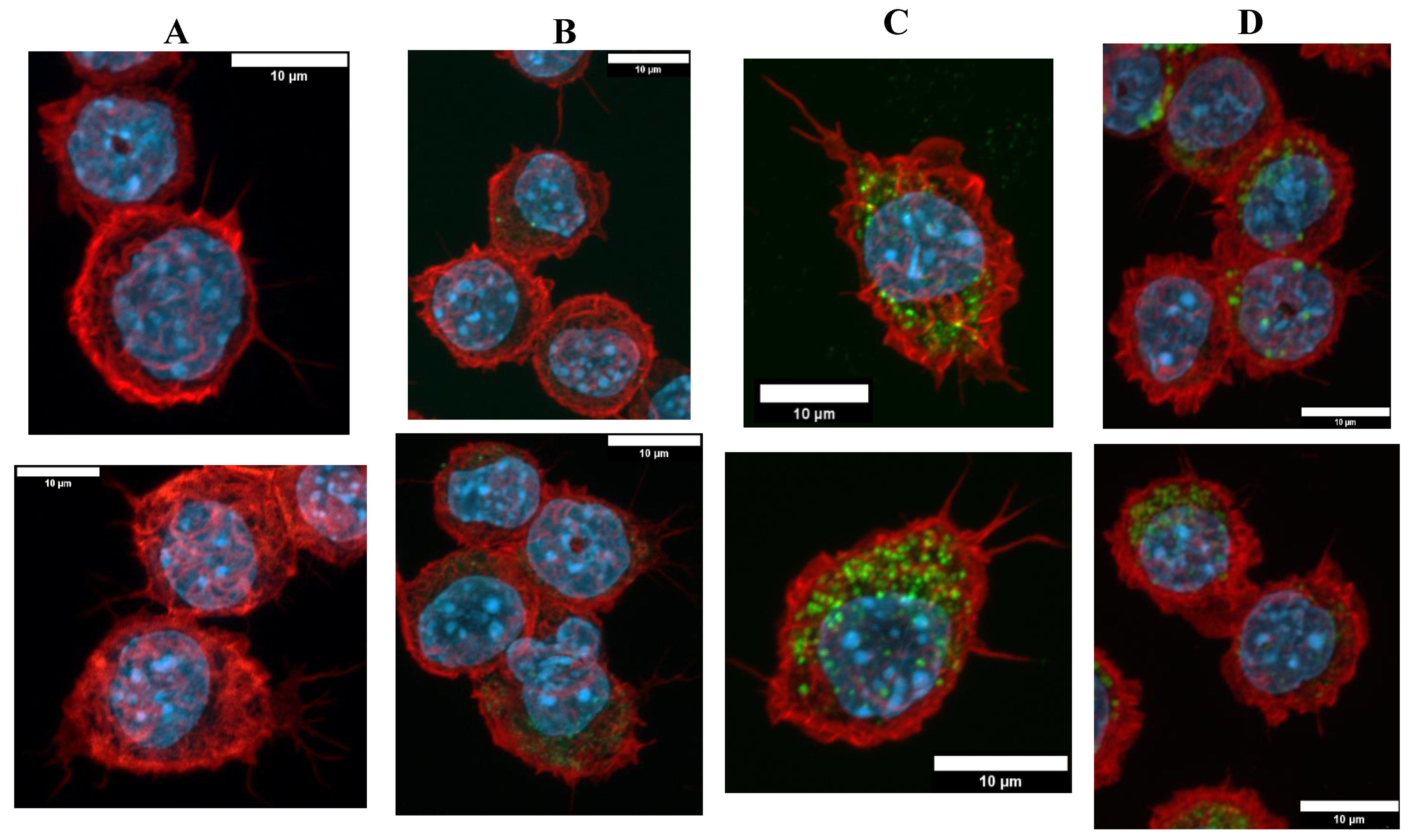
3.2.1. Internalization and Fluorescence Measurement in Acute Exposure
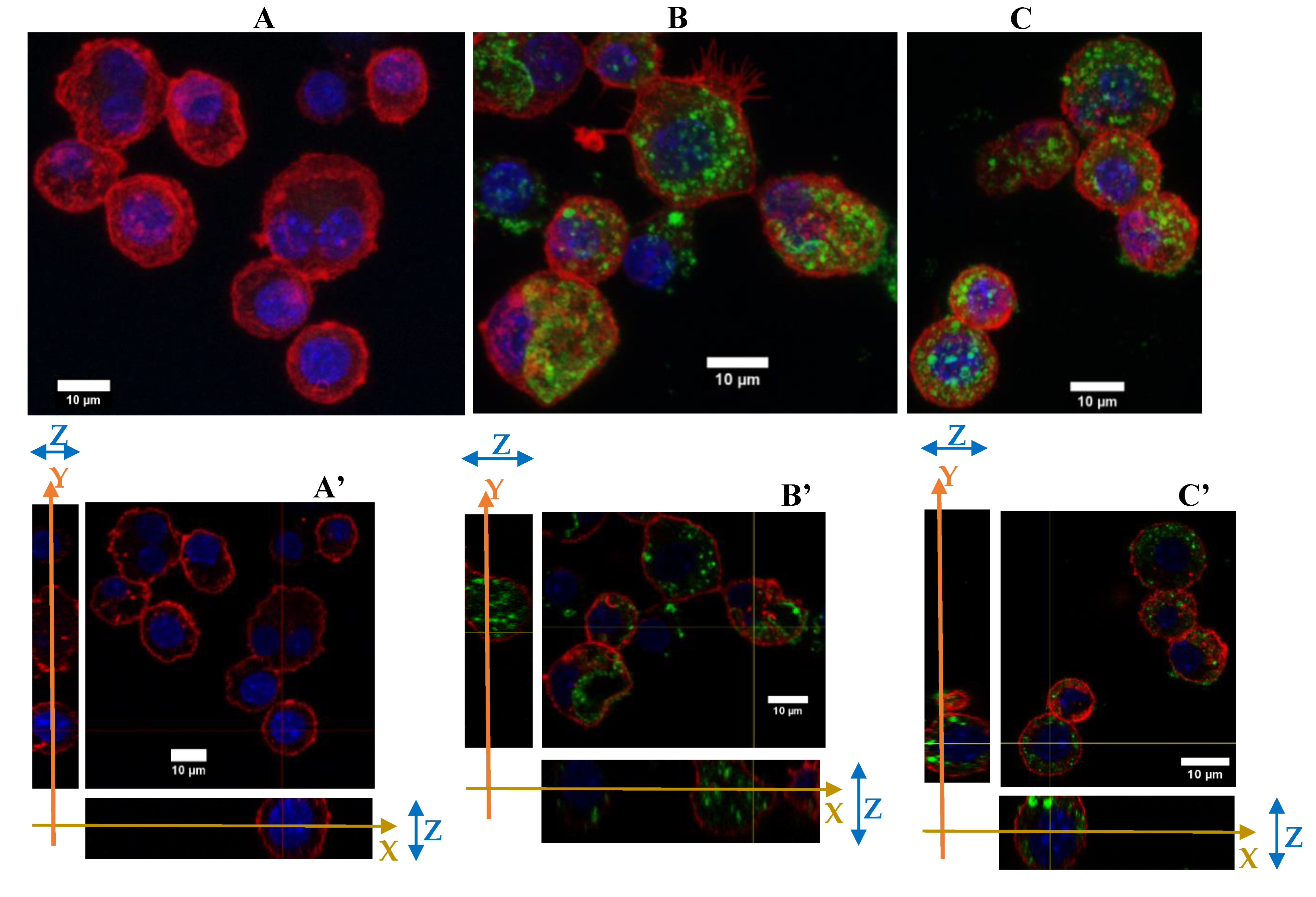
3.2.2. Silica Accumulation for a 4-Day Exposure
3.3. Biological Effect of the Silica Accumulation in Macrophages
3.3.1. Morphological Changes
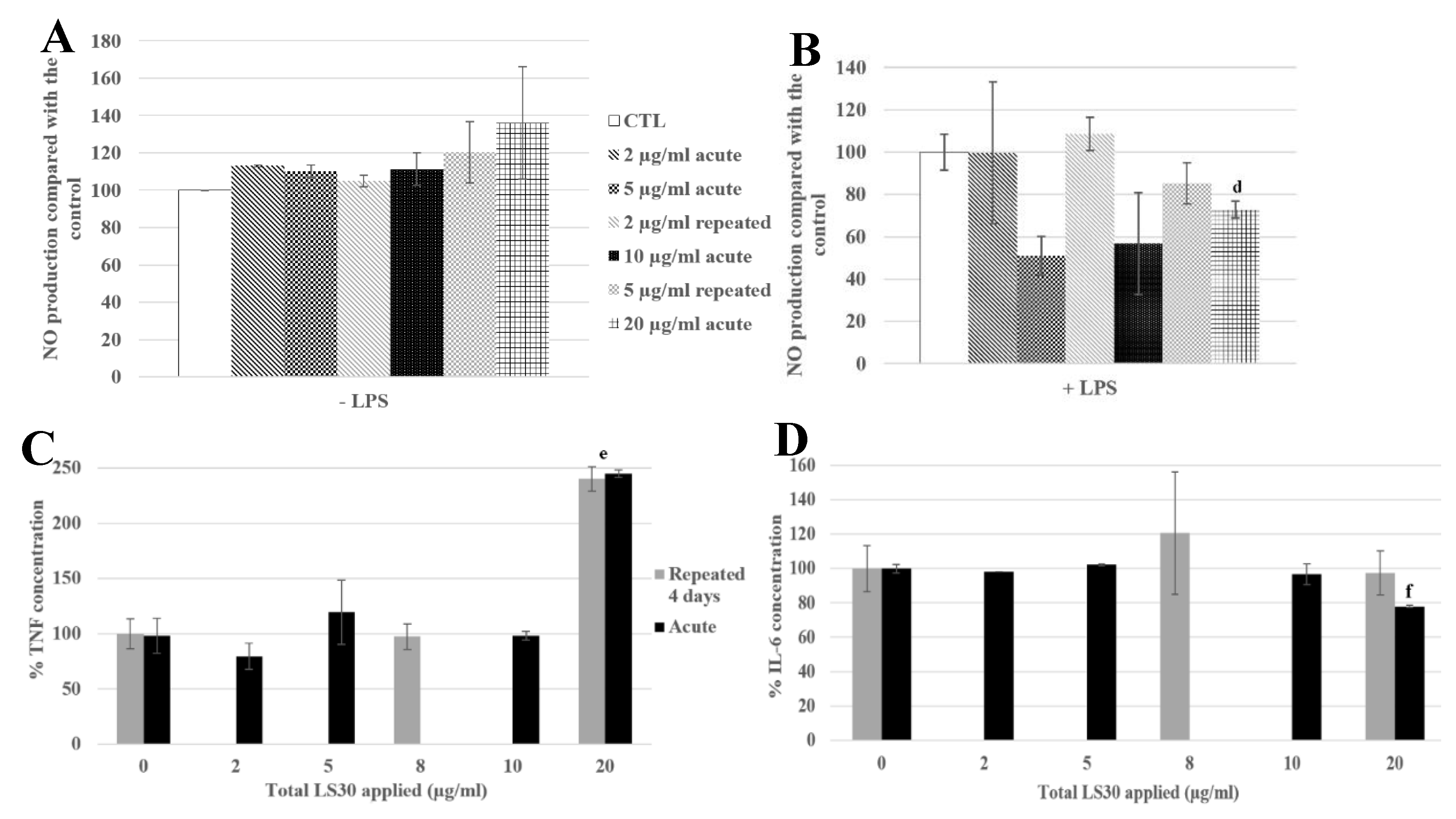
3.3.2. Functional Changes
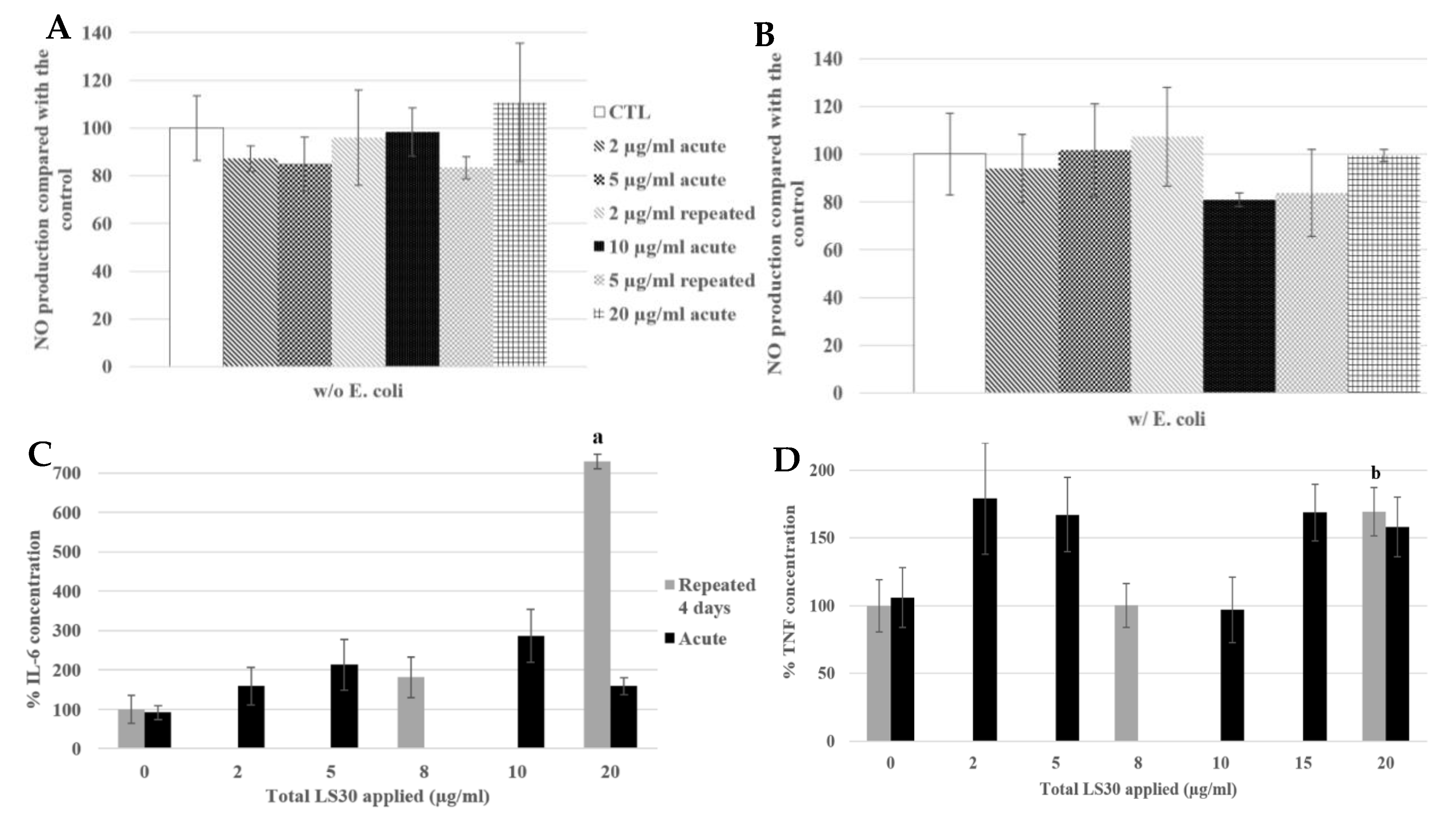
3.3.3. Physiological Situation of Co-Exposure: Industrial SiO2 and LPS
3.3.4. Physiological Situation of Co-Exposure: SiO2 and E. coli
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nie, S.; Xing, Y.; Kim, G.J.; Simons, J.W. Nanotechnology Applications in Cancer. Ann. Rev. Biomed. Eng. 2007, 9, 257–288. [Google Scholar] [CrossRef] [Green Version]
- Silica, amorphous [MAK Value Documentation, 1991]. MAK-Collect. Occup. Health Saf. 2012, 158–179. [CrossRef]
- Murphy, F.A.; Schinwald, A.; Poland, C.A.; Donaldson, K. The mechanism of pleural inflammation by long carbon nanotubes: Interaction of long fibres with macrophages stimulates them to amplify pro-inflammatory responses in mesothelial cells. Part Fibre Toxicol. 2012, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Flörke, O.W.; Graetsch, H.; Brunk, F.; Benda, L.; Paschen, S.; Bergna, H.E.; Roberts, W.O.; Welsh, W.A.; Chapman, D.M.; Ettlinger, M.; et al. Silica. Ullmanns Encycl. Ind. Chem. 2000. [Google Scholar] [CrossRef]
- Dekkers, S.; Krystek, P.; Peters, R.J.B.; Lankveld, D.P.K.; Bokkers, B.G.H.; van Hoeven-Arentzen, P.H.; Bouwmeester, H.; Oomen, A.G. Presence and risks of nanosilica in food products. Nanotoxicology 2011, 5, 393–405. [Google Scholar] [CrossRef]
- Powell, J.J.; Faria, N.; Thomas-McKay, E.; Pele, L.C. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J. Autoimmun. 2010, 34, J226–J233. [Google Scholar] [CrossRef]
- Rees, D.; Murray, J. Silica, silicosis and tuberculosis [State of the Art Series. Occupational lung disease in high- and low-income countries, Edited by M. Chan-Yeung. Number 4 in the series]. Int. J. Tuberc. Lung Dis. 2007, 11, 474–484. [Google Scholar]
- Work Safely with Silica n.d. Available online: https://www.silica-safe.org/ (accessed on 15 April 2019).
- Johnston, C.J.; Driscoll, K.E.; Finkelstein, J.N.; Baggs, R.; O’Reilly, M.A.; Carter, J.; Gelein, R.; Oberdorster, G. Pulmonary Chemokine and Mutagenic Responses in Rats after Subchronic Inhalation of Amorphous and Crystalline Silica. Toxicol. Sci. 2000, 56, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Joshi, G.; Gilberti, R.; Knecht, D. Single Cell Analysis of Phagocytosis, Phagosome Maturation, Phagolysosomal Leakage, and Cell Death Following Exposure of Macrophages to Silica Particles. Methods Mol. Biol. Clifton NJ 2017, 1519, 55–77. [Google Scholar] [CrossRef]
- Joshi, G.N.; Goetjen, A.M.; Knecht, D.A. Silica particles cause NADPH oxidase–independent ROS generation and transient phagolysosomal leakage. Mol. Biol. Cell 2015, 26, 3150–3164. [Google Scholar] [CrossRef]
- Park, E.-J.; Park, K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol. Lett. 2009, 184, 18–25. [Google Scholar] [CrossRef]
- Di Cristo, L.; Movia, D.; Bianchi, M.G.; Allegri, M.; Mohamed, B.M.; Bell, A.P.; Moore, C.; Pinelli, S.; Rasmussen, K.; Riego-Sintes, J.; et al. Proinflammatory Effects of Pyrogenic and Precipitated Amorphous Silica Nanoparticles in Innate Immunity Cells. Toxicol. Sci. 2016, 150, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Geppert, M.; Sigg, L.; Schirmer, K. A novel two-compartment barrier model for investigating nanoparticle transport in fish intestinal epithelial cells. Environ. Sci. Nano 2016, 3, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Hodges, G.M.; Carr, E.A.; Hazzard, R.A.; Carr, K.E. Uptake and translocation of microparticles in small intestine. Dig. Dis. Sci. 1995, 40, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.R.; Frevert, C.W. Innate Immunity in the Lungs. Proc. Am. Thorac Soc. 2005, 2, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Aude-Garcia, C.; Villiers, F.; Collin-Faure, V.; Pernet-Gallay, K.; Jouneau, P.-H.; Sorieul, S.; Mure, G.; Gerdil, A.; Herlin-Boime, N.; Carrière, M.; et al. Different in vitro exposure regimens of murine primary macrophages to silver nanoparticles induce different fates of nanoparticles and different toxicological and functional consequences. Nanotoxicology 2016, 10, 586–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annangi, B.; Rubio Lorente, L.; Alaraby, M.; Bach Griera, J.; Marcos, R.; Hernández, A. Acute and long-term in vitro effects of zinc oxide nanoparticles. Arch. Toxicol. 2015, 90. [Google Scholar] [CrossRef] [PubMed]
- Armand, L.; Tarantini, A.; Beal, D.; Biola-Clier, M.; Bobyk, L.; Sorieul, S.; Pernet-Gallay, K.; Marie-Desvergne, C.; Lynch, I.; Herlin-Boime, N.; et al. Long-term exposure of A549 cells to titanium dioxide nanoparticles induces DNA damage and sensitizes cells towards genotoxic agents. Nanotoxicology 2016, 10, 913–923. [Google Scholar] [CrossRef]
- Armand, L.; Biola-Clier, M.; Bobyk, L.; Collin-Faure, V.; Diemer, H.; Strub, J.M.; Cianferani, S.; Van Dorsselaer, A.; Herlin-Boime, N.; Rabilloud, T.; et al. Molecular responses of alveolar epithelial A549 cells to chronic exposure to titanium dioxide nanoparticles: A proteomic view. J. Proteomics 2016, 134, 163–173. [Google Scholar] [CrossRef]
- Dalzon, B.; Aude-Garcia, C.; Collin-Faure, V.; Diemer, H.; Béal, D.; Dussert, F.; Fenel, D.; Schoehn, G.; Cianférani, S.; Carrière, M.; et al. Differential proteomics highlights macrophage-specific responses to amorphous silica nanoparticles. Nanoscale 2017, 9, 9641–9658. [Google Scholar] [CrossRef] [Green Version]
- Triboulet, S. Etude des effets de deux types de nanoparticules métalliques sur des macrophages murins par une approche protéomique. Ph.D. Thesis, Université de Grenoble, Grenoble, France, 2013. [Google Scholar]
- Teller JDRNH, Grüttner CD rer nat, Rudershausen SDRN, Westphal FD-P. Verfahren zur Herstellung gefärbter und fluoreszenter Polykieselsäure-Partikel. EP1036763A1, 2000.
- Jones, K.H.; Senft, J.A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J. Histochem. Cytochem. 1985, 33, 77–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fede, C.; Selvestrel, F.; Compagnin, C.; Mognato, M.; Mancin, F.; Reddi, E.; Celloti, L. The toxicity outcome of silica nanoparticles (Ludox®) is influenced by testing techniques and treatment modalities. Anal. Bioanal. Chem. 2012, 404, 1789–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Aude-Garcia, C.; Dalzon, B.; Ravanat, J.-L.; Collin-Faure, V.; Diemer, H.; Strub, J.M.; Cianferani, S.; Van Dorsselaer, A.; Carrière, M.; Rabilloud, T. A combined proteomic and targeted analysis unravels new toxic mechanisms for zinc oxide nanoparticles in macrophages. J. Proteomics 2016, 134, 174–185. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Periasamy, V.S.; Alsaif, M.A.; Al-Warthan, A.A.; Alshatwi, A.A. Presence of nanosilica (E551) in commercial food products: TNF-mediated oxidative stress and altered cell cycle progression in human lung fibroblast cells. Cell Biol. Toxicol. 2014, 30, 89–100. [Google Scholar] [CrossRef]
- Keely, S.; Talley, N.J.; Hansbro, P.M. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 2012, 5, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Arts, J.H.E.; Muijser, H.; Duistermaat, E.; Junker, K.; Kuper, C.F. Five-day inhalation toxicity study of three types of synthetic amorphous silicas in Wistar rats and post-exposure evaluations for up to 3months. Food Chem. Toxicol. 2007, 45, 1856–1867. [Google Scholar] [CrossRef]
- Zhang, Y.; Nguyen, K.C.; Caldwell, D.; Fine, J.H.; Lefebvre, D.E.; Tayabali, A.F. Immune responses during single and repeated murine endotracheal exposures of zinc oxide nanoparticles. NanoImpact 2017, 7, 54–65. [Google Scholar] [CrossRef]
- Delaval, M.; Boland, S.; Solhonne, B.; Nicola, M.-A.; Mornet, S.; Baeza-Squiban, A.; Sallenave, J.-M.; Garcia-Verdugo, I.; et al. Acute exposure to silica nanoparticles enhances mortality and increases lung permeability in a mouse model of Pseudomonas aeruginosa pneumonia. Part Fibre Toxicol. 2015, 12. [Google Scholar] [CrossRef] [Green Version]
- Braakhuis, H.M.; Giannakou, C.; Peijnenburg, W.J.; Vermeulen, J.; van Loveren, H.; Park, M.V. Simple in vitro models can predict pulmonary toxicity of silver nanoparticles. Nanotoxicology 2016, 10, 770–779. [Google Scholar] [CrossRef]
- Toybou, D.; Celle, C.; Aude-Garcia, C.; Rabilloud, T.; Simonato, J.-P. A toxicology-informed, safer by design approach for the fabrication of transparent electrodes based on silver nanowires. Environ. Sci. 2019, 6, 684–694. [Google Scholar] [CrossRef]





| Nanoparticle | Solvant | Peak 1 | Peak 2 | Peak 3 |
|---|---|---|---|---|
| SiG (40 µg/mL) | H2O | 33.8 nm (100%) | 0 | 0 |
| RPMI | 34.4 nm (100%) | 0 | 0 | |
| RPMI 10% FBS | 7.99 nm (22.8%) | 43.5 m (74.4%) | 527 nm (2.9%) | |
| LS30 (20 µg/mL) | H2O | 24 nm (100%) | 0 | 0 |
| RPMI | 22.6 nm (100%) | 0 | 0 | |
| RPMI 10% FBS | 10.3 nm (17.7%) | 63.3 nm (82.3%) | 0 | |
| Media | H2O | 0 | 0 | 0 |
| RPMI | 0 | 0 | 0 | |
| RPMI 10% FBS | 39.7 nm (100%) | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, A.; Dalzon, B.; Collin-Faure, V.; Rabilloud, T. Repeated vs. Acute Exposure of RAW264.7 Mouse Macrophages to Silica Nanoparticles: A Bioaccumulation and Functional Change Study. Nanomaterials 2020, 10, 215. https://doi.org/10.3390/nano10020215
Torres A, Dalzon B, Collin-Faure V, Rabilloud T. Repeated vs. Acute Exposure of RAW264.7 Mouse Macrophages to Silica Nanoparticles: A Bioaccumulation and Functional Change Study. Nanomaterials. 2020; 10(2):215. https://doi.org/10.3390/nano10020215
Chicago/Turabian StyleTorres, Anaëlle, Bastien Dalzon, Véronique Collin-Faure, and Thierry Rabilloud. 2020. "Repeated vs. Acute Exposure of RAW264.7 Mouse Macrophages to Silica Nanoparticles: A Bioaccumulation and Functional Change Study" Nanomaterials 10, no. 2: 215. https://doi.org/10.3390/nano10020215
APA StyleTorres, A., Dalzon, B., Collin-Faure, V., & Rabilloud, T. (2020). Repeated vs. Acute Exposure of RAW264.7 Mouse Macrophages to Silica Nanoparticles: A Bioaccumulation and Functional Change Study. Nanomaterials, 10(2), 215. https://doi.org/10.3390/nano10020215





