A Highly Sensitive SERS and RRS Coupled Di-Mode Method for CO Detection Using Nanogolds as Catalysts and Bifunctional Probes
Abstract
1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Reagents
2.3. Experiment Methods
3. Results and Discussion
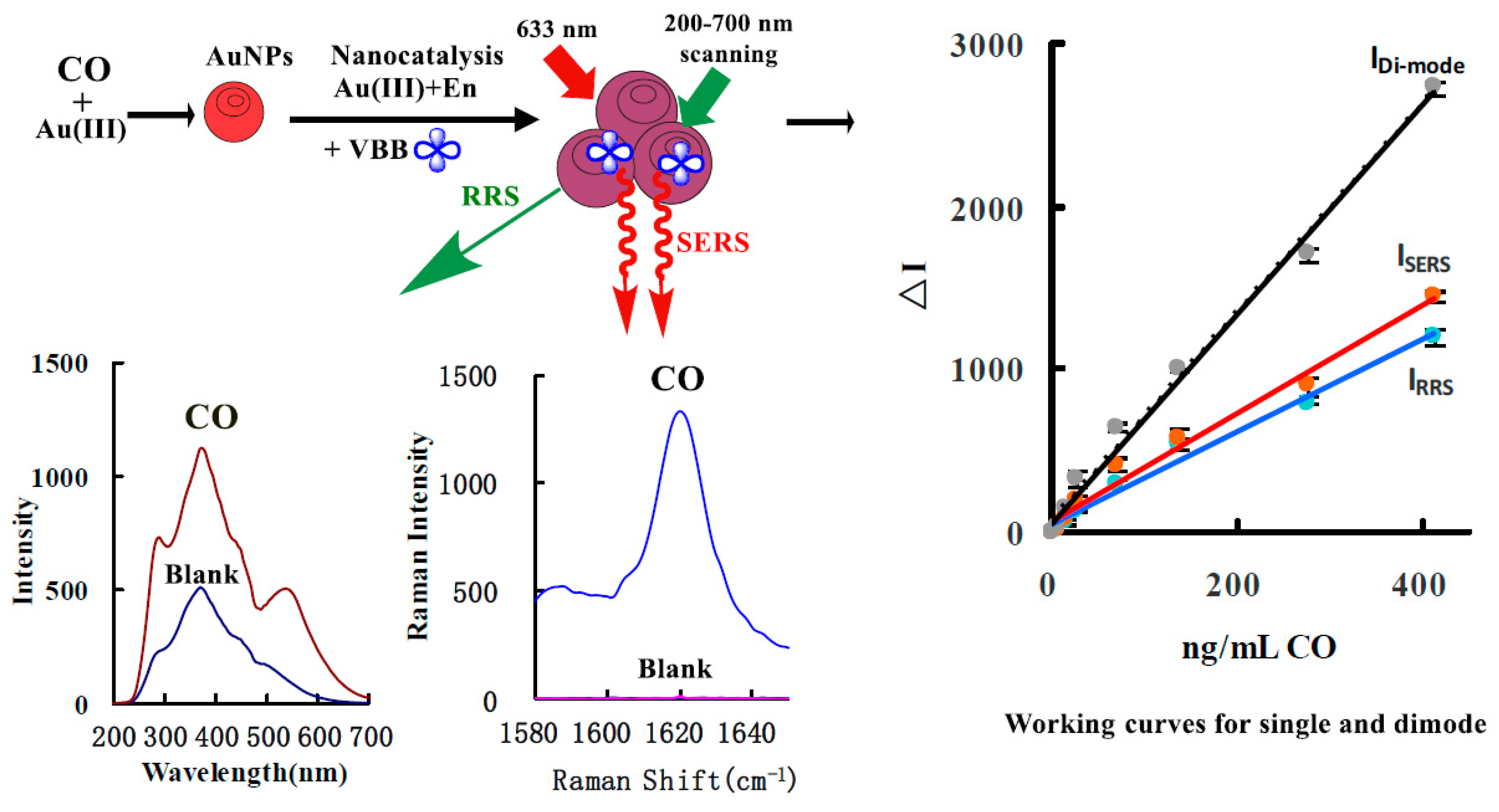
3.1. Analytical Principle
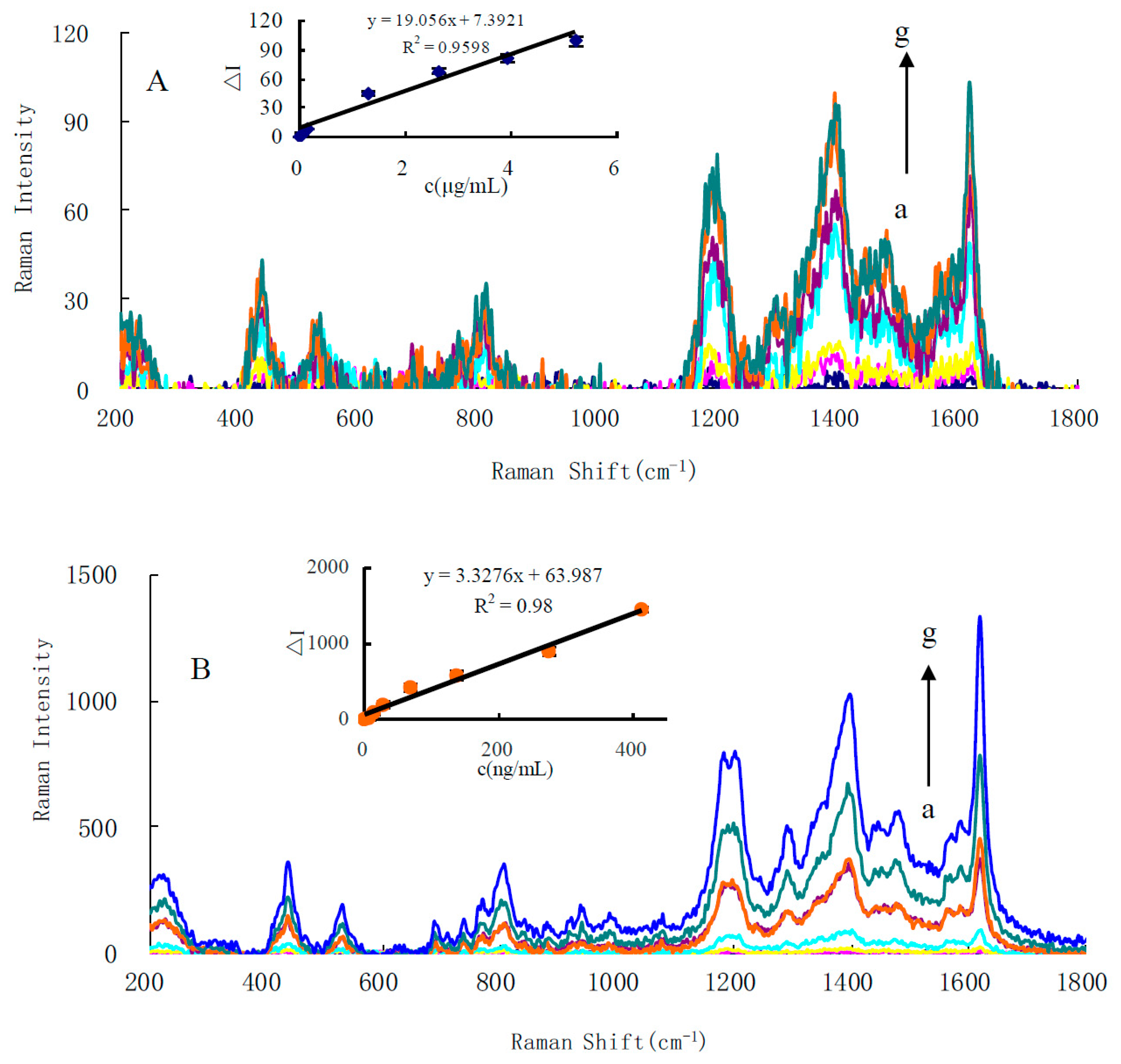
3.2. SERS Spectra
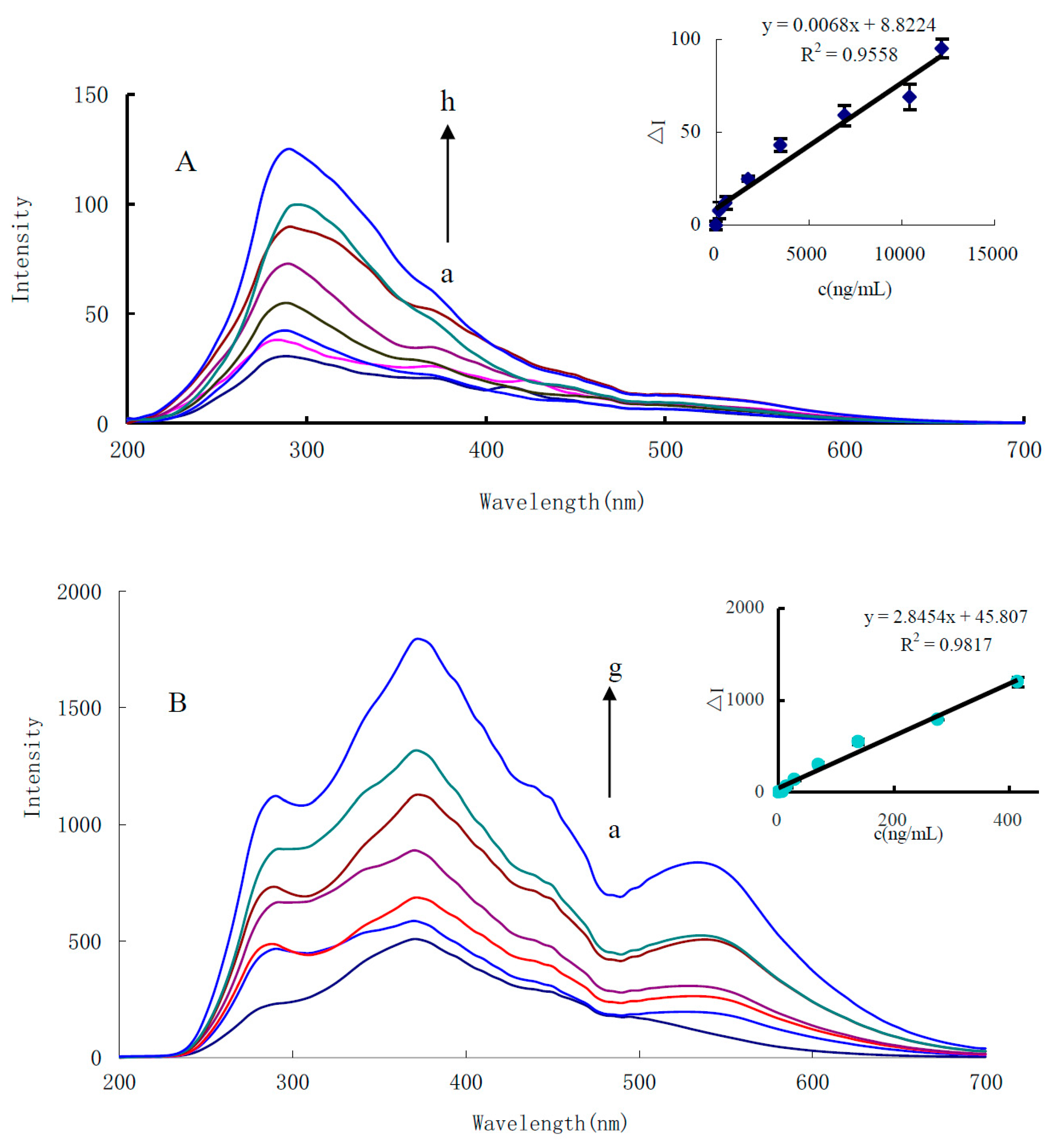
3.3. RRS Spectra
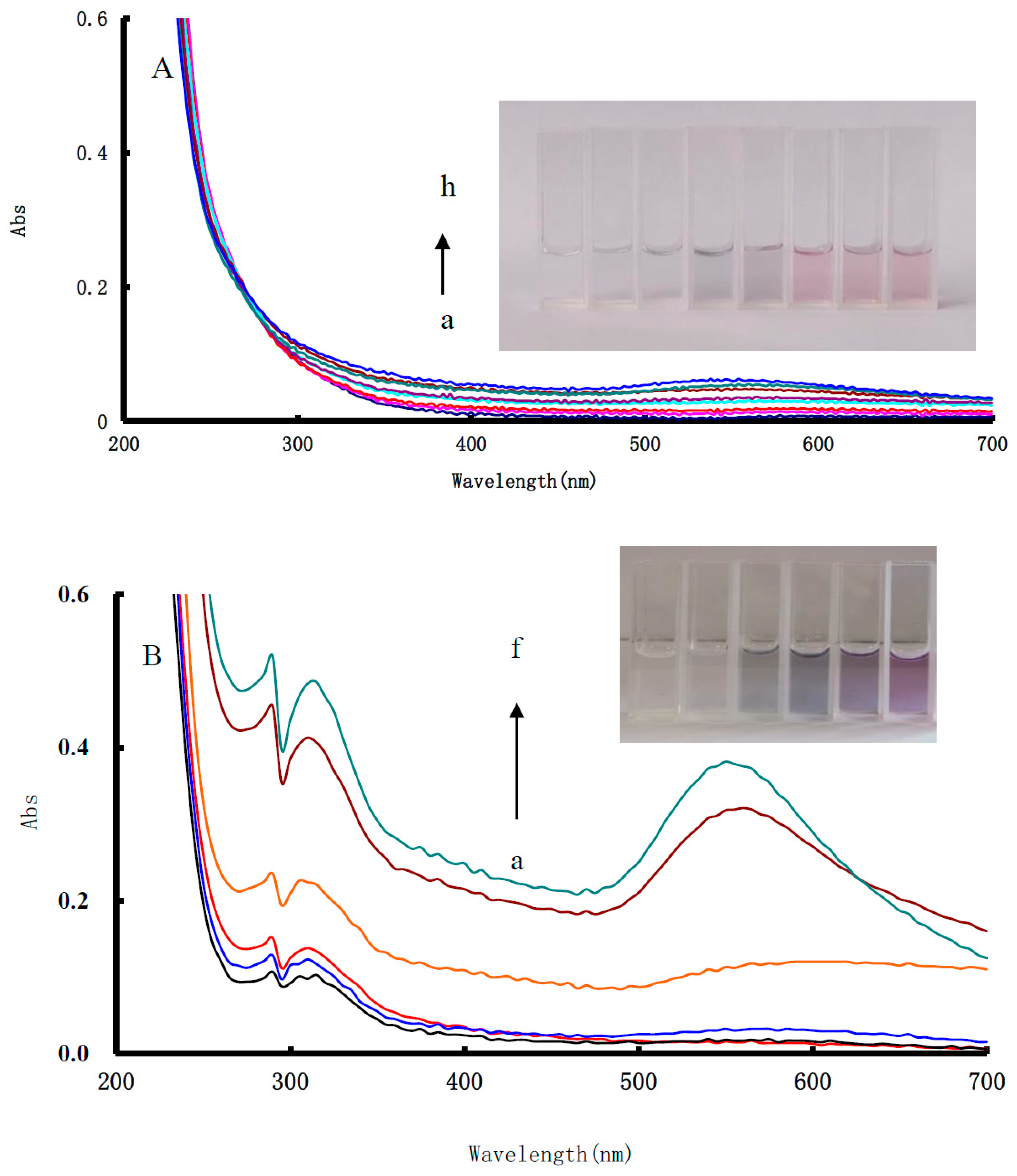
3.4. UV Absorption Spectra
3.5. Gold Nano-Enzyme Catalysis
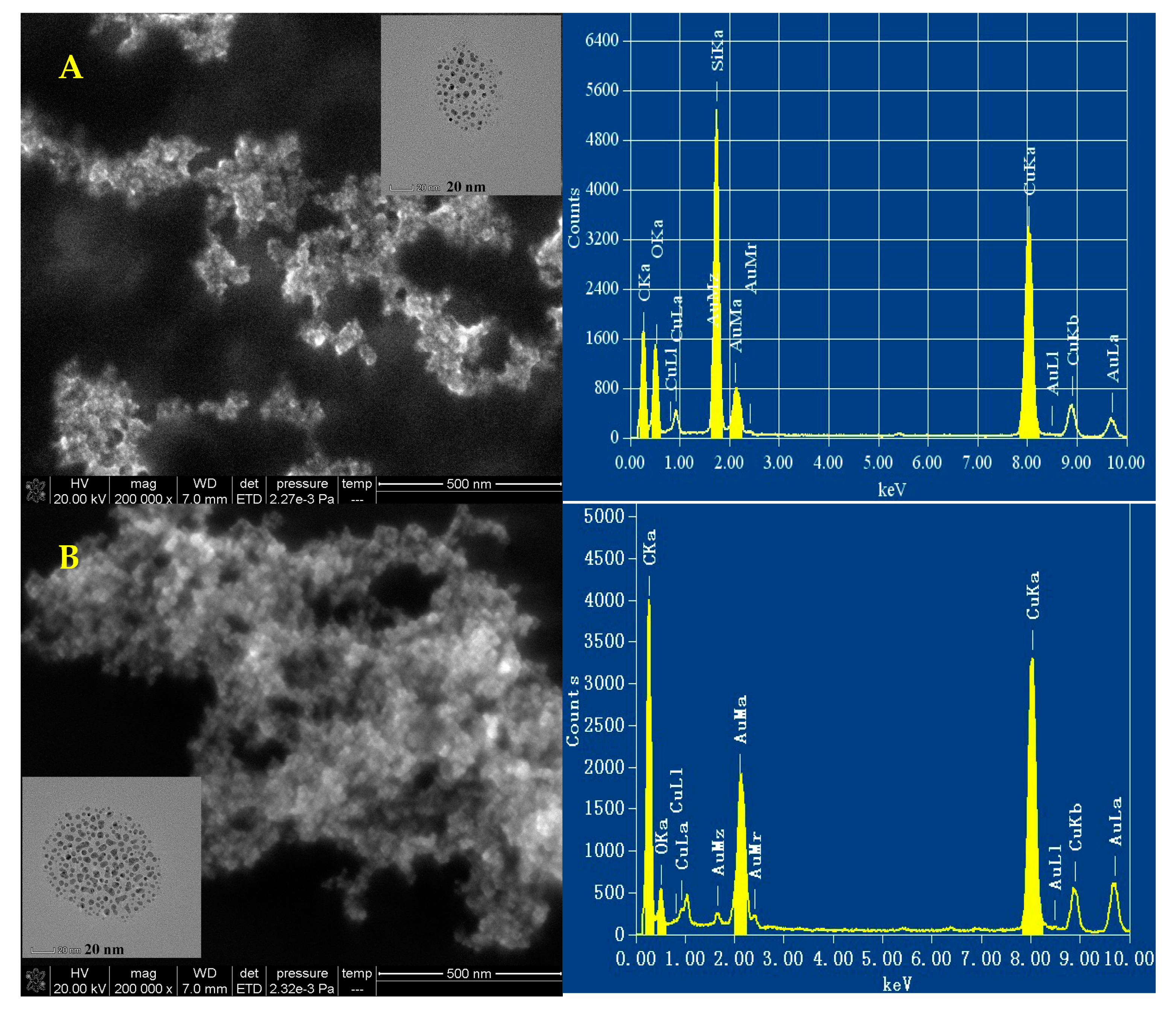
3.6. Scanning Electron Microscopy (SEM), Transmission Electron Microscope (TEM) and Energy Spectrum
3.7. Conditions Optimization
3.8. Calibration Curve
3.9. The Influence of Coexisting Substance
3.10. Analysis of Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shi, W.; Zhang, X.; He, S.; Li, J.; Huang, Y. Fast screening of the nanoparticles-based enzyme mimetics by a chemiluminescence method. Sci. Sin. Chim. 2013, 43, 1591–1598. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, C.P.; Wu, T.H.; Yang, C.H.; Lin, C.W.; Chen, C.Y. Gold nanoparticle-based Colorimetric Strategies for chemical and biological sensing applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef] [PubMed]
- Mandal, T.K.; Hou, Y.; Gao, Z.; Ning, H.; Yang, W.; Gao, M. Graphene oxide-based Sensor for ultrasensitive visual detection of fluoride. Adv. Sci. 2016, 3, 1600217–1600222. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Qiu, Q.M.; Sharif, S.; Ying, S.N.; Wang, Y.X.; Ying, Y.B. Solution-phase synthesis of platinum nanoparticle-decorated metal-organic framework hybrid nanomaterials as biomimetic nano-enzymes for biosensing applications. ACS Appl. Mater. Interfaces 2018, 10, 24108–24115. [Google Scholar] [CrossRef]
- Gao, L.Z.; Zhuang, J.; Nie, L. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Wu, T.T.; Ma, Z.Y.; Li, P.P.; Lu, Q.J.; Liu, M.L.; Li, H.T.; Zhang, Y.Y.; Yao, S.Z. Bifunctional colorimetric biosensors via regulation of the dual nano-enzyme activity of carbonized FeCo-ZIF. Sens. Actuators B 2019, 290, 357–363. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Q.; Liu, Z.P.; Wang, X.Y.; Su, X.G. A novel enzyme-mimic nanosensor based on quantum dot-Au nanoparticle@silica mesoporous microsphere for the detection of glucose. Anal. Chim. Acta 2014, 840, 68–74. [Google Scholar] [CrossRef]
- Zeng, H.H.; Qiu, W.B.; Zhang, L.; Liang, R.P.; Qiu, J.D. Lanthanide coordination polymer nanoparticles as an excellent artificial peroxidase for hydrogen peroxide detection. Anal. Chem. 2016, 88, 6342–6348. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, C.J.; Guo, Y. Peroxidase-like activity of apoferritin paired gold clusters for glucose detection. Biosens. Bioelectron. 2015, 64, 165–170. [Google Scholar] [CrossRef]
- Liu, J.B.; Hu, X.N.; Hou, S. Au@Pt core/shell nanorods with peroxidase- and ascorbate oxidase-like activities for improved detection of glucose. Sens. Actuators B 2012, 166–167, 708–714. [Google Scholar] [CrossRef]
- Liang, A.H.; Li, C.N.; Li, D.; Luo, Y.H.; Wen, G.Q.; Jiang, Z.L. A facile and sensitive peptide-modulating graphene oxide nanoribbon catalytic nanoplasmon analytical platform for human chorionic gonadotropin. Inter. J. Nanomed. 2017, 12, 8725–8734. [Google Scholar] [CrossRef] [PubMed]
- Li, C.N.; Peng, Y.T.; Wang, H.D.; Liang, A.H.; Jiang, Z.L. A nanosol SERS method for quantitative analysis of trace potassium based on aptamer recognition and silver nanorod catalysis of Ag(I)-glucose reaction. Sens. Actuators B 2019, 281, 53–59. [Google Scholar] [CrossRef]
- Xu, M.; Tu, G.; Ji, M.; Wan, X.; Liu, J.; Liu, J.; Rong, H.; Yang, Y.; Wang, C.; Zhang, J. Vacuum-tuned-atmosphere induced assembly of Au@Ag core/shell nanocubes into multi-dimensional superstructures and the ultrasensitive IAPP proteins SERS detection. Nano Res. 2019, 12, 1375–1379. [Google Scholar] [CrossRef]
- Li, D.; Li, C.N.; Liang, A.H.; Jiang, Z.L. SERS and fluorescence dual-mode sensing trace hemin and K+ based on Gquarplex/hemin DNAzyme catalytic amplification. Sens. Actuators B 2019, 297, 126799. [Google Scholar] [CrossRef]
- Ji, W.; Li, L.; Song, W.; Wang, X.; Zhao, B.; Ozaki, Y. Enhanced Raman scattering by ZnO superstructures: Synergistic effect of charge transfer and mie resonances. Angew. Chem. Int. Ed. 2019, 58, 14773. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, G.Q.; Zhang, H.W.; Li, Y.; Cai, W.P. Porous zeolite imidazole framework-wrapped urchin-like Au-Ag nanocrystals for SERS detection of trace hexachlorocyclohexane pesticides via efficient enrichment. J. Hazard. Mater. 2019, 368, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Pandeeswar, M.; Rohilla, S.; Ahmad, E.K.; Selvakannan, R.P.; Ylias, M.S.; Chilakapati, M.; Suresh, K.B.; Thimmaiah, G. SERS and fluorescence-based ultrasensitive detection of mercury in water. Biosens. Bioelectron. 2018, 100, 556–564. [Google Scholar]
- Ren, W.; Zhang, Y.; Fan, Y.Z.; Dong, J.X.; Li, N.B.; Luo, H.Q. A resonance Rayleigh scattering sensor for detection of Pb2+ ions via cleavage-induced G-wire formation. J. Hazard. Mater. 2017, 336, 195–201. [Google Scholar] [CrossRef]
- Wang, H.; Huang, X.; Wen, G.; Jiang, Z.L. A dual-model SERS and RRS analytical platform for Pb(II) based on Ag-doped carbon dot catalytic amplification and aptamer regulation. Sci. Rep. 2019, 9, 9991. [Google Scholar] [CrossRef]
- Jiang, Z.L.; Liao, X.J.; Deng, A.P.; Liang, A.H.; Li, J.S.; Pan, H.C.; Li, J.F.; Wang, S.M.; Huang, Y.J. Catalytic effect of nanogold on Cu(II)-N2H4 reaction and its application to resonance scattering immunoassay. Anal. Chem. 2008, 80, 8681–8687. [Google Scholar] [CrossRef]
- Liang, A.H.; Zhang, Y.; Fan, Y.Y.; Chen, Q.; Wen, G.Q.; Liu, Q.Y.; Kang, C.Y.; Jiang, Z.L. Catalysis of aptarmer-modified AuPdNPs nanoalloy probe and its application to resonance scattering detection of trace UO22+. Nanoscale 2011, 3, 3178–3184. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhang, Y.; Chen, H.G.; Gao, Z.F.; Li, N.B.; Luo, H.Q. Ultrasensitive label-free resonance Rayleigh scattering aptasensor for Hg2+ using Hg2+-triggered exonuclease III-assisted target recycling and growth of G-wires for signal amplification. Anal. Chem. 2016, 88, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ling, Y.; Li, N.B.; Luo, H.Q. Fluorometric and resonance Rayleigh scattering dual-mode bioprobe for determination of the activity of alkaline phosphatase based on the use of CoOOH nanoflakes and cobalt(II)-dependent DNAzyme-assisted amplification. Microchim. Acta 2019, 186, 437. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Jiang, X.; Zhang, Z.H.; Li, J.; Liang, A.H.; Jiang, Z.L. A fluorescence and resonance Rayleigh scattering di-model probe was developed for trace K+ coupled N-doped carbon dot and aptamer. J. Lumin. 2019, 214, 116559. [Google Scholar] [CrossRef]
- Wen, G.Q.; Yang, D.; Jiang, Z.L. A new resonance Rayleigh scattering spectral method for determination of O3 with victoria blue B. Spectrochim. Acta Part A 2014, 117, 170–174. [Google Scholar] [CrossRef]
- Guo, Z.P. The pollution and detection of carbon monoxide. Environ. Sci. Techn. 2000, 13, 44. [Google Scholar]
- Phawachalotorn, C.; Sanguanruang, O.; Ishihara, T. Highly selective amperometric sensors for carbon monoxide detection in exhaust gas. Sens. Actuators B 2012, 161, 635–640. [Google Scholar] [CrossRef]
- Blondeau-Patissier, V.; Vanotti, M.; Pretre, T.; Rabus, D.; Tortora, L.; Barbe, J.M.; Ballandras, S. Detection and monitoring of carbon monoxide using cobalt corroles film on love wave devices with delay line configuration. Procedia Eng. 2011, 25, 1085–1088. [Google Scholar] [CrossRef]
- Kim, B.; Lu, Y.J.; Hannon, A.; Meyyappan, M.; Li, J. Low temperature Pd/SnO2 sensor for carbon monoxide detection. Sens. Actuators B 2013, 177, 770–775. [Google Scholar] [CrossRef]
- Boehm, G.; Bachmann, A.; Rosskopf, J.; Ortsiefer, M.; Chen, J.; Hangauer, A.; Meyer, R.; Strzoda, R.; Amann, M.C. Comparison of InP- and GaSb-based VCSELs emitting at 2.3 μm suitable for carbon monoxide detection. J. Crystal Growth 2011, 323, 442–445. [Google Scholar] [CrossRef]
- Wang, R.X.; Zhang, D.J.; Liu, C.B. The germanium-doped boron nitride nanotube serving as a potential resource for the detection of carbon monoxide and nitric oxide. Comput. Mater. Sci. 2014, 82, 361–366. [Google Scholar] [CrossRef]
- Ghosh, S.; Narjinary, M.; Sen, A.; Bandyopadhyay, R.; Roy, S. Fast detection of low concentration carbon monoxide using calcium-loaded tin oxide sensors. Sens. Actuators B 2014, 203, 490–496. [Google Scholar] [CrossRef]
- Chen, D.; Sun, Y.; Li, J.; Ren, J. Determination of carbon monoxide content in water during euthanasia of tilapia by dual-wavelength spectrophotometry method. Food Sci. Technol. 2011, 36, 283–286. [Google Scholar]
- Chen, C.; Ren, Q.; Piao, H.; Wang, P.; Wang, Y. A trace carbon monoxide sensor based on differential absorption spectroscopy using mid-infrared quantum cascade laser. Micromachines 2018, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- Zoltan, B.; Andras, G.; Krisztian, H.; Janos, M. Determination of carbon monoxide concentration and total pressure in gas cavities in the silica glass body of light bulbs by FT-IR spectrometry. Anal. Chem. 2006, 78, 2382–2387. [Google Scholar]
- Wang, P.; Huang, L.; Zhou, Y.; Zhao, H.; Fang, Y.; Xue, C. Determination of carbon monoxide in aquatic products by headspace gas chromatography. J. Zhejiang Ocean Univ. 2012, 31, 518–520. [Google Scholar]
- Wang, Z.; Liu, C.; Wang, X.; Duan, Q.; Jia, P.; Zhu, H.; Li, Z.; Zhang, X.; Ren, X.; Zhu, B.; et al. A metal-free near-infrared fluorescent probe for tracking the glucose induced fluctuations of carbon monoxide in living cells and zebrafish. Sens. Actuators B 2019, 291, 329–336. [Google Scholar] [CrossRef]
- Hao, H.X.; Zhou, H.; Liu, X.P.; Zhang, Z.; Yu, Z.S. An accurate method for microanalysis of carbon monoxide in putrid postmortem blood by head-space gas chromatography-mass spectrometry (HS/GC/MS). Forensic Sci. Int. 2013, 229, 116–121. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; MeGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- Dong, L.; Lewicki, R.; Liu, K.; Buerki, P.R.; Weida, M.J.; Tittel, F.K. Ultra-sensitive carbon monoxide detection by using EC-QCL based quartz-enhanced photoacoustic spectroscopy. Appl. Phys. B 2012, 107, 275–283. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, S.M.; Chang, J.B.; Leonidas, E.O.; Chen, J.H. Highly sensitive room temperature carbon monoxide detection using SnO2 nanoparticle-decorated semiconducting single–walled carbon nanotubes. Nanotechnology 2013, 24, 477–487. [Google Scholar]
- Khalil, A.I.; Gondal, A.M.; Al-Suliman, N. Resonant photo-acoustic detection of carbon monoxide with UV Laser at 213 nm. Appl. Phys. B 2011, 103, 441–450. [Google Scholar] [CrossRef]
- Antonaroli, S.; Crociani, B.; Natale, C.D.; Nardis, S.; Stefanelli, M.; Paolesse, R. Palladium complexes based nano gravimetric sensors for carbon monoxide detection. Sens. Actuators B 2015, 208, 334–338. [Google Scholar] [CrossRef]
- Selvapathy, P. Rapid spectrophotometric determination of carbon monoxide in ambient air. Environ. Protection Xinjiang 1992, 14, 44–48. [Google Scholar] [CrossRef]

| System | Methods | Regression Equation | LR (ng/mL) | Coefficient | DL (ng/mL) |
|---|---|---|---|---|---|
| HAuCl4 | SERS | ΔI = 0.019C + 7.4 | 30–5230 | 0.9598 | 20 ± 0.04 |
| RRS | ΔI = 0.0068C + 8.8 | 100–1500 | 0.9558 | 80 ± 0.13 | |
| HAuCl4-En | SERS | ΔI = 3.33C + 63.9 | 5.0–413 | 0.9800 | 3 ± 0.02 |
| RRS | ΔI = 2.84C + 45.8 | 10–413 | 0.9817 | 6 ± 0.05 | |
| Di-mode | ΔI = 6.33C + 62 | 3.0–413 | 0.9851 | 1 ± 0.02 |
| Samples | Sampling Temperature (°C) | Measured Value (mg/m3) | Average (mg/m3) | RSD (%) | Ref. Results (mg/m3) |
|---|---|---|---|---|---|
| 1 | 18 | Not detected | Not detected | - | Not detected |
| 2 | 20 | Not detected | Not detected | - | Not detected |
| 3 | 18 | Not detected | Not detected | - | Not detected |
| 4 | 20 | 0.0076 0.0074 0.0073 0.0075 0.0073 | 0.00752 | 1.8 | 0.00810 |
| 5 | 20 | 0.0039 0.004 0.0038 0.0039 0.0041 | 0.00394 | 2.6 | 0.0035 |
| 6 | 18 | 0.0011 0.0012 0.0012 0.0011 0.0012 | 0.00116 | 4.2 | 0.00120 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, D.; Wen, G.; Gong, L.; Li, C.; Liang, A.; Jiang, Z. A Highly Sensitive SERS and RRS Coupled Di-Mode Method for CO Detection Using Nanogolds as Catalysts and Bifunctional Probes. Nanomaterials 2020, 10, 450. https://doi.org/10.3390/nano10030450
Yao D, Wen G, Gong L, Li C, Liang A, Jiang Z. A Highly Sensitive SERS and RRS Coupled Di-Mode Method for CO Detection Using Nanogolds as Catalysts and Bifunctional Probes. Nanomaterials. 2020; 10(3):450. https://doi.org/10.3390/nano10030450
Chicago/Turabian StyleYao, Dongmei, Guiqing Wen, Lingbo Gong, Chongning Li, Aihui Liang, and Zhiliang Jiang. 2020. "A Highly Sensitive SERS and RRS Coupled Di-Mode Method for CO Detection Using Nanogolds as Catalysts and Bifunctional Probes" Nanomaterials 10, no. 3: 450. https://doi.org/10.3390/nano10030450
APA StyleYao, D., Wen, G., Gong, L., Li, C., Liang, A., & Jiang, Z. (2020). A Highly Sensitive SERS and RRS Coupled Di-Mode Method for CO Detection Using Nanogolds as Catalysts and Bifunctional Probes. Nanomaterials, 10(3), 450. https://doi.org/10.3390/nano10030450





