Recent Advances in TiO2-Based Photocatalysts for Reduction of CO2 to Fuels
Abstract
:1. Introduction
2. Fundamentals of CO2 Photocatalytic Reduction
3. Advances in TiO2-Based Photocatalysts for CO2 Reduction
3.1. Nanostructured TiO2 Design
3.1.1. Crystal Phases
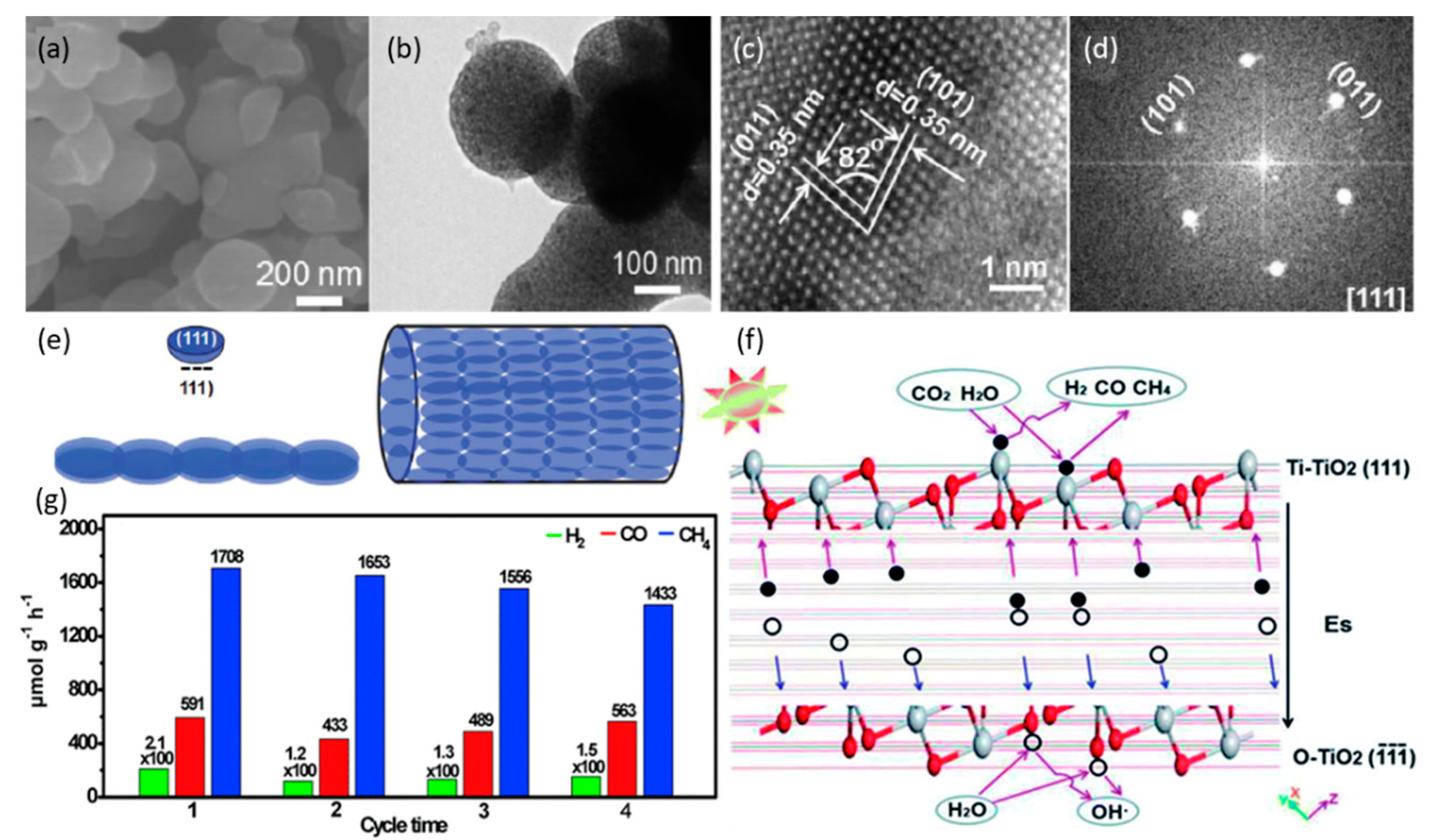
3.1.2. Effect of Facets
3.1.3. Well-Dispersed TiO2 on Porous Materials
3.2. Modification on TiO2 Surface
3.2.1. Oxygen Vacancies
3.2.2. Surfaces of Basic Functional Sites
3.3. Cocatalysts
3.3.1. Metal and Metal-Oxide Cocatalysts
3.3.2. Plasmonic Effect
3.4. Hybrid TiO2 Nanocomposites
3.4.1. Carbon-Containing Composites
3.4.2. Other Composites
4. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Meinshausen, M.; Meinshausen, N.; Hare, W.; Raper, S.C.B.; Frieler, K.; Knutti, R.; Frame, D.J.; Allen, M.R. Greenhouse-gas emission targets for limiting global warming to 2 °C. Nature 2009, 458, 1158–1162. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for Selective Photoreduction of CO2 into Solar Fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.; Plattner, G.-K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goeppert, A.; Czaun, M.; Jones, J.-P.; Surya Prakash, G.K.; Olah, G.A. Recycling of carbon dioxide to methanol and derived products—closing the loop. Chem. Soc. Rev. 2014, 43, 7995–8048. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Zhou, Y.; Zou, Z. Photocatalytic Conversion of CO2 into Renewable Hydrocarbon Fuels: State-of-the-Art Accomplishment, Challenges, and Prospects. Adv. Mater. 2014, 26, 4607–4626. [Google Scholar] [CrossRef]
- Marques, M.F.; Kim, D.H. From CO2 methanation to ambitious long-chain hydrocarbons: Alternative fuels paving the path to sustainability. Chem. Soc. Rev. 2019, 48, 205–259. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Wang, D. Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef] [Green Version]
- Kibria, M.G.; Edwards, J.P.; Gabardo, C.M.; Dinh, C.-T.; Seifitokaldani, A.; Sinton, D.; Sargent, E.H. Electrochemical CO2 Reduction into Chemical Feedstocks: From Mechanistic Electrocatalysis Models to System Design. Adv. Mater. 2019, 31, 1807166. [Google Scholar] [CrossRef]
- Marques, M.F.; Nguyen, D.L.T.; Lee, J.-E.; Piao, H.; Choy, J.-H.; Hwang, Y.J.; Kim, D.H. Toward an Effective Control of the H2 to CO Ratio of Syngas through CO2 Electroreduction over Immobilized Gold Nanoparticles on Layered Titanate Nanosheets. ACS Catal. 2018, 8, 4364–4374. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Kim, Y.; Hwang, Y.J.; Won, D.H. Progress in development of electrocatalyst for CO2 conversion to selective CO production. Carbon Energy 2019. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Jiang, K.; Wang, H. Recent Advances in Electrochemical CO2-to-CO Conversion on Heterogeneous Catalysts. Adv. Mater. 2018, 30, 1802066. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Jee, M.S.; Won, D.H.; Jung, H.; Oh, H.-S.; Min, B.K.; Hwang, Y.J. Selective CO2 Reduction on Zinc Electrocatalyst: The Effect of Zinc Oxidation State Induced by Pretreatment Environment. ACS Sustain. Chem. Eng. 2017, 5, 11377–11386. [Google Scholar] [CrossRef]
- Hasani, A.; Tekalgne, M.; Le, Q.V.; Jang, H.W.; Kim, S.Y. Two-dimensional materials as catalysts for solar fuels: Hydrogen evolution reaction and CO2 reduction. J. Mater. Chem. A 2019, 7, 430–454. [Google Scholar] [CrossRef]
- Chae, S.Y.; Choi, J.Y.; Kim, Y.; Nguyen, D.L.T.; Joo, O.-S. Photoelectrochemical CO2 Reduction with a Rhenium Organometallic Redox Mediator at Semiconductor/Aqueous Liquid Junction Interfaces. Angew. Chem. 2019, 131, 16547–16551. [Google Scholar] [CrossRef]
- Shaner, M.R.; Atwater, H.A.; Lewis, N.S.; McFarland, E.W. A comparative technoeconomic analysis of renewable hydrogen production using solar energy. Energy Environ. Sci. 2016, 9, 2354–2371. [Google Scholar] [CrossRef] [Green Version]
- Lewis, N.S. Research opportunities to advance solar energy utilization. Science 2016, 351, aad1920. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.-H.; Wu, J.C.S. Recent developments in the design of photoreactors for solar energy conversion from water splitting and CO2 reduction. Appl. Catal. A Gen. 2018, 550, 122–141. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.; Bard, A.J. Novel Carbon-Doped TiO2 Nanotube Arrays with High Aspect Ratios for Efficient Solar Water Splitting. Nano Lett. 2006, 6, 24–28. [Google Scholar] [CrossRef]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B. Efficient Photochemical Water Splitting by a Chemically Modified n-TiO2. Science 2002, 297, 2243. [Google Scholar] [CrossRef] [PubMed]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J. Photochem. Photobiol. C 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Epling, G.A.; Lin, C. Photoassisted bleaching of dyes utilizing TiO2 and visible light. Chemosphere 2002, 46, 561–570. [Google Scholar] [CrossRef]
- Bet-moushoul, E.; Mansourpanah, Y.; Farhadi, K.; Tabatabaei, M. TiO2 nanocomposite based polymeric membranes: A review on performance improvement for various applications in chemical engineering processes. Chem. Eng. J. 2016, 283, 29–46. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Hahn, C.; Liu, B.; Yang, P. Photoelectrochemical Properties of TiO2 Nanowire Arrays: A Study of the Dependence on Length and Atomic Layer Deposition Coating. ACS Nano 2012, 6, 5060–5069. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Boukai, A.; Yang, P. High Density n-Si/n-TiO2 Core/Shell Nanowire Arrays with Enhanced Photoactivity. Nano Lett. 2009, 9, 410–415. [Google Scholar] [CrossRef] [Green Version]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Navalon, S.; Corma, A.; Garcia, H. Photocatalytic CO2 reduction by TiO2 and related titanium containing solids. Energy Environ. Sci. 2012, 5, 9217–9233. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Lo, C.-C.; Hung, C.-H.; Yuan, C.-S.; Wu, J.-F. Photoreduction of carbon dioxide with H2 and H2O over TiO2 and ZrO2 in a circulated photocatalytic reactor. Sol. Energy Mater. Sol. Cells 2007, 91, 1765–1774. [Google Scholar] [CrossRef]
- Sarkar, A.; Gracia-Espino, E.; Wågberg, T.; Shchukarev, A.; Mohl, M.; Rautio, A.-R.; Pitkänen, O.; Sharifi, T.; Kordas, K.; Mikkola, J.-P. Photocatalytic reduction of CO2 with H2O over modified TiO2 nanofibers: Understanding the reduction pathway. Nano Res. 2016, 9, 1956–1968. [Google Scholar] [CrossRef]
- Marszewski, M.; Cao, S.; Yu, J.; Jaroniec, M. Semiconductor-based photocatalytic CO2 conversion. Mater. Horiz. 2015, 2, 261–278. [Google Scholar] [CrossRef]
- Wang, W.-N.; An, W.-J.; Ramalingam, B.; Mukherjee, S.; Niedzwiedzki, D.M.; Gangopadhyay, S.; Biswas, P. Size and Structure Matter: Enhanced CO2 Photoreduction Efficiency by Size-Resolved Ultrafine Pt Nanoparticles on TiO2 Single Crystals. J. Am. Chem. Soc. 2012, 134, 11276–11281. [Google Scholar] [CrossRef]
- Xu, H.; Ouyang, S.; Liu, L.; Wang, D.; Kako, T.; Ye, J. Porous-structured Cu2O/TiO2 nanojunction material toward efficient CO2 photoreduction. Nanotechnology 2014, 25, 165402. [Google Scholar] [CrossRef]
- An, X.; Li, K.; Tang, J. Cu2O/Reduced Graphene Oxide Composites for the Photocatalytic Conversion of CO2. ChemSusChem 2014, 7, 1086–1093. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.-C.; Shown, I.; Wei, H.-Y.; Chang, Y.-C.; Du, H.-Y.; Lin, Y.-G.; Tseng, C.-A.; Wang, C.-H.; Chen, L.-C.; Lin, Y.-C.; et al. Graphene oxide as a promising photocatalyst for CO2 to methanol conversion. Nanoscale 2013, 5, 262–268. [Google Scholar] [CrossRef]
- Yu, J.; Jin, J.; Cheng, B.; Jaroniec, M. A noble metal-free reduced graphene oxide—CdS nanorod composite for the enhanced visible-light photocatalytic reduction of CO2 to solar fuel. J. Mater. Chem. A 2014, 2, 3407–3416. [Google Scholar] [CrossRef]
- In, S.-I.; Vaughn Ii, D.D.; Schaak, R.E. Hybrid CuO-TiO2−xNx Hollow Nanocubes for Photocatalytic Conversion of CO2 into Methane under Solar Irradiation. Angew. Chem. Int. Ed. 2012, 51, 3915–3918. [Google Scholar] [CrossRef]
- He, H.; Zapol, P.; Curtiss, L.A. Computational screening of dopants for photocatalytic two-electron reduction of CO2 on anatase (101) surfaces. Energy Environ. Sci. 2012, 5, 6196–6205. [Google Scholar] [CrossRef]
- Xiong, Z.; Lei, Z.; Li, Y.; Dong, L.; Zhao, Y.; Zhang, J. A review on modification of facet-engineered TiO2 for photocatalytic CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2018, 36, 24–47. [Google Scholar] [CrossRef]
- Wang, Y.; He, D.; Chen, H.; Wang, D. Catalysts in electro-, photo- and photoelectrocatalytic CO2 reduction reactions. J. Photochem. Photobiol. C Photochem. Rev. 2019, 40, 117–149. [Google Scholar] [CrossRef]
- Kawahara, T.; Konishi, Y.; Tada, H.; Tohge, N.; Nishii, J.; Ito, S. A Patterned TiO2(Anatase)/TiO2(Rutile) Bilayer-Type Photocatalyst: Effect of the Anatase/Rutile Junction on the Photocatalytic Activity. Angew. Chem. 2002, 114, 2935–2937. [Google Scholar] [CrossRef]
- Ohno, T.; Tokieda, K.; Higashida, S.; Matsumura, M. Synergism between rutile and anatase TiO2 particles in photocatalytic oxidation of naphthalene. Appl. Catal. A Gen. 2003, 244, 383–391. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Dillert, R.; Feldhoff, A.; Bahnemann, D.W. Direct Synthesis of Photocatalytically Active Rutile TiO2 Nanorods Partly Decorated with Anatase Nanoparticles. J. Phys. Chem. C 2010, 114, 4909–4915. [Google Scholar] [CrossRef]
- Tan, L.-L.; Ong, W.-J.; Chai, S.-P.; Mohamed, A.R. Visible-light-activated oxygen-rich TiO2 as next generation photocatalyst: Importance of annealing temperature on the photoactivity toward reduction of carbon dioxide. Chem. Eng. J. 2016, 283, 1254–1263. [Google Scholar] [CrossRef]
- Reñones, P.; Moya, A.; Fresno, F.; Collado, L.; Vilatela, J.J.; de la Peña O’Shea, V.A. Hierarchical TiO2 nanofibres as photocatalyst for CO2 reduction: Influence of morphology and phase composition on catalytic activity. J. CO2 Util. 2016, 15, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yu, J.; Jaroniec, M. Tunable Photocatalytic Selectivity of Hollow TiO2 Microspheres Composed of Anatase Polyhedra with Exposed {001} Facets. J. Am. Chem. Soc. 2010, 132, 11914–11916. [Google Scholar] [CrossRef]
- He, H.; Zapol, P.; Curtiss, L.A. A Theoretical Study of CO2 Anions on Anatase (101) Surface. J. Phys. Chem. C 2010, 114, 21474–21481. [Google Scholar] [CrossRef]
- Han, X.; Kuang, Q.; Jin, M.; Xie, Z.; Zheng, L. Synthesis of Titania Nanosheets with a High Percentage of Exposed (001) Facets and Related Photocatalytic Properties. J. Am. Chem. Soc. 2009, 131, 3152–3153. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced Photocatalytic CO2-Reduction Activity of Anatase TiO2 by Coexposed {001} and {101} Facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yu, J.; Zhang, J.; Zhang, J.; Liu, G. Cubic anatase TiO2 nanocrystals with enhanced photocatalytic CO2 reduction activity. Chem. Commun. 2015, 51, 7950–7953. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wen, L.; Wang, D.; Xue, Y.; Lu, Q.; Wu, C.; Chen, J.; Song, S. Photocatalytic Reduction of CO2 in Aqueous Solution on Surface-Fluorinated Anatase TiO2 Nanosheets with Exposed {001} Facets. Energy Fuels 2014, 28, 3982–3993. [Google Scholar] [CrossRef]
- Jiao, W.; Wang, L.; Liu, G.; Lu, G.Q.; Cheng, H.-M. Hollow Anatase TiO2 Single Crystals and Mesocrystals with Dominant {101} Facets for Improved Photocatalysis Activity and Tuned Reaction Preference. ACS Catal. 2012, 2, 1854–1859. [Google Scholar] [CrossRef]
- Kar, P.; Zeng, S.; Zhang, Y.; Vahidzadeh, E.; Manuel, A.; Kisslinger, R.; Alam, K.M.; Thakur, U.K.; Mahdi, N.; Kumar, P.; et al. High rate CO2 photoreduction using flame annealed TiO2 nanotubes. Appl. Catal. B 2019, 243, 522–536. [Google Scholar] [CrossRef]
- Liu, J.; Liu, B.; Ren, Y.; Yuan, Y.; Zhao, H.; Yang, H.; Liu, S. Hydrogenated nanotubes/nanowires assembled from TiO2 nanoflakes with exposed {111} facets: Excellent photo-catalytic CO2 reduction activity and charge separation mechanism between (111) and polar surfaces. J. Mater. Chem. A 2019, 7, 14761–14775. [Google Scholar] [CrossRef]
- Anpo, M.; Yamashita, H.; Ichihashi, Y.; Fujii, Y.; Honda, M. Photocatalytic Reduction of CO2 with H2O on Titanium Oxides Anchored within Micropores of Zeolites: Effects of the Structure of the Active Sites and the Addition of Pt. J. Phys. Chem. B 1997, 101, 2632–2636. [Google Scholar] [CrossRef]
- Anpo, M.; Yamashita, H.; Ikeue, K.; Fujii, Y.; Zhang, S.G.; Ichihashi, Y.; Park, D.R.; Suzuki, Y.; Koyano, K.; Tatsumi, T. Photocatalytic reduction of CO2 with H2O on Ti-MCM-41 and Ti-MCM-48 mesoporous zeolite catalysts. Catal. Today 1998, 44, 327–332. [Google Scholar] [CrossRef]
- Anpo, M. Photocatalytic reduction of CO2 with H2O on highly dispersed Ti-oxide catalysts as a model of artificial photosynthesis. J. CO2 Util. 2013, 1, 8–17. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Chang, J.-S.; Park, S.-E.; Ikeue, K.; Anpo, M. Photoreduction of Carbondioxide on Surface Functionalized Nanoporous Catalysts. Top. Catal. 2005, 35, 311–319. [Google Scholar] [CrossRef]
- Xie, K.; Umezawa, N.; Zhang, N.; Reunchan, P.; Zhang, Y.; Ye, J. Self-doped SrTiO3−δ photocatalyst with enhanced activity for artificial photosynthesis under visible light. Energy Environ. Sci. 2011, 4, 4211–4219. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, H.; Andino, J.M.; Li, Y. Photocatalytic CO2 Reduction with H2O on TiO2 Nanocrystals: Comparison of Anatase, Rutile, and Brookite Polymorphs and Exploration of Surface Chemistry. ACS Catal. 2012, 2, 1817–1828. [Google Scholar] [CrossRef]
- Lee, J.; Sorescu, D.C.; Deng, X. Electron-Induced Dissociation of CO2 on TiO2(110). J. Am. Chem. Soc. 2011, 133, 10066–10069. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-Y.; Gong, X.-Q.; Alexandrova, A.N. Mechanism of CO2 Photocatalytic Reduction to Methane and Methanol on Defected Anatase TiO2 (101): A Density Functional Theory Study. J. Phys. Chem. C 2019, 123, 3505–3511. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Y.; Zhao, H.; Chen, J.; Cheng, J.; Yang, K.; Li, Y. Engineering Coexposed {001} and {101} Facets in Oxygen-Deficient TiO2 Nanocrystals for Enhanced CO2 Photoreduction under Visible Light. ACS Catal. 2016, 6, 1097–1108. [Google Scholar] [CrossRef]
- Fang, W.; Khrouz, L.; Zhou, Y.; Shen, B.; Dong, C.; Xing, M.; Mishra, S.; Daniele, S.; Zhang, J. Reduced {001}-TiO2−x photocatalysts: Noble-metal-free CO2 photoreduction for selective CH4 evolution. Phys. Chem. Chem. Phys. 2017, 19, 13875–13881. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhao, C.; Li, Y. Spontaneous Dissociation of CO2 to CO on Defective Surface of Cu(I)/TiO2–x Nanoparticles at Room Temperature. J. Phys. Chem. C 2012, 116, 7904–7912. [Google Scholar] [CrossRef]
- Liu, L.; Gao, F.; Zhao, H.; Li, Y. Tailoring Cu valence and oxygen vacancy in Cu/TiO2 catalysts for enhanced CO2 photoreduction efficiency. Appl. Catal. B 2013, 134–135, 349–358. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Zhu, Y.; Wang, Y.; Wang, C. Enhanced CO2 photoreduction activity of black TiO2−coated Cu nanoparticles under visible light irradiation: Role of metallic Cu. Appl. Catal. A Gen. 2016, 510, 34–41. [Google Scholar] [CrossRef]
- Pham, T.-D.; Lee, B.-K. Novel capture and photocatalytic conversion of CO2 into solar fuels by metals co-doped TiO2 deposited on PU under visible light. Appl. Catal. A Gen. 2017, 529, 40–48. [Google Scholar] [CrossRef]
- Pham, T.-D.; Lee, B.-K. Novel photocatalytic activity of Cu@V co-doped TiO2/PU for CO2 reduction with H2O vapor to produce solar fuels under visible light. J. Catal. 2017, 345, 87–95. [Google Scholar] [CrossRef]
- Meng, X.; Ouyang, S.; Kako, T.; Li, P.; Yu, Q.; Wang, T.; Ye, J. Photocatalytic CO2 conversion over alkali modified TiO2 without loading noble metal cocatalyst. Chem. Commun. 2014, 50, 11517–11519. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wang, Y.; Zhang, Q.; Fan, W.; Deng, W.; Wang, Y. Photocatalytic reduction of CO2 with H2O: Significant enhancement of the activity of Pt–TiO2 in CH4 formation by addition of MgO. Chem. Commun. 2013, 49, 2451–2453. [Google Scholar] [CrossRef]
- Xie, S.; Wang, Y.; Zhang, Q.; Deng, W.; Wang, Y. MgO- and Pt-Promoted TiO2 as an Efficient Photocatalyst for the Preferential Reduction of Carbon Dioxide in the Presence of Water. ACS Catal. 2014, 4, 3644–3653. [Google Scholar] [CrossRef]
- Feng, X.; Pan, F.; Zhao, H.; Deng, W.; Zhang, P.; Zhou, H.-C.; Li, Y. Atomic layer deposition enabled MgO surface coating on porous TiO2 for improved CO2 photoreduction. Appl. Catal. B 2018, 238, 274–283. [Google Scholar] [CrossRef]
- Liao, Y.; Cao, S.-W.; Yuan, Y.; Gu, Q.; Zhang, Z.; Xue, C. Efficient CO2 Capture and Photoreduction by Amine-Functionalized TiO2. Chem. Eur. J. 2014, 20, 10220–10222. [Google Scholar] [CrossRef]
- Liu, S.; Xia, J.; Yu, J. Amine-Functionalized Titanate Nanosheet-Assembled Yolk@Shell Microspheres for Efficient Cocatalyst-Free Visible-Light Photocatalytic CO2 Reduction. ACS Appl. Mater. Interfaces 2015, 7, 8166–8175. [Google Scholar] [CrossRef]
- Tanaka, K.; White, J.M. Dissociative adsorption of carbon dioxide on oxidized and reduced platinum/titanium dioxide. J. Phys. Chem. 1982, 86, 3977–3980. [Google Scholar] [CrossRef]
- Raskó, J. FTIR study of the photoinduced dissociation of CO2 on titania-supported noble metals. Catal. Lett. 1998, 56, 11–15. [Google Scholar] [CrossRef]
- Lan, Y.; Xie, Y.; Chen, J.; Hu, Z.; Cui, D. Selective photocatalytic CO2 reduction on copper–titanium dioxide: A study of the relationship between CO production and H2 suppression. Chem. Commun. 2019, 55, 8068–8071. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, M.; Yang, B.; Shu, W.; Shen, Q.; Liu, M.; Zhou, J. Enhanced Photocatalytic Performance toward CO2 Hydrogenation over Nanosized TiO2-Loaded Pd under UV Irradiation. J. Phys. Chem. C 2017, 121, 2923–2932. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, C.; Yang, P.; He, Y.; Feng, J.; Li, D. Synergetic promotional effect of oxygen vacancy-rich ultrathin TiO2 and photochemical induced highly dispersed Pt for photoreduction of CO2 with H2O. Appl. Catal. B 2019, 244, 919–930. [Google Scholar] [CrossRef]
- Gonell, F.; Puga, A.V.; Julián-López, B.; García, H.; Corma, A. Copper-doped titania photocatalysts for simultaneous reduction of CO2 and production of H2 from aqueous sulfide. Appl. Catal. B 2016, 180, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Fang, B.; Xing, Y.; Bonakdarpour, A.; Zhang, S.; Wilkinson, D.P. Hierarchical CuO–TiO2 Hollow Microspheres for Highly Efficient Photodriven Reduction of CO2 to CH4. ACS Sustain. Chem. Eng. 2015, 3, 2381–2388. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, W.; Miao, W.; Yuan, Z.; Yang, G.; Kong, F.; Yan, T.; Chen, J.; Huang, B.; An, C.; et al. Living Atomically Dispersed Cu Ultrathin TiO2 Nanosheet CO2 Reduction Photocatalyst. Adv. Sci. 2019, 6, 1900289. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Q.; Xie, S.; Fan, W.; Zhang, Q.; Wang, Y.; Deng, W.; Wang, Y. Photocatalytic Conversion of Carbon Dioxide with Water into Methane: Platinum and Copper(I) Oxide Co-catalysts with a Core–Shell Structure. Angew. Chem. Int. Ed. 2013, 52, 5776–5779. [Google Scholar] [CrossRef]
- Tan, D.; Zhang, J.; Shi, J.; Li, S.; Zhang, B.; Tan, X.; Zhang, F.; Liu, L.; Shao, D.; Han, B. Photocatalytic CO2 Transformation to CH4 by Ag/Pd Bimetals Supported on N-Doped TiO2 Nanosheet. ACS Appl. Mater. Interfaces 2018, 10, 24516–24522. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. TiO2–MnOx–Pt Hybrid Multiheterojunction Film Photocatalyst with Enhanced Photocatalytic CO2-Reduction Activity. ACS Appl. Mater. Interfaces 2019, 11, 5581–5589. [Google Scholar] [CrossRef]
- Kang, Q.; Wang, T.; Li, P.; Liu, L.; Chang, K.; Li, M.; Ye, J. Photocatalytic Reduction of Carbon Dioxide by Hydrous Hydrazine over Au–Cu Alloy Nanoparticles Supported on SrTiO3/TiO2 Coaxial Nanotube Arrays. Angew. Chem. Int. Ed. 2015, 54, 841–845. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, S.W.; Moon, S.Y.; Park, J.Y. The effect of hot electrons and surface plasmons on heterogeneous catalysis. J. Phys. Condens. Matter 2016, 28, 254002. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Chung, K.; Nguyen, T.-T.H.; Kim, D.H. Plasmon-Mediated Electrocatalysis for Sustainable Energy: From Electrochemical Conversion of Different Feedstocks to Fuel Cell Reactions. ACS Energy Lett. 2018, 3, 1415–1433. [Google Scholar] [CrossRef]
- Jang, Y.H.; Jang, Y.J.; Kim, S.; Quan, L.N.; Chung, K.; Kim, D.H. Plasmonic Solar Cells: From Rational Design to Mechanism Overview. Chem. Rev. 2016, 116, 14982–15034. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Qiu, S.; Xu, D.; Jiang, C.; Cheng, B. Direct evidence and enhancement of surface plasmon resonance effect on Ag-loaded TiO2 nanotube arrays for photocatalytic CO2 reduction. Appl. Surf. Sci. 2018, 434, 423–432. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Cao, S.-W.; Xue, C. Au/Pt Nanoparticle-Decorated TiO2 Nanofibers with Plasmon-Enhanced Photocatalytic Activities for Solar-to-Fuel Conversion. J. Phys. Chem. C 2013, 117, 25939–25947. [Google Scholar] [CrossRef]
- Tahir, M. Synergistic effect in MMT-dispersed Au/TiO2 monolithic nanocatalyst for plasmon-absorption and metallic interband transitions dynamic CO2 photo-reduction to CO. Appl. Catal. B 2017, 219, 329–343. [Google Scholar] [CrossRef]
- Neaţu, Ş.; Maciá-Agulló, J.A.; Concepción, P.; Garcia, H. Gold–Copper Nanoalloys Supported on TiO2 as Photocatalysts for CO2 Reduction by Water. J. Am. Chem. Soc. 2014, 136, 15969–15976. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, X.; Yuan, Z.; Chen, J.; Huang, B.; Dionysiou, D.D.; Yang, G. Enhanced photocatalytic CO2 reduction via the synergistic effect between Ag and activated carbon in TiO2/AC-Ag ternary composite. Chem. Eng. J. 2018, 348, 592–598. [Google Scholar] [CrossRef]
- Liang, Y.T.; Vijayan, B.K.; Gray, K.A.; Hersam, M.C. Minimizing Graphene Defects Enhances Titania Nanocomposite-Based Photocatalytic Reduction of CO2 for Improved Solar Fuel Production. Nano Lett. 2011, 11, 2865–2870. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Liu, Q.; Tian, Z.; Gao, J.; Chen, X.; Zhang, H.; Liu, J.; Zou, Z. Robust Hollow Spheres Consisting of Alternating Titania Nanosheets and Graphene Nanosheets with High Photocatalytic Activity for CO2 Conversion into Renewable Fuels. Adv. Funct. Mater. 2012, 22, 1215–1221. [Google Scholar] [CrossRef]
- Wang, W.; Xu, D.; Cheng, B.; Yu, J.; Jiang, C. Hybrid carbon@TiO2 hollow spheres with enhanced photocatalytic CO2 reduction activity. J. Mater. Chem. A 2017, 5, 5020–5029. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Liu, Q.; Yan, S.; Bao, S.; Wang, X.; Xiao, M.; Zou, Z. An In Situ Simultaneous Reduction-Hydrolysis Technique for Fabrication of TiO2-Graphene 2D Sandwich-Like Hybrid Nanosheets: Graphene-Promoted Selectivity of Photocatalytic-Driven Hydrogenation and Coupling of CO2 into Methane and Ethane. Adv. Funct. Mater. 2013, 23, 1743–1749. [Google Scholar] [CrossRef]
- Jin, B.; Yao, G.; Jin, F.; Hu, Y.H. Photocatalytic conversion of CO2 over C3N4-based catalysts. Catal. Today 2018, 316, 149–154. [Google Scholar] [CrossRef]
- Wada, K.; Ranasinghe, C.S.K.; Kuriki, R.; Yamakata, A.; Ishitani, O.; Maeda, K. Interfacial Manipulation by Rutile TiO2 Nanoparticles to Boost CO2 Reduction into CO on a Metal-Complex/Semiconductor Hybrid Photocatalyst. ACS Appl. Mater. Interfaces 2017, 9, 23869–23877. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wei, Y.; Wu, X.; Zheng, H.; Zhao, Z.; Liu, J.; Li, J. Graphene-wrapped Pt/TiO2 photocatalysts with enhanced photogenerated charges separation and reactant adsorption for high selective photoreduction of CO2 to CH4. Appl. Catal. B 2018, 226, 360–372. [Google Scholar] [CrossRef]
- Crake, A.; Christoforidis, K.C.; Godin, R.; Moss, B.; Kafizas, A.; Zafeiratos, S.; Durrant, J.R.; Petit, C. Titanium dioxide/carbon nitride nanosheet nanocomposites for gas phase CO2 photoreduction under UV-visible irradiation. Appl. Catal. B 2019, 242, 369–378. [Google Scholar] [CrossRef]
- Brunetti, A.; Pomilla, F.R.; Marcì, G.; Garcia-Lopez, E.I.; Fontananova, E.; Palmisano, L.; Barbieri, G. CO2 reduction by C3N4-TiO2 Nafion photocatalytic membrane reactor as a promising environmental pathway to solar fuels. Appl. Catal. B 2019, 255, 117779. [Google Scholar] [CrossRef]
- Wu, J.; Feng, Y.; Li, D.; Han, X.; Liu, J. Efficient photocatalytic CO2 reduction by P–O linked g-C3N4/TiO2-nanotubes Z-scheme composites. Energy 2019, 178, 168–175. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, J.; Zhu, B.; Yu, J.; Xu, J. CuInS2 sensitized TiO2 hybrid nanofibers for improved photocatalytic CO2 reduction. Appl. Catal. B 2018, 230, 194–202. [Google Scholar] [CrossRef]
- Low, J.; Zhang, L.; Tong, T.; Shen, B.; Yu, J. TiO2/MXene Ti3C2 composite with excellent photocatalytic CO2 reduction activity. J. Catal. 2018, 361, 255–266. [Google Scholar] [CrossRef]











| Product | Reaction | n | Eo (V vs. NHE) |
|---|---|---|---|
| Carbon monoxide | CO2 + 2H+ + 2e−→CO + H2O | 2 | −0.51 |
| Formic acid | CO2 + 2H+ + 2e−→HCOOH | 2 | −0.58 |
| Oxalate | 2CO2 + 2H+ + 2e−→H2C2O4 | 2 | −0.87 |
| Methanol | CO2 + 6H+ + 6e−→CH3OH + H2O | 6 | −0.39 |
| Methane | CO2 + 8H+ + 8e−→CH4 + 2H2O | 8 | −0.24 |
| Ethanol | 2CO2 + 12H+ + 12e−→C2H5OH + 3H2O | 12 | −0.33 |
| Ethane | 2CO2 + 14H+ + 14e−→C2H6 + 4H2O | 14 | −0.27 |
| Hydrogen | H2O + 2e−→2OH− + H2 | 2 | −0.41 |
| Photocatalysts | Reaction Conditions | Production Rate (μmol g−1 h−1) | Reference (Year) |
|---|---|---|---|
| Anatase TiO2 by coexposed {001} and {101} facets | 300 W Xe lamp TiO2-NaHCO3-HCl (solid-liquid) | CH4: 1.35 | [52] (2014) |
| Cubic anatase TiO2 nanocrystals (100 ± 13 nm) | 300 W Xe lamp TiO2-H2O-CO2 (solid-liquid) | CH4: 4.56 CH3OH: 1.48 | [53] (2015) |
| TiO2 nanosheets with exposed {001} facet | 2 × 18 W Hg lamps TiO2-H2O-CO2-NaOH (solid-liquid) | CH4: 0.2 CO: 0.12 CH3OH: 0.19 HCHO: 0.066 | [54] (2014) |
| Flame-annealed TiO2 nanotubes formed in aqueous electrolyte | AM 1.5G, 100 mW cm−2 Catalysts-H2O-CO2 (solid-gas) | CH4: 156.5 | [56] (2019) |
| Nanotubes/nanowires assembled from TiO2 nanoflakes with {111} facet | 300-W Xe lamp TiO2-H2O-CO2-(CH3)2CHOH (solid-liquid) | CH4: 1708.1 CO: 463.2 | [57] (2019) |
| Highly dispersed titanium oxide on mesoporous SBA-15 (Ti-SBA-15) | 100-W Hg lamp (>250 nm) TiO2-H2O-CO2 (solid-gas) | CH4: 106 CH3OH: 27.7 | [61] (2005) |
| Oxygen-deficient blue TiO2 nanocrystals with coexposed {101} and {001} facets | 100-W Hg lamp 450-W Xe lamp TiO2-H2O-CO2 (solid-gas) | CO: 55 (UV-VIS) CO: 27 (Visible light) | [66] (2016) |
| Cu and V co-doped TiO2 (P25) deposited on polyurethane Cu@V/TiO2-PU | Visible light (2 × 20 W white bulbs) Catalysts-H2O-CO2 (solid-gas) | CH4: 933 CO: 588 | [72] (2017) |
| 3% NaOH-surface modification TiO2 (ST01) | 300-W Xe lamp Catalysts-H2O-CO2 (solid-gas) | CH4: 8.7 | [73] (2014) |
| 5 ultrathin MgO layers deposited on porous TiO2 mixed anatase-rutile phases by atomic layer deposition | 450-W Xe lamp Catalysts-H2O-CO2 (solid-gas) | CO: 54 | [76] (2019) |
| TiO2-0.5% Ag | 100-W Xe lamp (320–780 nm) Catalysts-H2O-CO2 (solid-gas) | CH4: 2.1 | [75] (2014) |
| TiO2-0.5% Au | 100-W Xe lamp (320–780 nm) Catalysts-H2O-CO2 (solid-gas) | CH4: 3.1 | [75] (2014) |
| TiO2-0.5% Rh | 100-W Xe lamp (320–780 nm) Catalysts-H2O-CO2 (solid-gas) | CH4: 3.5 | [75] (2014) |
| TiO2-0.5% Pd | 100-W Xe lamp (320–780 nm) Catalysts-H2O-CO2 (solid-gas) | CH4: 4.3 | [75] (2014) |
| TiO2-0.5% Pt | 100-W Xe lamp (320–780 nm) Catalysts-H2O-CO2 (solid-gas) | CH4: 4.3 | [75] (2014) |
| 1-D nanostructured TiO2 single crystals loaded with Pt nanoparticles | 400-W Xe lamp Catalysts-H2O-CO2 (solid-gas) | CH4: 1361 CO: 200 | [35] (2012) |
| Ag-Pd on N-doped TiO2 NSs | 300-W Xe lamp Simulated sunlight Catalysts-H2O-CO2 (solid-liquid) | CH4: 79 | [88] (2018) |
| Montmorillonite (MMT) dispersed Au/TiO2 nanocatalyst | Simulated sunlight Catalysts-H2O-CO2 (solid-gas) | CO: 1223 | [95] (2017) |
| TiO2 powder (Degussa P25) loaded with Au–Cu alloy nanoparticles | 1000-W Xe lamp Catalysts-H2O-CO2 (solid-gas) | CH4: 2200 | [96] (2014) |
| Au–Cu bimetal as cocatalyst supported on SrTiO3/TiO2 | 300-W Xe lamp Catalysts-H2O-CO2 (solid-gas) | CO: 3770 CH4: 421.2 C2H6: 190.1 C2H4: 73.3 C3H6: 40.8 | [97] (2015) |
| TiO2-graphene 2D sandwich-like hybrid nanosheets | 500-W Xe lamp Catalysts-H2O-CO2 (solid-gas) | C2H6: 16.8 CH4: 8 | [102] (2013) |
| 2.5% CuInS2/TiO2 | 350-W Xe lamp Catalysts-H2O-CO2 (solid-gas) | CH4: 2.5 CH3OH: 0.86 | [109] (2018) |
| TiO2/carbon nitride nanosheet nanocomposites | 300-W Xe lamp (>325 nm) Catalysts-H2O-CO2 (solid-gas) | CO: 1.96 | [106] (2019) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.P.; Nguyen, D.L.T.; Nguyen, V.-H.; Le, T.-H.; Vo, D.-V.N.; Trinh, Q.T.; Bae, S.-R.; Chae, S.Y.; Kim, S.Y.; Le, Q.V. Recent Advances in TiO2-Based Photocatalysts for Reduction of CO2 to Fuels. Nanomaterials 2020, 10, 337. https://doi.org/10.3390/nano10020337
Nguyen TP, Nguyen DLT, Nguyen V-H, Le T-H, Vo D-VN, Trinh QT, Bae S-R, Chae SY, Kim SY, Le QV. Recent Advances in TiO2-Based Photocatalysts for Reduction of CO2 to Fuels. Nanomaterials. 2020; 10(2):337. https://doi.org/10.3390/nano10020337
Chicago/Turabian StyleNguyen, Thang Phan, Dang Le Tri Nguyen, Van-Huy Nguyen, Thu-Ha Le, Dai-Viet N. Vo, Quang Thang Trinh, Sa-Rang Bae, Sang Youn Chae, Soo Young Kim, and Quyet Van Le. 2020. "Recent Advances in TiO2-Based Photocatalysts for Reduction of CO2 to Fuels" Nanomaterials 10, no. 2: 337. https://doi.org/10.3390/nano10020337







