High-Performance Ultraviolet Photodetector Based on a Zinc Oxide Nanoparticle@Single-Walled Carbon Nanotube Heterojunction Hybrid Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZnO NPs
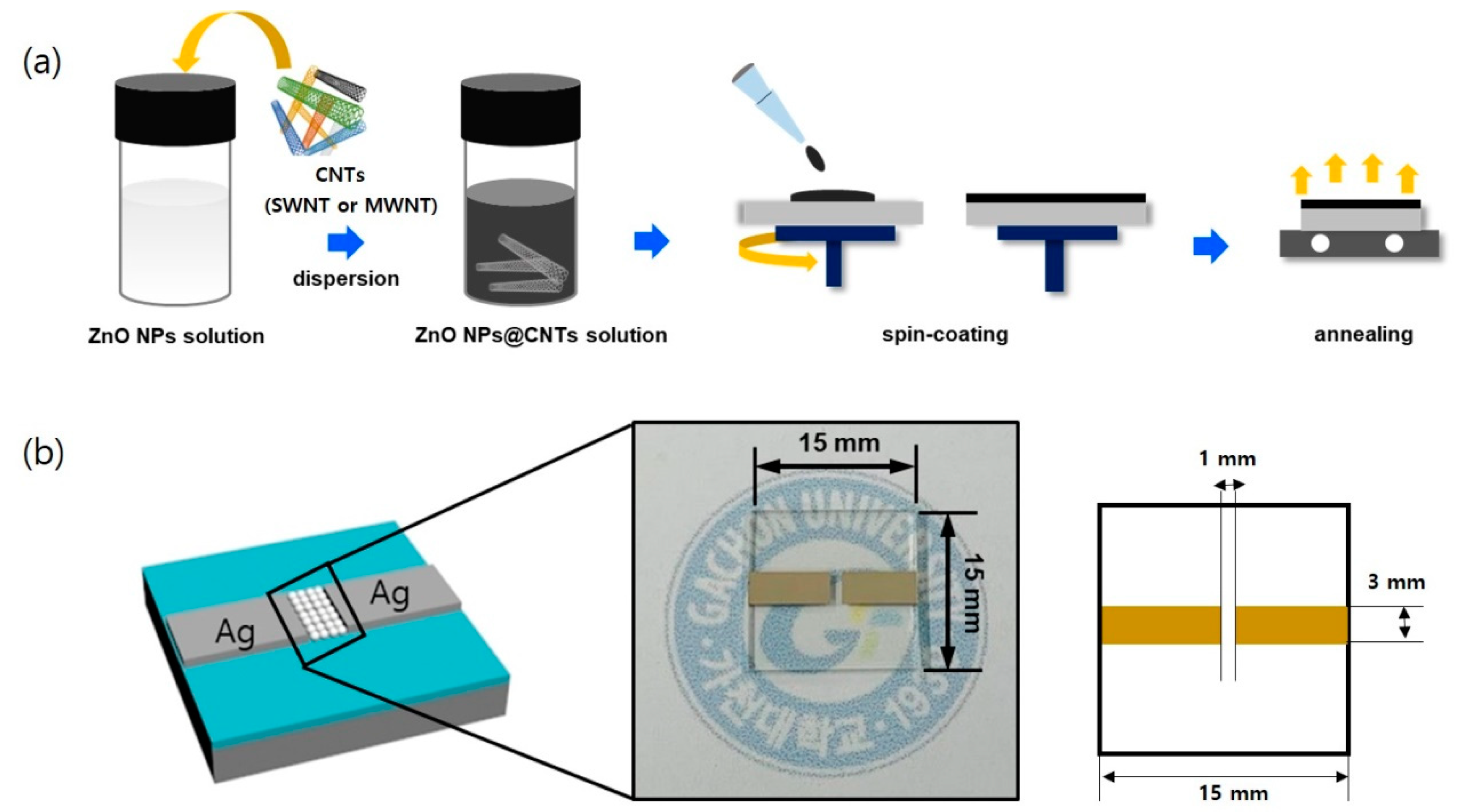
2.3. Preparation of a UV Sensor Based on ZnO NPs/CNT Hybrid Films
2.4. Characterization and Device Measurement
3. Results and Discussion
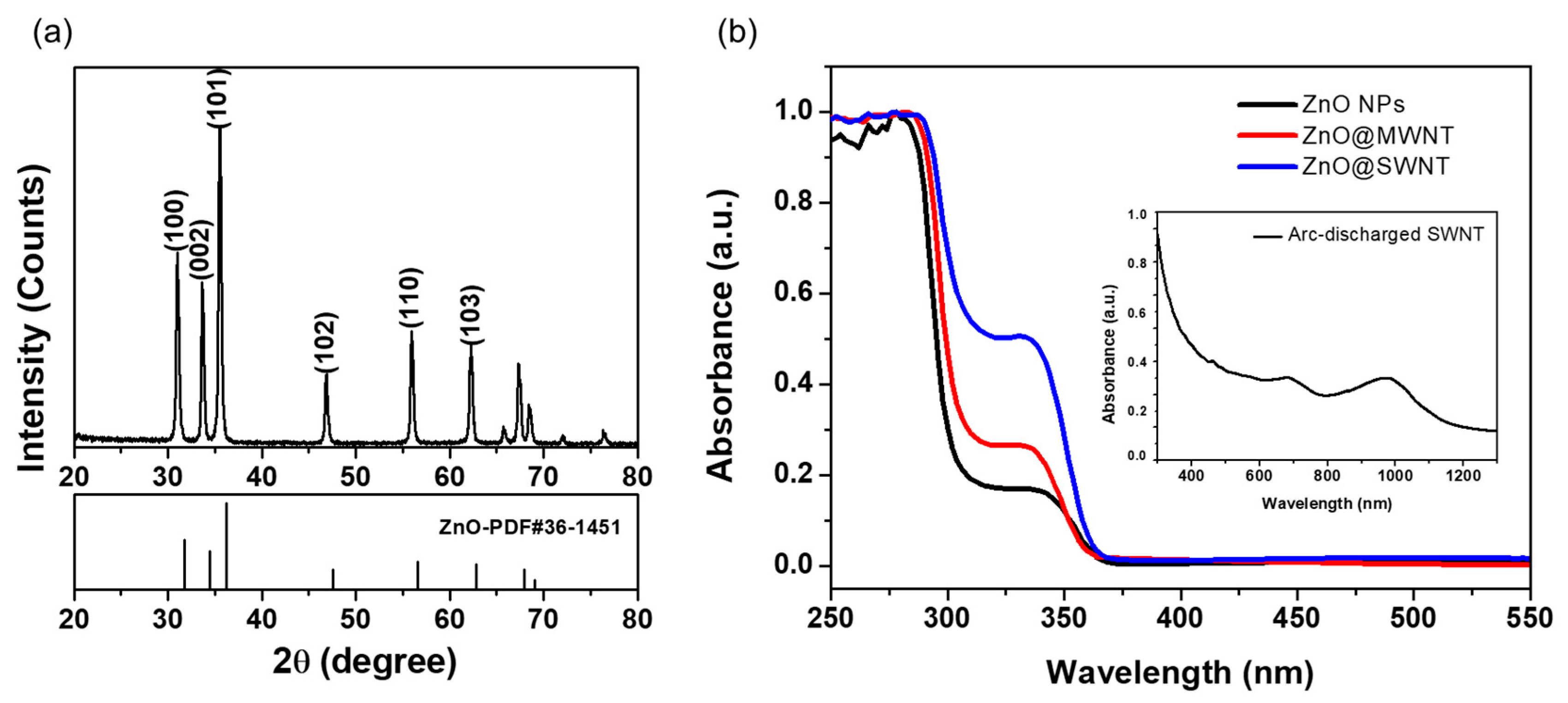
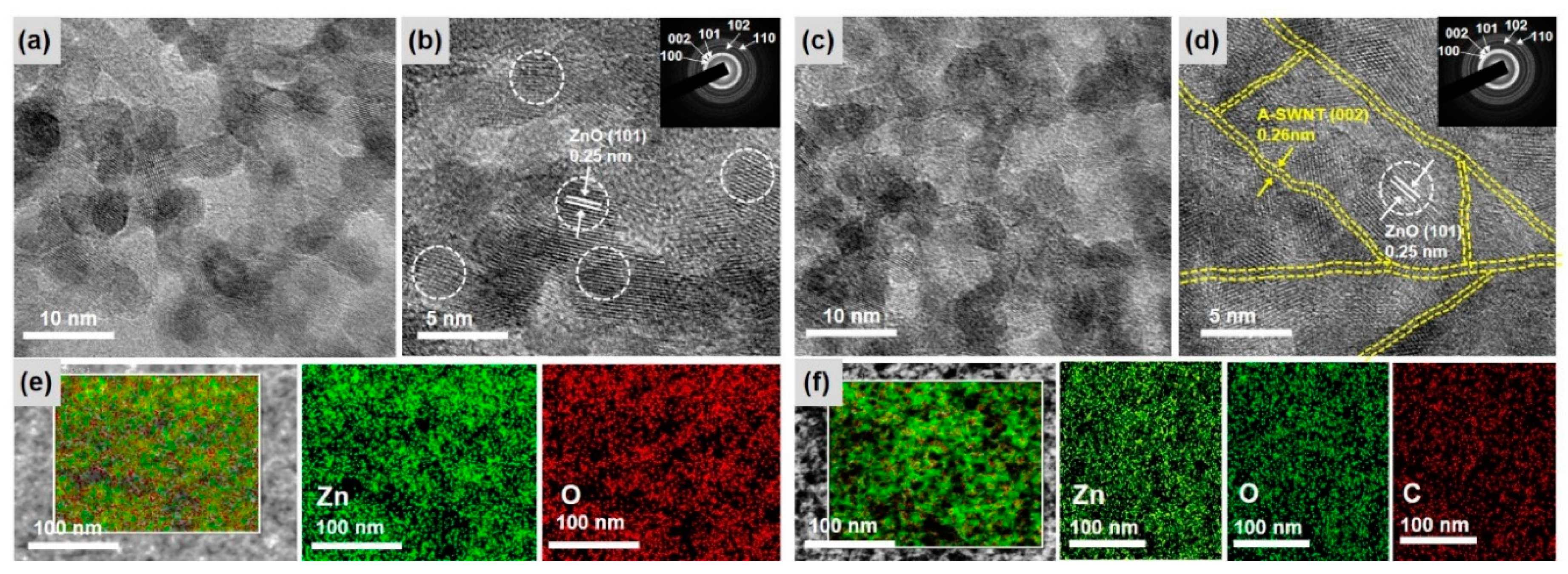
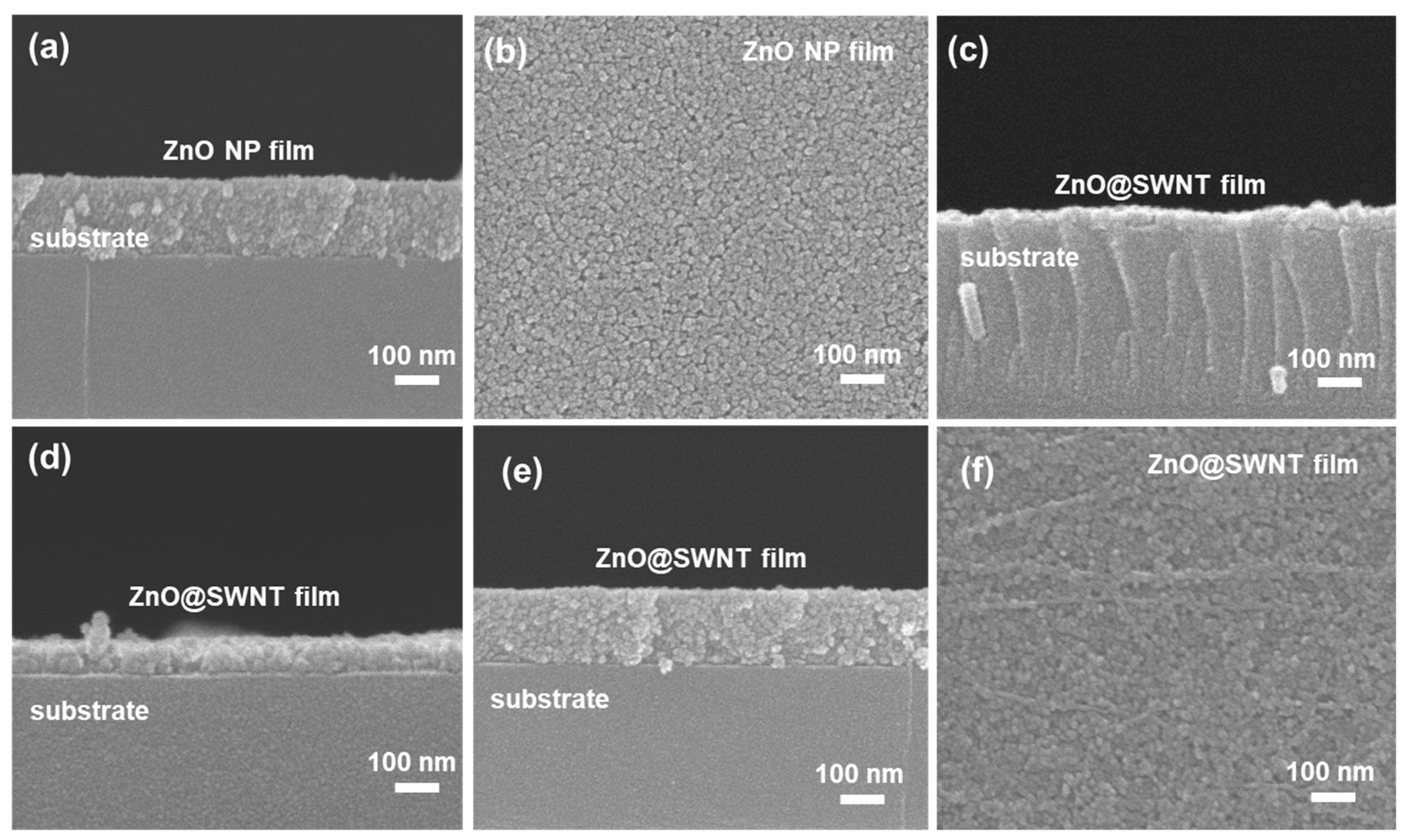
3.1. Characterization of the As-Prepared ZnO NPs and the ZnO NP/CNT Film
3.2. Characterization of ZnO and ZnO@CNT Film
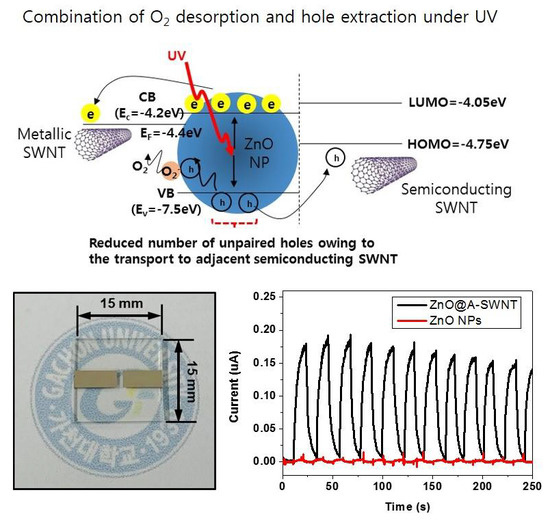
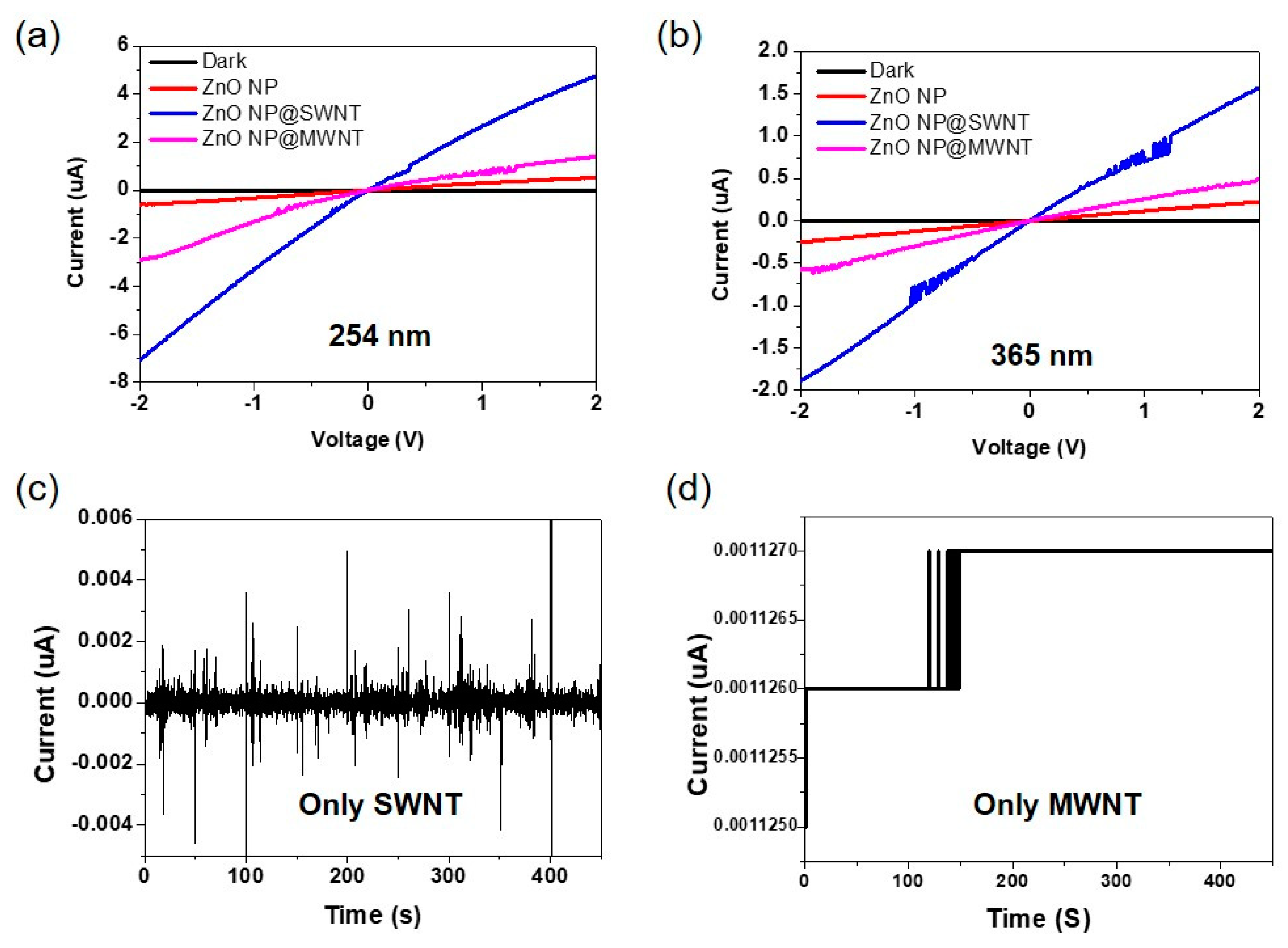
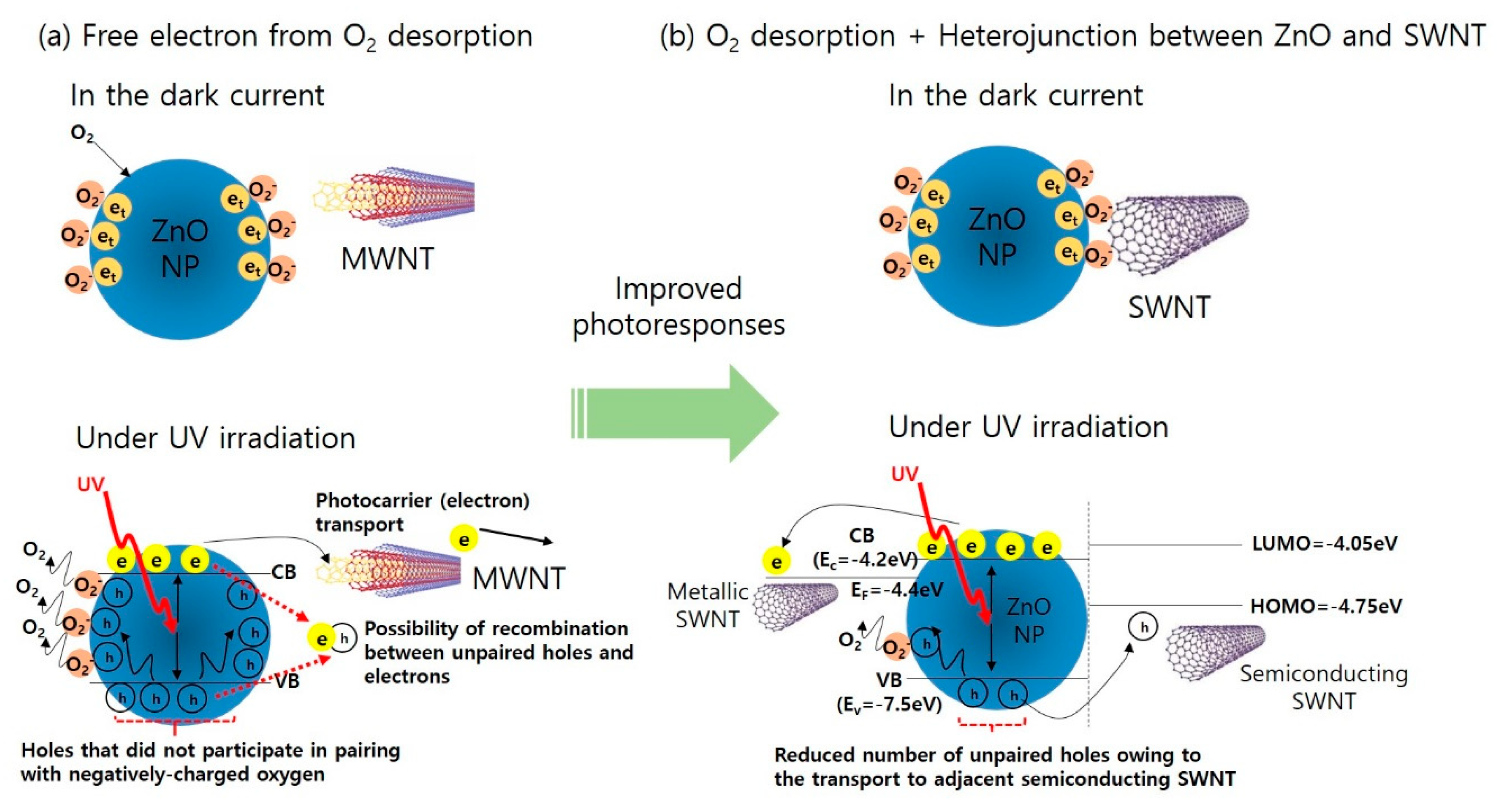
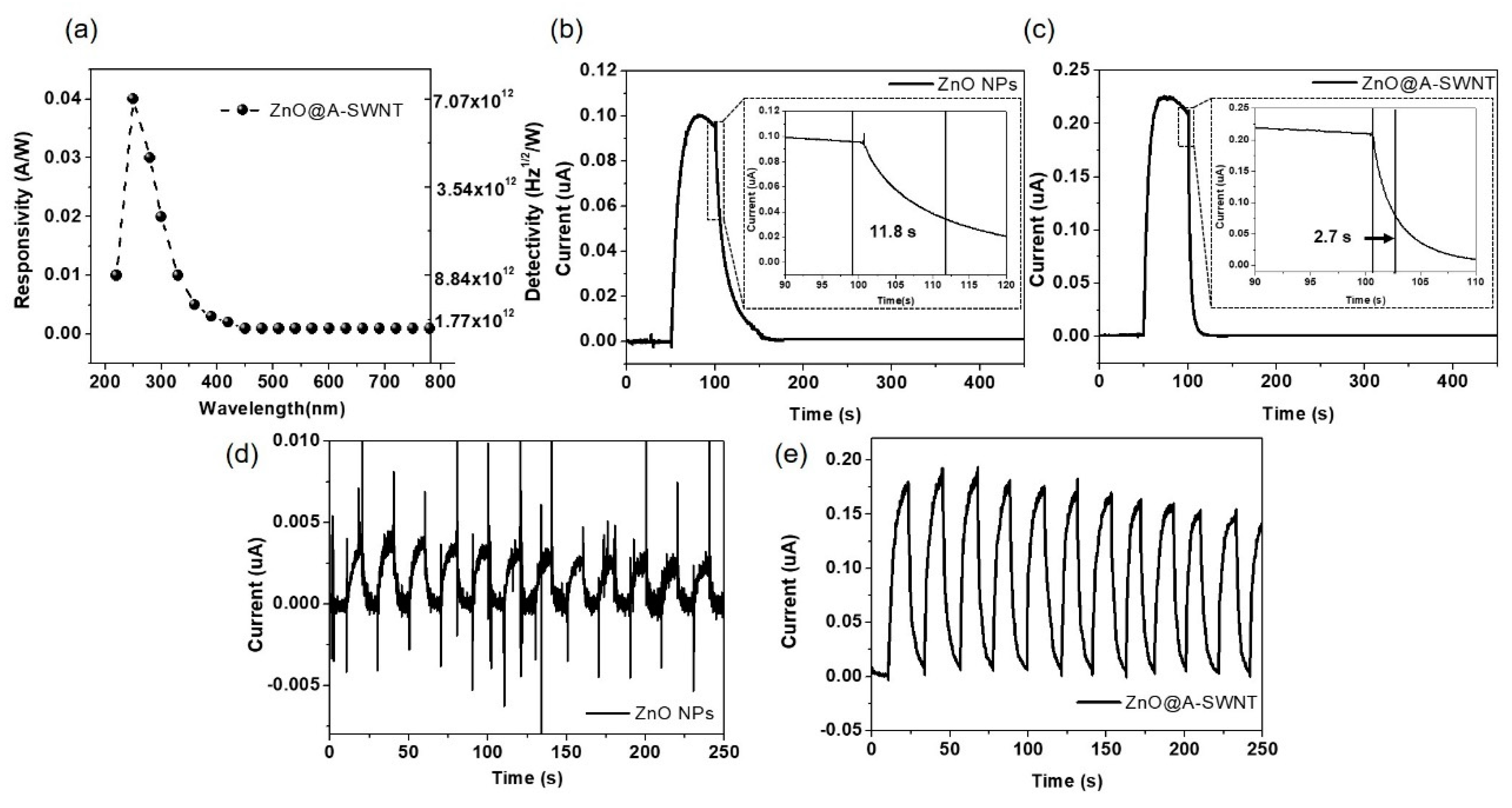
3.3. Electrical Characteristics of ZnO, ZnO@MWNT Film, and ZnO@SWNT Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hatch, S.M.; Briscoe, J.; Dunn, S. A self-powered ZnO-nanorod/CuSCN UV photodetector exhibiting rapid response. Adv. Mater. 2013, 25, 867–871. [Google Scholar] [CrossRef]
- Ardakani, A.G.; Pazoki, M.; Mahdavi, S.M.; Bahrampour, A.R.; Taghavinia, N. Ultraviolet photodetectors based on ZnO sheets: the effect of sheet size on photoresponse properties. In Appl. Surf. Sci.; 2012; Volume 258, pp. 5405–5411. [Google Scholar]
- Yu, Y.-Q.; Luo, L.-B.; Wang, M.-Z.; Wang, B.; Zeng, L.-H.; Wu, C.-Y.; Jie, J.-S.; Liu, J.-W.; Wang, L.; Yu, S.-H. Interfacial state induced ultrasensitive ultraviolet light photodetector with resolved flux down to 85 photons per second. Nano Res. 2015, 8, 1098–1107. [Google Scholar] [CrossRef]
- Curiel, M.; Nedev, N.; Paz, J.; Perez, O.; Valdez, B.; Mateos, D.; Arias, A.; Nesheva, D.; Manolov, E.; Nedev, R. UV Sensitivity of MOS Structures with Silicon Nanoclusters. Sensors 2019, 19, 2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.; Wu, H.; Zhang, W.; Li, H.; Pan, W. Enhanced UV photoresponse from heterostructured Ag–ZnO nanowires. Appl. Phys. Lett. 2009, 94, 172103. [Google Scholar] [CrossRef]
- Seong, H.; Yun, J.; Jun, J.H.; Cho, K.; Kim, S. The transfer of charge carriers photogenerated in ZnO nanoparticles into a single ZnO nanowire. Nanotechnology 2009, 20, 245201. [Google Scholar] [CrossRef] [PubMed]
- Silva da, S.C.; Ricardo, Y.; Kuroda, R.; Sugawa, S. A Highly Robust Silicon Ultraviolet Selective Radiation Sensor Using Differential Spectral Response Method. Sensors 2019, 19, 2755. [Google Scholar]
- Liu, X.; Du, H.; Wang, P.; Lim, T.-T.; Sun, X.W. A high-performance UV/visible photodetector of Cu2O/ZnO hybrid nanofilms on SWNT-based flexible conducting substrates. J. Mater. Chem. C 2014, 2, 9536–9542. [Google Scholar] [CrossRef]
- Kennedy, J.; Fang, F.; Futter, J.; Leveneur, J.; Murmu, P.; Panin, G.; Kang, T.; Manikandan, E. Synthesis and enhanced field emission of zinc oxide incorporated carbon nanotubes. Diamond Relat. Mater. 2017, 71, 79–84. [Google Scholar] [CrossRef]
- Tian, C.; Jiang, D.; Zhao, Y.; Liu, Q.; Hou, J.; Zhao, J.; Liang, Q.; Gao, S.; Qin, J. Effects of continuous annealing on the performance of ZnO based metal-semiconductor-metal ultraviolet photodetectors. Mater. Sci. Eng. B 2014, 184, 67–71. [Google Scholar] [CrossRef]
- Dutta, M.; Basak, D. Multiwalled carbon nanotubes/ZnO nanowires composite structure with enhanced ultraviolet emission and faster ultraviolet response. Chem. Phys. Lett. 2009, 480, 253–257. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.M.; Behal, A.; Sushkova, S.N.; Mandzhieva, S.; Singh, R.; Gorovtsov, A.; Tsitsuashvili, V.S.; Purvis, W.O.; Ghazaryan, K.A.; et al. Effects of zinc-oxide nanoparticles on soil, plants, animals and soil organisms: A review. Environ. Nanotechnol. Monit. Manag. 2018, 9, 76–84. [Google Scholar] [CrossRef]
- Liang, S.; Sheng, H.; Liu, Y.; Huo, Z.; Lu, Y.; Shen, H. ZnO Schottky ultraviolet photodetectors. J. Cryst.Growth 2001, 225, 110–113. [Google Scholar] [CrossRef]
- Liu, K.; Sakurai, M.; Aono, M. ZnO-based ultraviolet photodetectors. Sensors 2010, 10, 8604–8634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Elim, H.I.; Foo, Y.-L.; Yu, T.; Liu, Y.; Ji, W.; Lee, J.Y.; Shen, Z.; Wee, A.T.-S.; Thong, J.T.-L. Multiwalled carbon nanotubes beaded with ZnO nanoparticles for ultrafast nonlinear optical switching. Adv. Mater. 2006, 18, 587–592. [Google Scholar] [CrossRef]
- Rangkooy, H.A.; Pour, M.N.; Dehaghi, B.F. Efficiency evaluation of the photocatalytic degradation of zinc oxide nanoparticles immobilized on modified zeolites in the removal of styrene vapor from air. Korean J. Chem. Eng. 2017, 34, 3142–3149. [Google Scholar] [CrossRef]
- Nie, B.; Hu, J.G.; Luo, L.B.; Xie, C.; Zeng, L.H.; Lv, P.; Li, F.Z.; Jie, J.S.; Feng, M.; Wu, C.Y. Monolayer Graphene Film on ZnO Nanorod Array for High-Performance Schottky Junction Ultraviolet Photodetectors. Small 2013, 9, 2872–2879. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, P.; Wang, N.; Ma, Y.; San, H. UV-assisted photochemical synthesis of reduced graphene oxide/ZnO nanowires composite for photoresponse enhancement in UV photodetectors. Nanomaterials 2018, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Ates, E.S.; Kucukyildiz, S.; Unalan, H.E. Zinc oxide nanowire photodetectors with single-walled carbon nanotube thin-film electrodes. ACS Appl. Mater. Interfaces 2012, 4, 5142–5146. [Google Scholar] [CrossRef]
- Park, Y.R.; Liu, N.; Lee, C.J. Photoluminescence enhancement from hybrid structures of metallic single-walled carbon nanotube/ZnO films. Curr. Appl. Phys. 2013, 13, 2026–2032. [Google Scholar] [CrossRef]
- Kim, S.J.; Han, J.-W.; Kim, B.; Meyyappan, M. Single walled carbon nanotube based air pocket encapsulated ultraviolet sensor. ACS Sens. 2017, 2, 1679–1683. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, C.; Li, K.; Chen, Y.; Ruan, S.; Zhang, X.; Li, C. Facilitated extrinsic majority carrier depletion and photogenerated exciton dissociation in an annealing-free ZnO: C photodetector. Nanoscale 2018, 10, 6459–6466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xiao, X.; Xue, H.; Pang, H. Zinc Oxide Based Composite Materials for Advanced Supercapacitors. ChemistrySelect 2018, 3, 550–565. [Google Scholar] [CrossRef]
- Jin, Z.; Zhou, Q.; Chen, Y.; Mao, P.; Li, H.; Liu, H.; Wang, J.; Li, Y. Graphdiyne: ZnO Nanocomposites for High-Performance UV Photodetectors. Adv. Mater. 2016, 28, 3697–3702. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.; Khalili, S.; Peyravi, M.; Jahanshahi, M.; Darabi, R.R.; Ardeshiri, F.; Rad, A.S. Sublayer assisted by hydrophilic and hydrophobic ZnO nanoparticles toward engineered osmosis process. Korean J. Chem. Eng. 2018, 35, 2256–2268. [Google Scholar] [CrossRef]
- Shao, D.; Sun, H.; Gao, J.; Xin, G.; Aguilar, M.A.; Yao, T.; Koratkar, N.; Lian, J.; Sawyer, S. Flexible, thorn-like ZnO-multiwalled carbon nanotube hybrid paper for efficient ultraviolet sensing and photocatalyst applications. Nanoscale 2014, 6, 13630–13636. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Wang, J.; Sun, B.; Blakesley, J.C.; Greenham, N.C. Solution-processed ultraviolet photodetectors based on colloidal ZnO nanoparticles. Nano lett. 2008, 8, 1649–1653. [Google Scholar] [CrossRef]
- Cai, Y.; Tang, L.; Xiang, J.; Ji, R.; Lai, S.K.; Lau, S.P.; Zhao, J.; Kong, J.; Zhang, K. High performance ultraviolet photodetectors based on ZnO nanoflakes/PVK heterojunction. Appl. Phys. Lett. 2016, 109, 073103. [Google Scholar] [CrossRef]
- Mridha, S.; Basak., D. ZnO/polyaniline based inorganic/organic hybrid structure: Electrical and photoconductivity properties. Appl. Phys. Lett. 2008, 92, 142111. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Zhang, L.; Yin, Z.; Wang, D.; Meng, J.; Jiang, Q.; Wang, Y.; You, J. A high-performance photodetector based on an inorganic perovskite–ZnO heterostructure. J. Mater. Chem. C 2017, 5, 6115–6122. [Google Scholar] [CrossRef]
- Lamastra, F.R.; Grilli, M.L.; Belardini, A.; Voti, L.R.; Sibilia, C.; Salvatori, D.; Cacciotti, I.; Nanni, F. Photoacoustic Spectroscopy Investigation of Zinc Oxide/Diatom Frustules Hybrid Powders. Int. J. Thermophys. 2018, 39, 110. [Google Scholar] [CrossRef]
- Lamastra, F.R.; Grilli, M.L.; Belardini, A.; Voti, L.R.; Sibilia, C.; Salvatori, D.; Cacciotti, I.; Nanni, F. Diatom frustules decorated with zinc oxide nanoparticles for enhanced optical properties. Nanotechnology 2017, 28, 375704. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56. [Google Scholar] [CrossRef]
- Arnold, M.S.; Green, A.A.; Hulvat, J.F.; Stupp, S.I.; Hersam, M.C. Sorting carbon nanotubes by electronic structure using density differentiation. Nat. Nanotechnol. 2006, 1, 60. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Resasco, D.E. Dispersion of single-walled carbon nanotubes of narrow diameter distribution. J. Phys. Chem. B 2005, 109, 14454–14460. [Google Scholar] [CrossRef] [PubMed]
- Leahu, G.; Li, V.R.; Larciprete, M.-C.; Sibilia, C.; Bertolotti, M.; Nefedov, I.; Anoshkin, I. Thermal Characterization of Carbon Nanotubes by Photothermal Techniques. Int. J. Thermophys. 2015, 36, 1349–1357. [Google Scholar] [CrossRef]
- Larciprete, M.C.; Paoloni, S.; Orazi, N.; Mercuri, F.; Orth, M.; Gloy, Y.; Centini, M.; Voti, L.R.; Sibilia, C. Infrared emissivity characterization of carbon nanotubes dispersed poly(ethylene terephthalate) fibers. Int. J. Therm. Sci. 2019, 146, 106–109. [Google Scholar] [CrossRef]
- Macias, J.D.; Bante-Guerra, J.; Cervantes-Alvarez, F.; Rodrìguez-Gattorno, G.; Arés-Muzio, O.; Romero-Paredes, H.; Arancibia-Bulnes, C.A.; Ramos-Sánchez, V.; Villafán-Vidales, H.I.; Ordonez-Miranda, J.; et al. Thermal Characterization of Carbon Fiber-Reinforced Carbon Composites. Appl. Compos. Mater. 2019, 26, 321–337. [Google Scholar] [CrossRef]
- Li, G.; Suja, M.; Chen, M.; Bekyarova, E.; Haddon, R.C.; Liu, J.; Itkis, M.E. Visible-blind UV photodetector based on single-walled carbon nanotube thin film/ZnO vertical heterostructures. ACS Appl. Mater. Interfaces 2017, 9, 37094–37104. [Google Scholar] [CrossRef]
- Beek, W.J.; Wienk, M.M.; Kemerink, M.; Yang, X.; Janssen, R.A. Hybrid zinc oxide conjugated polymer bulk heterojunction solar cells. J. Phys. Chem. B. 2005, 109, 9505–9516. [Google Scholar] [CrossRef]
- Liu, K.; Sakurai, M.; Liao, M.; Aono, M. Giant improvement of the performance of ZnO nanowire photodetectors by Au nanoparticles. J. Phys. Chem. C 2010, 114, 19835–19839. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, Y.; Xu, F.; Li, Q.; Zhu, Z.; Wan, Q. Significant improvement of field emission by depositing zinc oxide nanostructures on screen-printed carbon nanotube films. Appl. Phys. Lett. 2006, 88, 153123. [Google Scholar] [CrossRef]
- Zhang, J.; Gui, H.; Liu, B.; Liu, J.; Zhou, C. Comparative study of gel-based separated arcdischarge, HiPCO, and CoMoCAT carbon nanotubes for macroelectronic applications. Nano Res. 2013, 6, 906–920. [Google Scholar] [CrossRef]
- Selman, A.M.; Hassan, Z. Highly sensitive fast-response UV photodiode fabricated from rutile TiO2 nanorod array on silicon substrate. Sens. Actuators A 2015, 221, 15–21. [Google Scholar] [CrossRef]
- Alsultany, F.H.; Hassan, Z.; Ahmed, N.M. A high-sensitivity, fast-response, rapid-recovery UV photodetector fabricated based on catalyst-free growth of ZnO nanowire networks on glass substrate. Opt. Mater. 2016, 60, 30–37. [Google Scholar] [CrossRef]
- Nasiri, N.; Bo, R.; Wang, F.; Fu, L.A. Tricoli, Ultraporous Electron-Depleted ZnO Nanoparticle Networks for Highly Sensitive Portable Visible-Blind UV Photodetectors. Adv. Mater. 2015, 27, 4336–4343. [Google Scholar] [CrossRef] [PubMed]
- Kind, H.; Yan, H.; Messer, B.; Law, M.; Yang, P. Nanowire ultraviolet photodetectors and optical switches. Adv. Mater. 2002, 14, 158–160. [Google Scholar] [CrossRef]
- Svrcek, V.; Cook, S.; Kazaoui, S.; Kondo, M. Silicon nanocrystals and semiconducting single-walled carbon nanotubes applied to photovoltaic cells. J. Phys. Chem. Lett. 2011, 2, 1646–1650. [Google Scholar] [CrossRef]
- Jorio, A.; Araujo, P.T.; Doorn, S.K.; Maruyama, S.; Chacham, H.; Pimenta, M.A. The Kataura plot over broad energy and diameter ranges. Phys. Stat. Sol. 2006, 243, 3117–3121. [Google Scholar] [CrossRef]
- Huang, Z.; Gao, M.; Yan, Z.; Pan, T.; Liao, F.; Lin, Y. Flexible infrared detectors based on p–n junctions of multi-walled carbon nanotubes. Nanoscale 2016, 8, 9592–9599. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.; Yan, X.; Zhang, Z.; Shen, Y.; Zhao, Y.; Bai, Z.; Zhang, Y. Self-powered UV photosensor based on PEDOT: PSS/ZnO micro/nanowire with strain-modulated photoresponse. ACS Appl. Mater. Interfaces 2013, 5, 3671–3676. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, F.; Wang, X.; Wang, X.; Flahaut, E.; Liu, X.; Li, Y.; Wang, X.; Xu, Y.; Shi, Y. Planar carbon nanotube–graphene hybrid films for high-performance broadband photodetectors. Nat. Commun. 2015, 6, 8589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Han, C.; Tang, Z.-R.; Xu, Y.-J. Heterostructured semiconductor nanowire arrays for artificial photosynthesis. Mater. Horiz. 2016, 3, 270–282. [Google Scholar] [CrossRef]
- Costa, S.; Borowiak-Palen, E.; Kruszynska, M.; Bachmatiuk, A.; Kalenczuk, R. Characterization of carbon nanotubes by Raman spectroscopy. Mater. Sci. Pol. 2008, 26, 433–441. [Google Scholar]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Muramatsu, H.; Hayashi, T.; Endo, M.; Terrones, M.; Dresselhaus, M.S. Fabrication of high-purity, double-walled carbon nanotube buckypaper. Chem. Vap. Depos. 2006, 12, 327–330. [Google Scholar] [CrossRef]
- Dang, V.Q.; Trung, T.Q.; Kim, D.I.; Duy, L.T.; Hwang, B.U.; Lee, D.W.; Kim, B.Y.; Toan, L.D.; Lee, N.E. Ultrahigh responsivity in graphene–ZnO nanorod hybrid UV photodetector. Small 2015, 11, 3054–3065. [Google Scholar] [CrossRef]
- Wang, X.; Liu, K.; Chen, X.; Li, B.; Jiang, M.; Zhang, Z.; Zhao, H.; Shen, D. Highly wavelength-selective enhancement of responsivity in Ag nanoparticle-modified ZnO UV photodetector. ACS Appl. Mater. Interfaces 2017, 9, 5574–5579. [Google Scholar] [CrossRef]
- Bai, S.; Wu, W.; Qin, Y.; Cui, N.; Bayerl, D.J.; Wang, X. High-performance integrated ZnO nanowire UV sensors on rigid and flexible substrates. Adv. Funct. Mater. 2011, 21, 4464–4469. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, H.; Zhao, D.; Li, B.; Zhang, Z.; Jiang, M.; Shen, D. High responsivity ZnO nanowires based UV detector fabricated by the dielectrophoresis method. Sens. Actuators B 2012, 166, 12–16. [Google Scholar] [CrossRef]
- Zhang, T.-F.; Li, Z.-P.; Wang, J.-Z.; Kong, W.-Y.; Wu, G.-A.; Zheng, Y.-Z.; Zhao, Y.-W.; Yao, E.-X.; Zhuang, N.-X.; Luo, L.-B. Broadband photodetector based on carbon nanotube thin film/single layer graphene Schottky junction. Sci. Rep. 2016, 6, 38569. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Zhang, Y.; Guo, R. ZnO microtube ultraviolet detectors. J. Cryst. Growth 2008, 310, 57–61. [Google Scholar] [CrossRef]
- Manekkathodi, A.; Lu, M.Y.; Wang, C.W.; Chen, L.J. Direct growth of aligned zinc oxide nanorods on paper substrates for low-cost flexible electronics. Adv. Mater. 2010, 22, 4059–4063. [Google Scholar] [CrossRef] [PubMed]
- Lupan, O.; Chai, G.; Chow, L.; Emelchenko, G.; Heinrich, H.; Ursaki, V.; Gruzintsev, A.; Tiginyanu, I.; Redkin, A. Ultraviolet photoconductive sensor based on single ZnO nanowire. Phys. Status Solidi A 2010, 207, 1735–1740. [Google Scholar] [CrossRef]
- Gan, L.; Liao, M.; Li, H.; Ma, Y.; Zhai, T. Geometry-induced hig performance of ultraviolet photodetectors in kinked SnO2 nanowires. J. Mater. Chem. C 2015, 3, 8300–8306. [Google Scholar] [CrossRef]
- Lou, Z.; Li, L.; Shen, G. High-performance rigid and flexible ultraviolet photodetectors with single-crystalline ZnGa2O4 nanowires. Nano Res. 2015, 8, 2162–2169. [Google Scholar] [CrossRef]
- Noothongkaew, S.; Thumthan, O.; An, K.S. Minimal layer graphene/TiO2 nanotube membranes used for enhancement of UV photodetectors. Mater. Lett. 2018, 218, 274–279. [Google Scholar] [CrossRef]
- Chong, H.; Wei, G.; Hou, H.; Yang, H.; Shang, M.; Gao, F.; Yang, W.; Shen, G. High-performance solar-blind ultraviolet photodetector based on electrospun TiO2-ZnTiO3 heterojunction nanowires. Nano Res. 2015, 8, 2822–2832. [Google Scholar] [CrossRef]



| Materials | Length (μm) | Diameter (nm) | Aspect ratio | Property | Sheet Resistance (Ω/□) |
|---|---|---|---|---|---|
| A-SWNTs | 5–10 | 1.3–1.5 | 3300–7700 | ~33% metallic (~67% semiconducting) | 515 |
| H-SWNTs | 0.1–1 | 0.8–1.0 | 100–1250 | ~33% metallic (~67% semiconducting) | 20620 |
| C-SWNTs | 0.1–1 | 0.7–0.8 | 120–1430 | ~10% metallic (~90% semiconducting) | 34791 |
| Material | Wavelength (nm) | Bias (V) | Intensity of Light (mW/cm2) | Iuv-Idark | Rise Time (s) | Decay Time (s) | Ref. |
|---|---|---|---|---|---|---|---|
| ZnO nanowires | 365 | 1 | 1.3 | 1000 | 40 | 300 | [60] |
| ZnO microtubes | 365 | 5 | 150 | 600 | 5.9 | 638 | [62] |
| ZnO nanorods | 365 | 5 | - | 85 | >500 | 500 | [63] |
| ZnO-coated ZnO arrays | 365 | 3 | 150 | 1.25 | 120 | 180 | [64] |
| ZnO@A-SWNT | 365 | 2 | 1.25 | 49 | 11.3 | 5.97 | This Work |
| 254 | 2 | 1.25 | 950 | (@365nm) | (@365nm) |
| Material | Wavelength (nm) | Bias (V) | Intensity of Light (mW/cm2) | Iuv-Idark | Rise Time (s) | Decay Time (s) | Ref. |
|---|---|---|---|---|---|---|---|
| SnO2 nanowire | 250 | 3 | - | 10 | >20 | >10 | [65] |
| ZnGa2O4 nanowire | 350 | 5 | 0.53 | 130 | 15 | >10 | [66] |
| Graphene/TiO2 | 365 | 5 | - | - | >150 | >150 | [67] |
| TiO2-ZnTiO2 nanowires | 320 | 2 | 0.186 | >300 | >10 | 10 | [68] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.-S.; Park, T.; Kim, W.-J.; Hur, J. High-Performance Ultraviolet Photodetector Based on a Zinc Oxide Nanoparticle@Single-Walled Carbon Nanotube Heterojunction Hybrid Film. Nanomaterials 2020, 10, 395. https://doi.org/10.3390/nano10020395
Choi M-S, Park T, Kim W-J, Hur J. High-Performance Ultraviolet Photodetector Based on a Zinc Oxide Nanoparticle@Single-Walled Carbon Nanotube Heterojunction Hybrid Film. Nanomaterials. 2020; 10(2):395. https://doi.org/10.3390/nano10020395
Chicago/Turabian StyleChoi, Myung-Soo, Taehyun Park, Woo-Jae Kim, and Jaehyun Hur. 2020. "High-Performance Ultraviolet Photodetector Based on a Zinc Oxide Nanoparticle@Single-Walled Carbon Nanotube Heterojunction Hybrid Film" Nanomaterials 10, no. 2: 395. https://doi.org/10.3390/nano10020395