Gold Nanoclusters as Electrocatalysts for Energy Conversion
Abstract
:1. Introduction
2. Electrocatalytic Reaction in Water Splitting
2.1. Hydrogen Evolution Reaction
2.2. Oxygen Evolution Reaction
3. Electrocatalytic Reactions in Fuel Cells
4. Conclusions
5. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of Thiol-Derivatised Gold Nanoparticles in a Two-Phase Liquid–Liquid System. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef] [PubMed]
- Kurashige, W.; Niihori, Y.; Sharma, S.; Negishi, Y. Precise Synthesis, Functionalization and Application of Thiolate-Protected Gold Clusters. Coord. Chem. Rev. 2016, 320, 238–250. [Google Scholar] [CrossRef]
- Hossain, S.; Niihori, Y.; Nair, L.V.; Kumar, B.; Kurashige, W.; Negishi, Y. Alloy Clusters: Precise Synthesis and Mixing Effects. Acc. Chem. Res. 2018, 51, 3114–3124. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Xia, N.; Wu, Z. Discovery, Mechanism; Application of Antigalvanic Reaction. Acc. Chem. Res. 2018, 51, 2774–2783. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Pradeep, T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, T.; Yuan, X.; Xie, J. Toward Total Synthesis of Thiolate-Protected Metal Nanoclusters. Acc. Chem. Res. 2018, 51, 1338–1348. [Google Scholar] [CrossRef]
- Qian, H.; Zhu, M.; Wu, Z.; Jin, R. Quantum Sized Gold Nanoclusters with Atomic Precision. Acc. Chem. Res. 2012, 45, 1470–1479. [Google Scholar] [CrossRef]
- Whetten, R.L.; Weissker, H.-C.; Pelayo, J.J.; Mullins, S.M.; López-Lozano, X.; Garzón, I.L. Chiral-Icosahedral (I) Symmetry in Ubiquitous Metallic Cluster Compounds (145A,60X): Structure and Bonding Principles. Acc. Chem. Res. 2019, 52, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Aikens, C.M. Electronic and Geometric Structure, Optical Properties, and Excited State Behavior in Atomically Precise Thiolate-Stabilized Noble Metal Nanoclusters. Acc. Chem. Res. 2018, 51, 3065–3073. [Google Scholar] [CrossRef]
- Nieto-Ortega, B.; Bürgi, T. Vibrational Properties of Thiolate-Protected Gold Nanoclusters. Acc. Chem. Res. 2018, 51, 2811–2819. [Google Scholar] [CrossRef] [PubMed]
- Agrachev, M.; Ruzzi, M.; Venzo, A.; Maran, F. Nuclear and Electron Magnetic Resonance Spectroscopies of Atomically Precise Gold Nanoclusters. Acc. Chem. Res. 2019, 52, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Wang, P.; Ma, Z.; Xiong, L. Growth-Rule-Guided Structural Exploration of Thiolate-Protected Gold Nanoclusters. Acc. Chem. Res. 2019, 52, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Mohammed, O.F.; Bakr, O.M. Atomic-Level Doping of Metal Clusters. Acc. Chem. Res. 2018, 51, 3094–3103. [Google Scholar] [CrossRef] [Green Version]
- Bigioni, T.P.; Whetten, R.L.; Dag, Ö. Near-Infrared Luminescence from Small Gold Nanocrystals. J. Phys. Chem. B 2000, 104, 6983–6986. [Google Scholar] [CrossRef]
- Yan, J.; Teo, B.K.; Zheng, N. Surface Chemistry of Atomically Precise Coinage–Metal Nanoclusters: From Structural Control to Surface Reactivity and Catalysis. Acc. Chem. Res. 2018, 51, 3084–3093. [Google Scholar] [CrossRef]
- Sakthivel, N.A.; Dass, A. Aromatic Thiolate-Protected Series of Gold Nanomolecules and a Contrary Structural Trend in Size Evolution. Acc. Chem. Res. 2018, 51, 1774–1783. [Google Scholar] [CrossRef]
- Tang, Q.; Hu, G.; Fung, V.; Jiang, D.-E. Insights into Interfaces, Stability, Electronic Properties, and Catalytic Activities of Atomically Precise Metal Nanoclusters from First Principles. Acc. Chem. Res. 2018, 51, 2793–2802. [Google Scholar] [CrossRef]
- Negishi, Y.; Nobusada, K.; Tsukuda, T. Glutathione-Protected Gold Clusters Revisited: Bridging the Gap between Gold(I)−Thiolate Complexes and Thiolate-Protected Gold Nanocrystals. J. Am. Chem. Soc. 2005, 127, 5261–5270. [Google Scholar] [CrossRef]
- Jadzinsky, P.D.; Calero, G.; Ackerson, C.J.; Bushnell, D.A.; Kornberg, R.D. Structure of a Thiol Monolayer-Protected Gold Nanoparticle at 1.1 Å Resolution. Science 2007, 318, 430–433. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Li, Q.; Kang, X.; Zhu, M. Customizing the Structure, Composition, and Properties of Alloy Nanoclusters by Metal Exchange. Acc. Chem. Res. 2018, 51, 2784–2792. [Google Scholar] [CrossRef] [PubMed]
- Higaki, T.; Li, Q.; Zhou, M.; Zhao, S.; Li, Y.; Li, S.; Jin, R. Toward the Tailoring Chemistry of Metal Nanoclusters for Enhancing Functionalities. Acc. Chem. Res. 2018, 51, 2764–2773. [Google Scholar] [CrossRef] [PubMed]
- Negishi, Y.; Kurashige, W.; Niihori, Y.; Iwasa, T.; Nobusada, K. Isolation, Structure, and Stability of a Dodecanethiolate-Protected Pd1Au24 Cluster. Phys. Chem. Chem. Phys. 2010, 12, 6219–6225. [Google Scholar] [CrossRef]
- Negishi, Y.; Iwai, T.; Ide, M. Continuous Modulation of Electronic Structure of Stable Thiolate-Protected Au25 Cluster by Ag Doping. Chem. Commun. 2010, 46, 4713–4715. [Google Scholar] [CrossRef] [PubMed]
- Negishi, Y.; Igarashi, K.; Munakata, K.; Ohgake, W.; Nobusada, K. Palladium Doping of Magic Gold Cluster Au38(SC2H4Ph)24: Formation of Pd2Au36(SC2H4Ph)24 with Higher Stability than Au38(SC2H4Ph)24. Chem. Commun. 2012, 48, 660–662. [Google Scholar] [CrossRef] [PubMed]
- Negishi, Y.; Munakata, K.; Ohgake, W.; Nobusada, K. Effect of Copper Doping on Electronic Structure, Geometric Structure, and Stability of Thiolate-Protected Au25 Nanoclusters. J. Phys. Chem. Lett. 2012, 3, 2209–2214. [Google Scholar] [CrossRef]
- Niihori, Y.; Kurashige, W.; Matsuzaki, M.; Negishi, Y. Remarkable Enhancement in Ligand-Exchange Reactivity of Thiolate-Protected Au25 Nanoclusters by Single Pd Atom Doping. Nanoscale 2013, 5, 508–512. [Google Scholar] [CrossRef]
- Negishi, Y.; Kurashige, W.; Kobayashi, Y.; Yamazoe, S.; Kojima, N.; Seto, M.; Tsukuda, T. Formation of a Pd@Au12 Superatomic Core in Au24Pd1(SC12H25)18 Probed by 197Au Mössbauer and Pd K-edge EXAFS Spectroscopy. J. Phys. Chem. Lett. 2013, 4, 3579–3583. [Google Scholar] [CrossRef]
- Negishi, Y.; Kurashige, W.; Niihori, Y.; Nobusada, K. Toward the Creation of Stable, Functionalized Metal Clusters. Phys. Chem. Chem. Phys. 2013, 15, 18736–18751. [Google Scholar] [CrossRef]
- Niihori, Y.; Matsuzaki, M.; Uchida, C.; Negishi, Y. Advanced Use of High-Performance Liquid Chromatography for Synthesis of Controlled Metal Clusters. Nanoscale 2014, 6, 7889–7896. [Google Scholar] [CrossRef]
- Yamazoe, S.; Kurashige, W.; Nobusada, K.; Negishi, Y.; Tsukuda, T. Preferential Location of Coinage Metal Dopants (M = Ag or Cu) in [Au25-xMx(SC2H4Ph)18]− (x ~ 1) as Determined by Extended X-ray Absorption Fine Structure and Density Functional Theory Calculations. J. Phys. Chem. C 2014, 118, 25284–25290. [Google Scholar] [CrossRef]
- Sharma, S.; Kurashige, W.; Nobusada, K.; Negishi, Y. Effect of Trimetallization in Thiolate-Protected Au24−nCunPd Clusters. Nanoscale 2015, 7, 10606–10612. [Google Scholar] [CrossRef] [PubMed]
- Niihori, Y.; Eguro, M.; Kato, A.; Sharma, S.; Kumar, B.; Kurashige, W.; Nobusada, K.; Negishi, Y. Improvements in the Ligand-Exchange Reactivity of Phenylethanethiolate-Protected Au25 Nanocluster by Ag or Cu Incorporation. J. Phys. Chem. C 2016, 120, 14301–14309. [Google Scholar] [CrossRef]
- Niihori, Y.; Hossain, S.; Kumar, B.; Nair, L.V.; Kurashige, W.; Negishi, Y. Perspective: Exchange Reactions in Thiolate-Protected Metal Clusters. APL Mater. 2017, 5, 053201. [Google Scholar] [CrossRef]
- Niihori, Y.; Hossain, S.; Sharma, S.; Kumar, B.; Kurashige, W.; Negishi, Y. Understanding and Practical Use of Ligand and Metal Exchange Reactions in Thiolate-Protected Metal Clusters to Synthesize Controlled Metal Clusters. Chem. Rec. 2017, 17, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Niihori, Y.; Shima, D.; Yoshida, K.; Hamada, K.; Nair, L.V.; Hossain, S.; Kurashige, W.; Negishi, Y. High-Performance Liquid Chromatography Mass Spectrometry of Gold and Alloy Clusters Protected by Hydrophilic Thiolates. Nanoscale 2018, 10, 1641–1649. [Google Scholar] [CrossRef]
- Hossain, S.; Ono, T.; Yoshioka, M.; Hu, G.; Hosoi, M.; Chen, Z.; Nair, L.V.; Niihori, Y.; Kurashige, W.; Jiang, D.-E.; et al. Thiolate-Protected Trimetallic Au~20Ag~4Pd and Au~20Ag~4Pt Alloy Clusters with Controlled Chemical Composition and Metal Positions. J. Phys. Chem. Lett. 2018, 9, 2590–2594. [Google Scholar] [CrossRef]
- Yokoyama, T.; Hirata, N.; Tsunoyama, H.; Negishi, Y.; Nakajima, A. Characterization of Floating-gate Memory Device with Thiolate-Protected Gold and Gold-Palladium Nanoclusters. AIP Adv. 2018, 8, 065002. [Google Scholar] [CrossRef] [Green Version]
- Niihori, Y.; Koyama, Y.; Watanabe, S.; Hashimoto, S.; Hossain, S.; Nair, L.V.; Kumar, B.; Kurashige, W.; Negishi, Y. Atomic and Isomeric Separation of Thiolate-Protected Alloy Clusters. J. Phys. Chem. Lett. 2018, 9, 4930–4934. [Google Scholar] [CrossRef]
- Niihori, Y.; Hashimoto, S.; Koyama, Y.; Hossain, S.; Kurashige, W.; Negishi, Y. Dynamic Behavior of Thiolate-Protected Gold–Silver 38-Atom Alloy Clusters in Solution. J. Phys. Chem. C 2019, 123, 13324–13329. [Google Scholar] [CrossRef]
- Kurashige, W.; Hayashi, R.; Wakamatsu, K.; Kataoka, Y.; Hossain, S.; Iwase, A.; Kudo, A.; Yamazoe, S.; Negishi, Y. Atomic-Level Understanding of the Effect of Heteroatom Doping of the Cocatalyst on Water-Splitting Activity in AuPd or AuPt Alloy Cluster-Loaded BaLa4Ti4O15. ACS Appl. Energy Mater. 2019, 2, 4175–4187. [Google Scholar] [CrossRef]
- Hossain, S.; Imai, Y.; Suzuki, D.; Choi, W.; Chen, Z.; Suzuki, T.; Yoshioka, M.; Kawawaki, T.; Lee, D.; Negishi, Y. Elucidating Ligand Effects in Thiolate-Protected Metal Clusters Using Au24Pt(TBBT)18 as a Model Cluster. Nanoscale 2019, 11, 22089–22098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawawaki, T.; Negishi, Y.; Kawasaki, H. Photo/electrocatalysis and Photosensitization Using Metal Nanoclusters for Green Energy and Medical Applications. Nanoscale Adv. 2020, 2, 17–36. [Google Scholar] [CrossRef] [Green Version]
- Hossain, S.; Imai, Y.; Motohashi, Y.; Chen, Z.; Suzuki, D.; Suzuki, T.; Kataoka, Y.; Hirata, M.; Ono, T.; Kurashige, W.; et al. Understanding and Designing One-Dimensional Assemblies of Ligand-Protected Metal Nanoclusters. Mater. Horiz. in press. [CrossRef] [Green Version]
- Haruta, M. Spiers Memorial Lecture Role of Perimeter Interfaces in Catalysis by Gold Nanoparticles. Faraday Discuss. 2011, 152, 11–32. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Qian, H.; Ge, Q.; Xu, H.; Jin, R. CO Oxidation Catalyzed by Oxide-Supported Au25(SR)18 Nanoclusters and Identification of Perimeter Sites as Active Centers. ACS Nano 2012, 6, 6014–6022. [Google Scholar] [CrossRef]
- Nie, X.; Zeng, C.; Ma, X.; Qian, H.; Ge, Q.; Xu, H.; Jin, R. CeO2-Supported Au38(SR)24 Nanocluster Catalysts for CO Oxidation: A Comparison of Ligand-on and -off Catalysts. Nanoscale 2013, 5, 5912–5918. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, D.-e.; Mann, A.K.P.; Mullins, D.R.; Qiao, Z.-A.; Allard, L.F.; Zeng, C.; Jin, R.; Overbury, S.H. Thiolate Ligands as a Double-Edged Sword for CO Oxidation on CeO2 Supported Au25(SCH2CH2Ph)18 Nanoclusters. J. Am. Chem. Soc. 2014, 136, 6111–6122. [Google Scholar] [CrossRef]
- Li, W.; Ge, Q.; Ma, X.; Chen, Y.; Zhu, M.; Xu, H.; Jin, R. Mild Activation of CeO2-Supported Gold Nanoclusters and Insight into the Catalytic Behavior in CO Oxidation. Nanoscale 2016, 8, 2378–2385. [Google Scholar] [CrossRef]
- Gaur, S.; Miller, J.T.; Stellwagen, D.; Sanampudi, A.; Kumar, C.S.S.R.; Spivey, J.J. Synthesis, Characterization, and Testing of Supported Au Catalysts Prepared from Atomically-Tailored Au38(SC12H25)24 Clusters. Phys. Chem. Chem. Phys. 2012, 14, 1627–1634. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, G.; Jiang, D.-e.; Mullins, D.R.; Zhang, Q.-F.; Allard, L.F.; Wang, L.-S.; Overbury, S.H. Diphosphine-Protected Au22 Nanoclusters on Oxide Supports Are Active for Gas-Phase Catalysis without Ligand Removal. Nano Lett. 2016, 16, 6560–6567. [Google Scholar] [CrossRef]
- Wu, Z.; Mullins, D.R.; Allard, L.F.; Zhang, Q.; Wang, L. CO Oxidation over Ceria Supported Au22 Nanoclusters: Shape Effect of the Support. Chin. Chem. Lett. 2018, 29, 795–799. [Google Scholar] [CrossRef]
- Lin, J.; Li, W.; Liu, C.; Huang, P.; Zhu, M.; Ge, Q.; Li, G. One-Phase Controlled Synthesis of Au25 Nanospheres and Nanorods from 1.3 nm Au : PPh3 Nanoparticles: The Ligand Effects. Nanoscale 2015, 7, 13663–13670. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, C.; Abroshan, H.; Ge, Q.; Yang, X.; Xu, H.; Li, G. Catalytic CO Oxidation Using Bimetallic MXAu25–X Clusters: A Combined Experimental and Computational Study on Doping Effects. J. Phys. Chem. C 2016, 120, 10261–10267. [Google Scholar] [CrossRef]
- Good, J.; Duchesne, P.N.; Zhang, P.; Koshut, W.; Zhou, M.; Jin, R. On the Functional Role of the Cerium Oxide Support in the Au38(SR)24/CeO2 Catalyst for CO Oxidation. Catal. Today 2017, 280, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Sheng, H.; Astruc, D.; Zhu, M. Atomically Precise Noble Metal Nanoclusters as Efficient Catalysts: A Bridge between Structure and Properties. Chem. Rev. 2020, 120, 526–622. [Google Scholar] [CrossRef]
- Xie, S.; Tsunoyama, H.; Kurashige, W.; Negishi, Y.; Tsukuda, T. Enhancement in Aerobic Alcohol Oxidation Catalysis of Au25 Clusters by Single Pd Atom Doping. ACS Catal. 2012, 2, 1519–1523. [Google Scholar] [CrossRef]
- Yoskamtorn, T.; Yamazoe, S.; Takahata, R.; Nishigaki, J.-i.; Thivasasith, A.; Limtrakul, J.; Tsukuda, T. Thiolate-Mediated Selectivity Control in Aerobic Alcohol Oxidation by Porous Carbon-Supported Au25 Clusters. ACS Catal. 2014, 4, 3696–3700. [Google Scholar] [CrossRef]
- Lavenn, C.; Demessence, A.; Tuel, A. Au25(SPh-pNH2)17 Nanoclusters Deposited on SBA-15 as Catalysts for Aerobic Benzyl Alcohol Oxidation. J. Catal. 2015, 322, 130–138. [Google Scholar] [CrossRef]
- Deng, H.; Wang, S.; Jin, S.; Yang, S.; Xu, Y.; Liu, L.; Xiang, J.; Hu, D.; Zhu, M. Active Metal (Cadmium) Doping Enhanced the Stability of Inert Metal (Gold) Nanocluster under O2 Atmosphere and the Catalysis Activity of Benzyl Alcohol Oxidation. Gold Bull. 2015, 48, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Dou, L.; Zhang, H. Layered Double Hydroxide Supported Gold Nanoclusters by Glutathione-Capped Au Nanoclusters Precursor Method for Highly Efficient Aerobic Oxidation of Alcohols. Nanoscale 2014, 6, 3753–3763. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yin, S.; Chen, G.; Li, L.; Zhang, H. Nearly Atomic Precise Gold Nanoclusters on Nickel-Based Layered Double Hydroxides for Extraordinarily Efficient Aerobic Oxidation of Alcohols. Catal. Sci. Technol. 2016, 6, 4090–4104. [Google Scholar] [CrossRef]
- Yin, S.; Li, J.; Zhang, H. Hierarchical Hollow Nanostructured Core@Shell Recyclable Catalysts γ-Fe2O3@LDH@Au25-X for Highly Efficient Alcohol Oxidation. Green Chem. 2016, 18, 5900–5914. [Google Scholar] [CrossRef]
- Lee, K.E.; Shivhare, A.; Hu, Y.; Scott, R.W.J. Supported Bimetallic AuPd Clusters Using Activated Au25 Clusters. Catal. Today 2017, 280, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Tsunoyama, H.; Ichikuni, N.; Sakurai, H.; Tsukuda, T. Effect of Electronic Structures of Au Clusters Stabilized by Poly(N-vinyl-2-pyrrolidone) on Aerobic Oxidation Catalysis. J. Am. Chem. Soc. 2009, 131, 7086–7093. [Google Scholar] [CrossRef]
- Zhu, Y.; Qian, H.; Zhu, M.; Jin, R. Thiolate-Protected Aun Nanoclusters as Catalysts for Selective Oxidation and Hydrogenation Processes. Adv. Mater. 2010, 22, 1915–1920. [Google Scholar] [CrossRef]
- Zhu, Y.; Qian, H.; Jin, R. An Atomic-Level Strategy for Unraveling Gold Nanocatalysis from the Perspective of Aun(SR)m Nanoclusters. Chem. Eur. J. 2010, 16, 11455–11462. [Google Scholar] [CrossRef]
- Wang, S.; Jin, S.; Yang, S.; Chen, S.; Song, Y.; Zhang, J.; Zhu, M. Total Structure Determination of Surface Doping [Ag46Au24(SR)32](BPh4)2 Nanocluster and Its Structure-Related Catalytic Property. Science Adv. 2015, 1, e1500441. [Google Scholar] [CrossRef] [Green Version]
- Chai, J.; Chong, H.; Wang, S.; Yang, S.; Wu, M.; Zhu, M. Controlling the Selectivity of Catalytic Oxidation of Styrene over Nanocluster Catalysts. RSC Adv. 2016, 6, 111399–111405. [Google Scholar] [CrossRef]
- Turner, M.; Golovko, V.B.; Vaughan, O.P.H.; Abdulkin, P.; Berenguer-Murcia, A.; Tikhov, M.S.; Johnson, B.F.G.; Lambert, R.M. Selective Oxidation with Dioxygen by Gold Nanoparticle Catalysts Derived from 55-Atom Clusters. Nature 2008, 454, 981–983. [Google Scholar] [CrossRef]
- Zhang, B.; Kaziz, S.; Li, H.; Hevia, M.G.; Wodka, D.; Mazet, C.; Bürgi, T.; Barrabés, N. Modulation of Active Sites in Supported Au38(SC2H4Ph)24 Cluster Catalysts: Effect of Atmosphere and Support Material. J. Phys. Chem. C 2015, 119, 11193–11199. [Google Scholar] [CrossRef]
- Liu, Y.; Tsunoyama, H.; Akita, T.; Xie, S.; Tsukuda, T. Aerobic Oxidation of Cyclohexane Catalyzed by Size-Controlled Au Clusters on Hydroxyapatite: Size Effect in the Sub-2 nm Regime. ACS Catal. 2011, 1, 2–6. [Google Scholar] [CrossRef]
- Liu, C.; Yan, C.; Lin, J.; Yu, C.; Huang, J.; Li, G. One-Pot Synthesis of Au144(SCH2Ph)60 Nanoclusters and Their Catalytic Application. J. Mater. Chem. A 2015, 3, 20167–20173. [Google Scholar] [CrossRef]
- Li, G.; Qian, H.; Jin, R. Gold Nanocluster-Catalyzed Selective Oxidation of Sulfide to Sulfoxide. Nanoscale 2012, 4, 6714–6717. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Liu, C.; Li, Z.; Li, G. Kinetically Controlled Synthesis of Au102(SPh)44 Nanoclusters and Catalytic Application. Nanoscale 2016, 8, 10059–10065. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, D.R.; Alfonso, D.; Matranga, C.; Ohodnicki, P.; Deng, X.; Siva, R.C.; Zeng, C.; Jin, R. Probing Active Site Chemistry with Differently Charged Au25q Nanoclusters (q = −1, 0, +1). Chem. Sci. 2014, 5, 3151–3157. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Alfonso, D.; Matranga, C.; Qian, H.; Jin, R. Experimental and Computational Investigation of Au25 Clusters and CO2: A Unique Interaction and Enhanced Electrocatalytic Activity. J. Am. Chem. Soc. 2012, 134, 10237–10243. [Google Scholar] [CrossRef]
- Zhao, S.; Jin, R.; Jin, R. Opportunities and Challenges in CO2 Reduction by Gold- and Silver-Based Electrocatalysts: From Bulk Metals to Nanoparticles and Atomically Precise Nanoclusters. ACS Energy Lett. 2018, 3, 452–462. [Google Scholar] [CrossRef]
- Andrews, E.; Katla, S.; Kumar, C.; Patterson, M.; Sprunger, P.; Flake, J. Electrocatalytic Reduction of CO2 at Au Nanoparticle Electrodes: Effects of Interfacial Chemistry on Reduction Behavior. J. Electrochem. Soc. 2015, 162, F1373–F1378. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Thakkar, J.; Siva, R.; Matranga, C.; Ohodnicki, P.R.; Zeng, C.; Jin, R. Efficient Electrochemical CO2 Conversion Powered by Renewable Energy. ACS Appl. Mater. Inter. 2015, 7, 15626–15632. [Google Scholar] [CrossRef]
- Zhao, S.; Austin, N.; Li, M.; Song, Y.; House, S.D.; Bernhard, S.; Yang, J.C.; Mpourmpakis, G.; Jin, R. Influence of Atomic-Level Morphology on Catalysis: The Case of Sphere and Rod-Like Gold Nanoclusters for CO2 Electroreduction. ACS Catal. 2018, 8, 4996–5001. [Google Scholar] [CrossRef]
- Jupally, V.R.; Dharmaratne, A.C.; Crasto, D.; Huckaba, A.J.; Kumara, C.; Nimmala, P.R.; Kothalawala, N.; Delcamp, J.H.; Dass, A. Au137(SR)56 Nanomolecules: Composition, Optical Spectroscopy, Electrochemistry and Electrocatalytic Reduction of CO2. Chem. Commun. 2014, 50, 9895–9898. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, D.R.; Kauffman, D.; Matranga, C. Active Sites of Ligand-Protected Au25 Nanoparticle Catalysts for CO2 Electroreduction to CO. J. Chem. Phys. 2016, 144, 184705. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining Theory and Experiment in Electrocatalysis: Insights into Materials Design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of Electrocatalysts for Oxygen- and Hydrogen-Involving Energy Conversion Reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Sabatier, P. Hydrogénations Et Déshydrogénations Par Catalyse. Ber. Dtsch. Chem. Ges. 1911, 44, 1984–2001. [Google Scholar] [CrossRef] [Green Version]
- Parsons, R. The Rate of Electrolytic Hydrogen Evolution and the Heat of Adsorption of Hydrogen. Trans. Faraday Soc. 1958, 54, 1053–1063. [Google Scholar] [CrossRef]
- Benck, J.D.; Hellstern, T.R.; Kibsgaard, J.; Chakthranont, P.; Jaramillo, T.F. Catalyzing the Hydrogen Evolution Reaction (HER) with Molybdenum Sulfide Nanomaterials. ACS Catal. 2014, 4, 3957–3971. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.; Veith, G.M.; Neuefeind, J.C.; Adzic, R.R.; Khalifah, P.G. Mixed Close-Packed Cobalt Molybdenum Nitrides as Non-Noble Metal Electrocatalysts for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 19186–19192. [Google Scholar] [CrossRef]
- Popczun, E.J.; McKone, J.R.; Read, C.G.; Biacchi, A.J.; Wiltrout, A.M.; Lewis, N.S.; Schaak, R.E. Nanostructured Nickel Phosphide as an Electrocatalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. [Google Scholar] [CrossRef] [PubMed]
- Kibler, L.A.; El-Aziz, A.M.; Hoyer, R.; Kolb, D.M. Tuning Reaction Rates by Lateral Strain in a Palladium Monolayer. Angew. Chem. Int. Ed. 2005, 44, 2080–2084. [Google Scholar] [CrossRef] [PubMed]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Stoerzinger, K.A.; Qiao, L.; Biegalski, M.D.; Shao-Horn, Y. Orientation-Dependent Oxygen Evolution Activities of Rutile IrO2 and RuO2. J. Phys. Chem. Lett. 2014, 5, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zheng, X.; Voznyy, O.; Comin, R.; Bajdich, M.; García-Melchor, M.; Han, L.; Xu, J.; Liu, M.; Zheng, L.; et al. Homogeneously Dispersed Multimetal Oxygen-Evolving Catalysts. Science 2016, 352, 333–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity Benchmarks and Requirements for Pt, Pt-Alloy, and Non-Pt Oxygen Reduction Catalysts for PEMFCs. Appl. Catal. B 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Marković, N.M. Just a Dream−or Future Reality? Science 2009, 324, 48–49. [Google Scholar] [CrossRef]
- Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E.A.; Frydendal, R.; Hansen, T.W.; et al. Enabling Direct H2O2 Production through Rational Electrocatalyst Design. Nat. Mater. 2013, 12, 1137–1143. [Google Scholar] [CrossRef] [Green Version]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Z.; Cao, L.; Chen, Y.; Zhu, E.; Lin, Z.; Li, M.; Yan, A.; Zettl, A.; Wang, Y.M.; et al. High-Performance Transition Metal–Doped Pt3Ni Octahedra for Oxygen Reduction Reaction. Science 2015, 348, 1230–1234. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Yang, H. Synthesis and Oxygen Reduction Electrocatalytic Property of Pt-on-Pd Bimetallic Heteronanostructures. J. Am. Chem. Soc. 2009, 131, 7542–7543. [Google Scholar] [CrossRef] [PubMed]
- Kwak, K.; Choi, W.; Tang, Q.; Kim, M.; Lee, Y.; Jiang, D.-e.; Lee, D. A Molecule-Like PtAu24(SC6H13)18 Nanocluster as an Electrocatalyst for Hydrogen Production. Nat. Commun. 2017, 8, 14723. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Hu, G.; Kwak, K.; Kim, M.; Jiang, D.-e.; Choi, J.-P.; Lee, D. Effects of Metal-Doping on Hydrogen Evolution Reaction Catalyzed by MAu24 and M2Au36 Nanoclusters (M = Pt, Pd). ACS Appl. Mater. Inter. 2018, 10, 44645–44653. [Google Scholar] [CrossRef] [PubMed]
- Kwak, K.; Lee, D. Electrochemistry of Atomically Precise Metal Nanoclusters. Acc. Chem. Res. 2019, 52, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Tang, Q.; Lee, D.; Wu, Z.; Jiang, D.-e. Metallic Hydrogen in Atomically Precise Gold Nanoclusters. Chem. Mater. 2017, 29, 4840–4847. [Google Scholar] [CrossRef]
- Du, Y.; Xiang, J.; Ni, K.; Yun, Y.; Sun, G.; Yuan, X.; Sheng, H.; Zhu, Y.; Zhu, M. Design of Atomically Precise Au2Pd6 Nanoclusters for Boosting Electrocatalytic Hydrogen Evolution on MoS2. Inorg. Chem. Front. 2018, 5, 2948–2954. [Google Scholar] [CrossRef]
- Eguchi, D.; Sakamoto, M.; Teranishi, T. Ligand Effect on the Catalytic Activity of Porphyrin-Protected Gold Clusters in the Electrochemical Hydrogen Evolution Reaction. Chem. Sci. 2018, 9, 261–265. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Jin, R.; Song, Y.; Zhang, H.; House, S.D.; Yang, J.C.; Jin, R. Atomically Precise Gold Nanoclusters Accelerate Hydrogen Evolution over MoS2 Nanosheets: The Dual Interfacial Effect. Small 2017, 13, 1701519. [Google Scholar] [CrossRef]
- Kwak, K.; Choi, W.; Tang, Q.; Jiang, D.-e.; Lee, D. Rationally Designed Metal Nanocluster for Electrocatalytic Hydrogen Production from Water. J. Mater. Chem. A 2018, 6, 19495–19501. [Google Scholar] [CrossRef]
- Zhao, S.; Jin, R.; Abroshan, H.; Zeng, C.; Zhang, H.; House, S.D.; Gottlieb, E.; Kim, H.J.; Yang, J.C.; Jin, R. Gold Nanoclusters Promote Electrocatalytic Water Oxidation at the Nanocluster/CoSe2 Interface. J. Am. Chem. Soc. 2017, 139, 1077–1080. [Google Scholar] [CrossRef]
- Chen, W.; Chen, S. Oxygen Electroreduction Catalyzed by Gold Nanoclusters: Strong Core Size Effects. Angew. Chem. Int. Ed. 2009, 48, 4386–4389. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, Z.; Yan, W.; Yang, H.; Wang, Q.; Chen, S. Porous Carbon-Supported Gold Nanoparticles for Oxygen Reduction Reaction: Effects of Nanoparticle Size. ACS Appl. Mater. Inter. 2016, 8, 20635–20641. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.; Sakthivel, N.A.; Schrock, H.; Artyushkova, K.; Dass, A.; Chakraborty, S. Electrocatalytic Oxygen Reduction Activities of Thiol-Protected Nanomolecules Ranging in Size from Au28(SR)20 to Au279(SR)84. J. Phys. Chem. C 2018, 122, 24809–24817. [Google Scholar] [CrossRef]
- Jones, T.C.; Sumner, L.; Ramakrishna, G.; Hatshan, M.b.; Abuhagr, A.; Chakraborty, S.; Dass, A. Bulky t-Butyl Thiolated Gold Nanomolecular Series: Synthesis, Characterization, Optical Properties, and Electrocatalysis. J. Phys. Chem. C 2018, 122, 17726–17737. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, Y.; Gao, X.; Chen, W. Charge State-Dependent Catalytic Activity of [Au25(SC12H25)18] Nanoclusters for the Two-Electron Reduction of Dioxygen to Hydrogen Peroxide. Chem. Commun. 2014, 50, 8464–8467. [Google Scholar] [CrossRef]
- Kwak, K.; Azad, U.P.; Choi, W.; Pyo, K.; Jang, M.; Lee, D. Efficient Oxygen Reduction Electrocatalysts Based on Gold Nanocluster–Graphene Composites. ChemElectroChem 2016, 3, 1253–1260. [Google Scholar] [CrossRef]
- Skúlason, E.; Tripkovic, V.; Björketun, M.E.; Gudmundsdóttir, S.; Karlberg, G.; Rossmeisl, J.; Bligaard, T.; Jónsson, H.; Nørskov, J.K. Modeling the Electrochemical Hydrogen Oxidation and Evolution Reactions on the Basis of Density Functional Theory Calculations. J. Phys. Chem. C 2010, 114, 18182–18197. [Google Scholar] [CrossRef]
- Sakamoto, M.; Tanaka, D.; Tsunoyama, H.; Tsukuda, T.; Minagawa, Y.; Majima, Y.; Teranishi, T. Platonic Hexahedron Composed of Six Organic Faces with an Inscribed Au Cluster. J. Am. Chem. Soc. 2012, 134, 816–819. [Google Scholar] [CrossRef]
- Tanaka, D.; Inuta, Y.; Sakamoto, M.; Furube, A.; Haruta, M.; So, Y.-G.; Kimoto, K.; Hamada, I.; Teranishi, T. Strongest Π–Metal Orbital Coupling in a Porphyrin/Gold Cluster System. Chem. Sci. 2014, 5, 2007–2010. [Google Scholar] [CrossRef]
- Li, P.; Wang, M.; Duan, X.; Zheng, L.; Cheng, X.; Zhang, Y.; Kuang, Y.; Li, Y.; Ma, Q.; Feng, Z.; et al. Boosting Oxygen Evolution of Single-Atomic Ruthenium through Electronic Coupling with Cobalt-Iron Layered Double Hydroxides. Nat. Commun. 2019, 10, 1711. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, G.; Giannozzi, P.; Amore Bonapasta, A.; Guidoni, L. Reaction Pathways for Oxygen Evolution Promoted by Cobalt Catalyst. J. Am. Chem. Soc. 2013, 135, 15353–15363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frydendal, R.; Paoli, E.A.; Knudsen, B.P.; Wickman, B.; Malacrida, P.; Stephens, I.E.L.; Chorkendorff, I. Benchmarking the Stability of Oxygen Evolution Reaction Catalysts: The Importance of Monitoring Mass Losses. ChemElectroChem 2014, 1, 2075–2081. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Z.; Sheng, W.; Yan, Y. Synthesis of Monodispere Au@Co3O4 Core-Shell Nanocrystals and Their Enhanced Catalytic Activity for Oxygen Evolution Reaction. Adv. Mater. 2014, 26, 3950–3955. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, P.; Yan, Y.; Li, X.; Xing, Y.; Li, H.; Peng, Z.; Yang, J.; Zeng, J. Gold Atom-Decorated CoSe2 Nanobelts with Engineered Active Sites for Enhanced Oxygen Evolution. J. Mater. Chem. A 2017, 5, 20202–20207. [Google Scholar] [CrossRef]
- Li, Z.-y.; Ye, K.-h.; Zhong, Q.-s.; Zhang, C.-j.; Shi, S.-t.; Xu, C.-w. Au–Co3O4/C as an Efficient Electrocatalyst for the Oxygen Evolution Reaction. ChemPlusChem 2014, 79, 1569–1572. [Google Scholar] [CrossRef]
- Mills, G.; Gordon, M.S.; Metiu, H. Oxygen Adsorption on Au Clusters and a Rough Au(111) Surface: The Role of Surface Flatness, Electron Confinement, Excess Electrons, and Band Gap. J. Chem. Phys. 2003, 118, 4198–4205. [Google Scholar] [CrossRef] [Green Version]
- Okumura, M.; Kitagawa, Y.; Kawakami, T.; Haruta, M. Theoretical Investigation of the Hetero-Junction Effect in PVP-Stabilized Au13 Clusters. The Role of PVP in Their Catalytic Activities. Chem. Phys. Lett. 2008, 459, 133–136. [Google Scholar] [CrossRef]
- Yin, H.; Tang, H.; Wang, D.; Gao, Y.; Tang, Z. Facile Synthesis of Surfactant-Free Au Cluster/Graphene Hybrids for High-Performance Oxygen Reduction Reaction. ACS Nano 2012, 6, 8288–8297. [Google Scholar] [CrossRef]





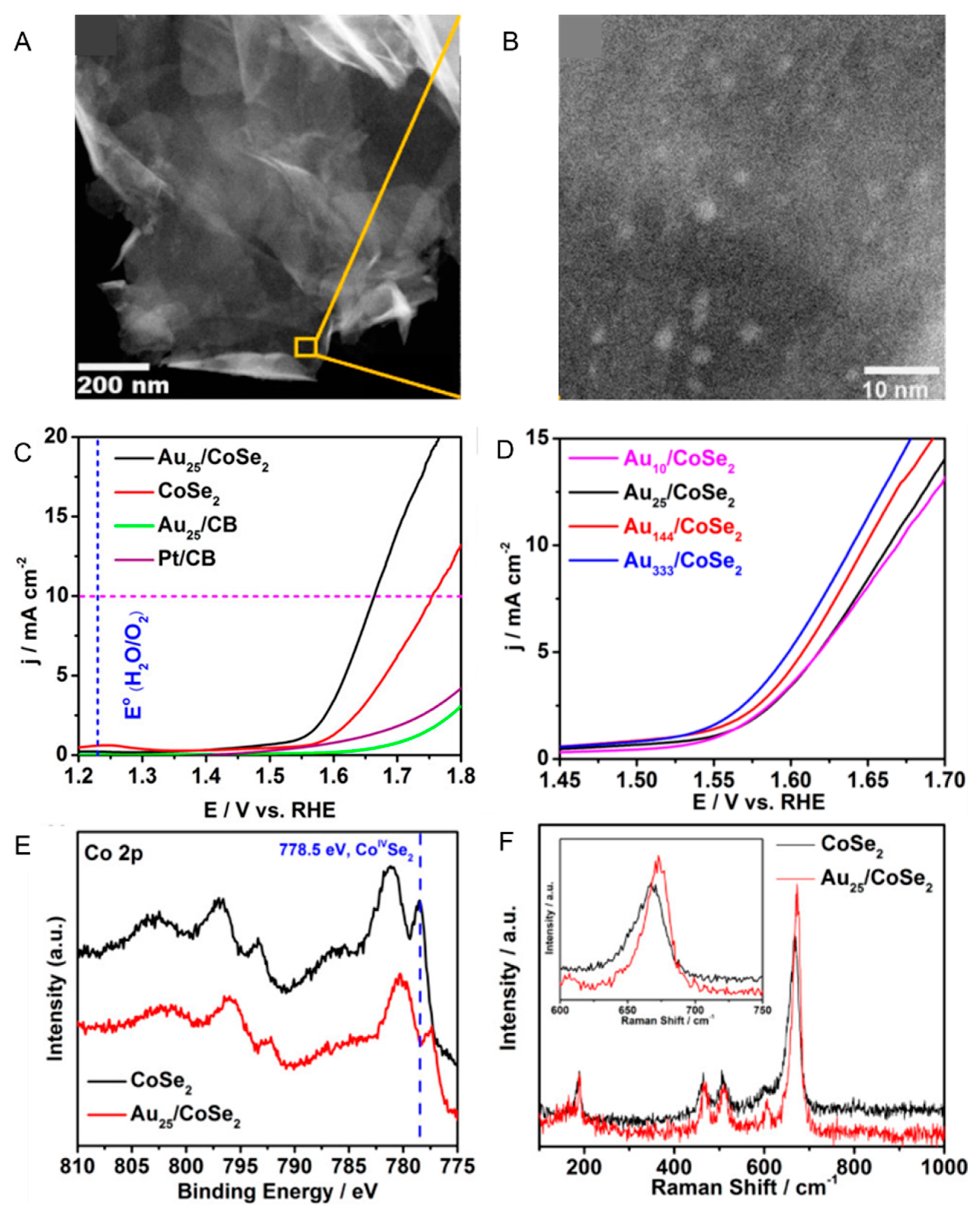


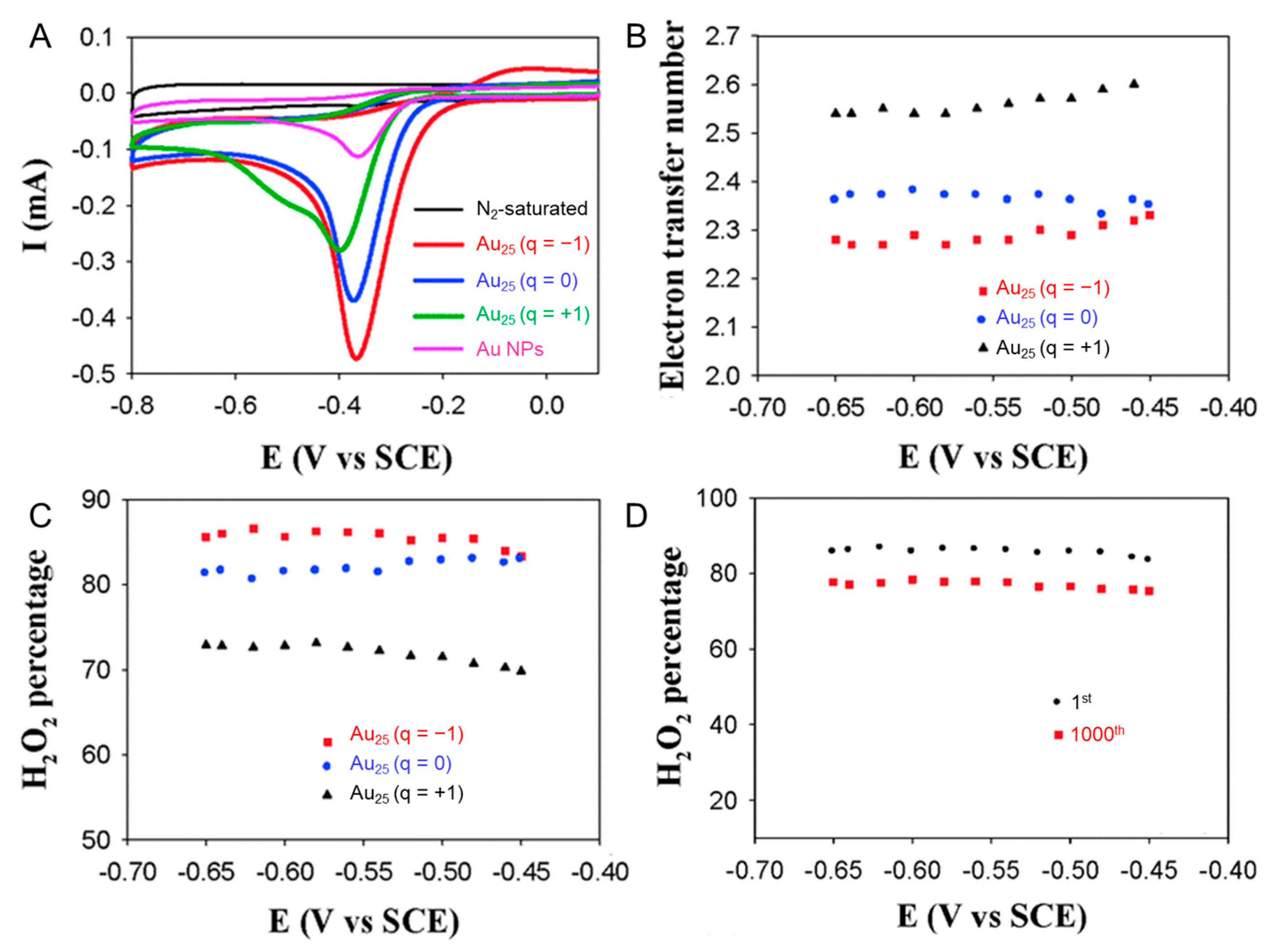
| Ligand | Support | Experimental Condition | Activity | Reference |
|---|---|---|---|---|
| SC6H13 | − | 1.0 M TFA and 0.1 M Bu4NPF6 in THF c | Au24Pt(SC6H13)18 > Au25(SC6H13)18 | [102] |
| SC6H13 | carbon black | 1 M Britton–Robinson buffer solution in 2 M KCl aq (pH 3) c,d | Au24Pt(SC6H13)18 > Au24Pd(SC6H13)18 > Au25(SC6H13)18 | [103] |
| SC6H13 | carbon black | 1 M Britton–Robinson buffer solution in 2 M KCl aq (pH 3) c,d | Au36Pt2(SC6H13)24 > Au36Pd2(SC6H13)24 > Au38(SC6H13)24 | [103] |
| PPh3 PPh2 a Cl b PhF2S | MoS2 | 0.5 M phosphate buffer solution (pH 6.7) c,d | Au2Pd6(S4(PPh3)4(PhF2S)6)/MoS2 > Mixture of Au2Cl2C(PPh2)2 and Pd3(Cl(PPh2)2(PPh3)3)/MoS2 > Pd3(Cl(PPh2)2(PPh3)3)/MoS2 > Au2Cl2C(PPh2)2/MoS2 > MoS2 | [104] |
| porphyrin SC1P porphyrin SC2P PET | − | 0.5 M H2SO4 aq e | Au(1.3 nm)(porphyrin SC1P) > Au(1.3 nm)(porphyrin SC2P) > Au(1.3 nm)(PET) | [107] |
| PET SePh | MoS2 | 0.5 M H2SO4 aq c,d | Au25(PET)18/MoS2 > Au25(SePh)18/MoS2 > MoS2 | [108] |
| SC6H13 MPA MPS | − | 0.1 M KCl aq c | Au24Pt(MPS)18 > Au25(MPS)18 > Au25(MPA)18 > Au25(SC6H13)18 | [109] |
| Ligand | Support | Experimental Condition | Activity | Reference |
|---|---|---|---|---|
| PET | CoSe2 | 0.l M KOH aq a, b | Au25(PET)18/CoSe2 > CoSe2 | [110] |
| Ligand | Support | Experimental Condition | Activity | Reference |
|---|---|---|---|---|
| PET SC6H13 Cl PPh3 | − | 0.1 M KOH aq a | Au11(PPh3)8Cl3 > Au25(PET)18 > Au55(PPh3)12Cl6 > Au140(SC6H13)53 | [101] |
| PET | Reduced graphene oxide | 0.1 M KOH aq a, b | Au25(PET)18 > Au38(PET)24 > Au144(PET)60 | [112] |
| TBBT | SWNTs | 0.1 M KOH aq a, b | Au36(TBBT)24 > Au133(TBBT)52 > Au279(TBBT)84 > Au28(TBBT)20 | [113] |
| S-tBu | SWNTs | 0.1 M KOH aq a, b | Au65(S-tBu)29 > Au46(S-tBu)24 > Au30(S-tBu)18 > Au23(S-tBu)16 | [114] |
| SC12H25 | − | 0.1 M KOH aq a, b | [Au25(SC12H25)18]− > [Au25(SC12H25)18]0 > [Au25(SC12H25)18]+ c | [115] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawawaki, T.; Negishi, Y. Gold Nanoclusters as Electrocatalysts for Energy Conversion. Nanomaterials 2020, 10, 238. https://doi.org/10.3390/nano10020238
Kawawaki T, Negishi Y. Gold Nanoclusters as Electrocatalysts for Energy Conversion. Nanomaterials. 2020; 10(2):238. https://doi.org/10.3390/nano10020238
Chicago/Turabian StyleKawawaki, Tokuhisa, and Yuichi Negishi. 2020. "Gold Nanoclusters as Electrocatalysts for Energy Conversion" Nanomaterials 10, no. 2: 238. https://doi.org/10.3390/nano10020238
APA StyleKawawaki, T., & Negishi, Y. (2020). Gold Nanoclusters as Electrocatalysts for Energy Conversion. Nanomaterials, 10(2), 238. https://doi.org/10.3390/nano10020238






