Effect of Dispersion Solvent on the Deposition of PVP-Silver Nanoparticles onto DBD Plasma-Treated Polyamide 6,6 Fabric and Its Antimicrobial Efficiency
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Dielectric Barrier Discharge (DBD) Plasma Treatment
2.3. Preparation of Silver Nanoparticles Dispersions and Its Deposition onto Polyamide 6,6 Fabric
2.4. Dynamic Light Scattering (DLS) Measurements
2.5. Diffuse Reflectance (%R)
2.6. X-ray Photoelectron Spectroscopy (XPS)
2.7. Scanning Electron Microscopy (SEM)
2.8. Antimicrobial Analyses
3. Results and Discussion
3.1. Dynamic Light Scattering (DLS)
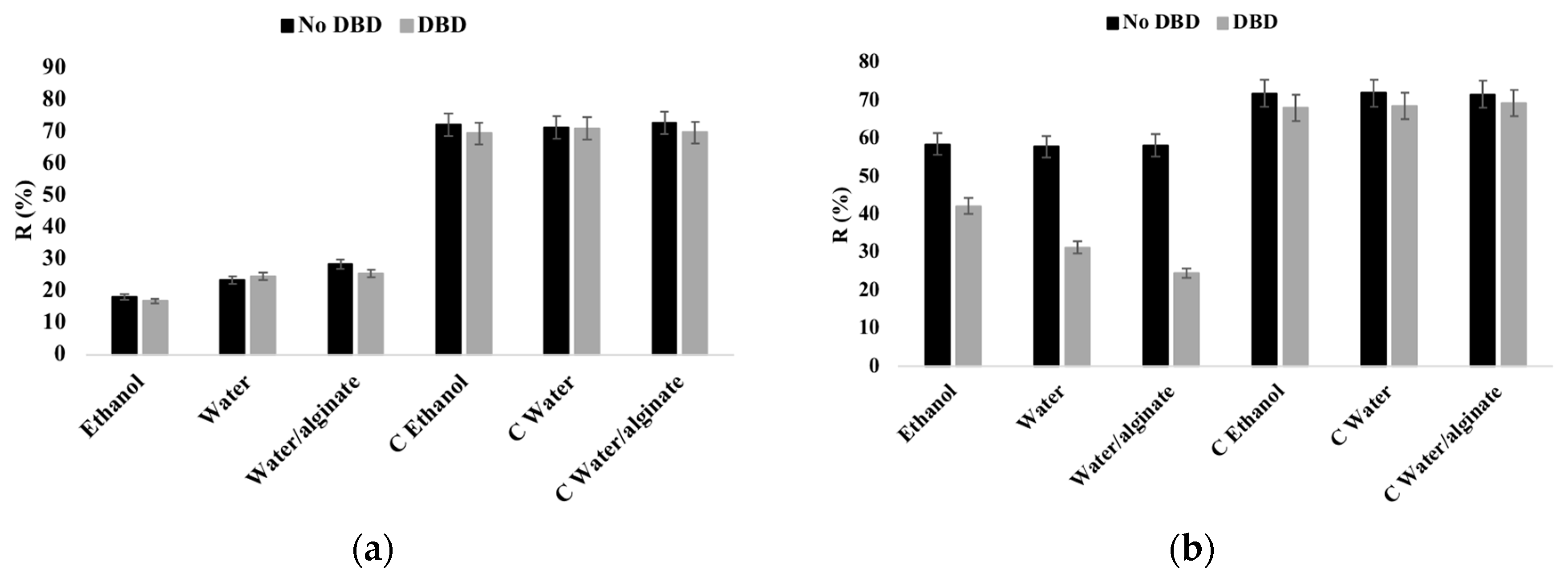
3.2. Reflectance
3.3. XPS
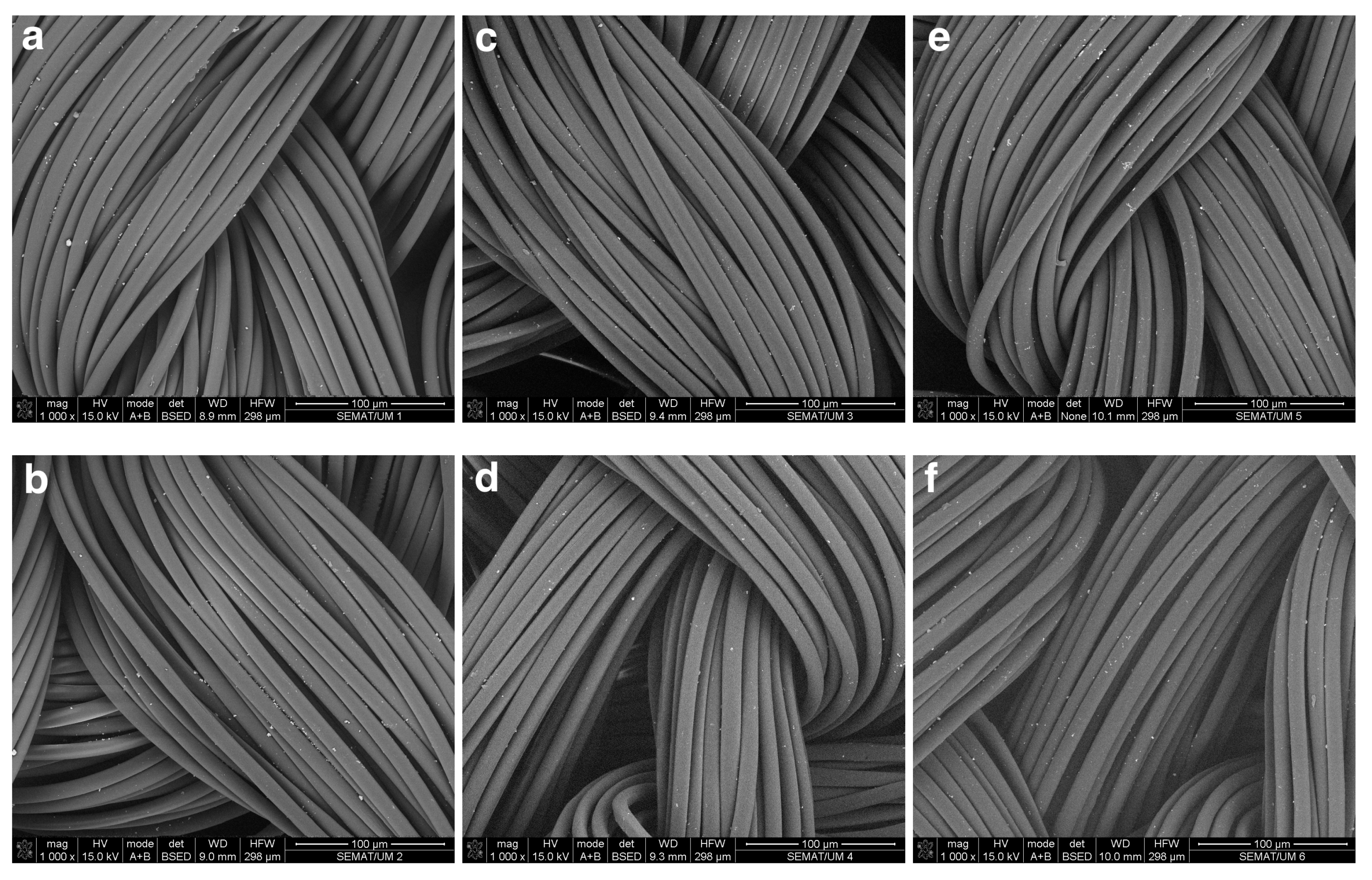
3.4. SEM
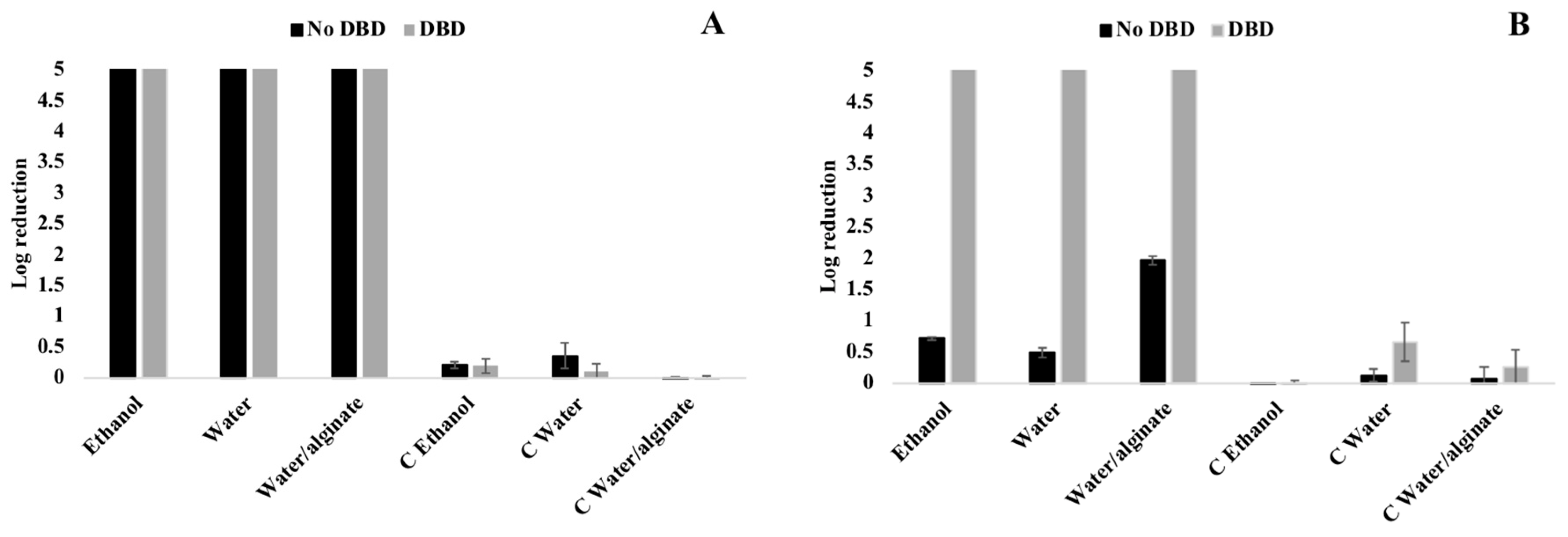
3.5. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Bapat, R.A.; Chaubal, T.V.; Joshi, C.P.; Bapat, P.R.; Choudhury, H.; Pandey, M.; Gorain, B.; Kesharwani, P. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater. Sci. Eng. C 2018, 91, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Zille, A.; Almeida, L.; Amorim, T.; Carneiro, N.; Esteves, M.F.; Silva, C.J.; Souto, A.P. Application of nanotechnology in antimicrobial finishing of biomedical textiles. Mater. Res. Express 2014, 1, 032003. [Google Scholar] [CrossRef] [Green Version]
- Zia, R.; Riaz, M.; Farooq, N.; Qamar, A.; Anjum, S. Antibacterial activity of Ag and Cu nanoparticles synthesized by chemical reduction method: A comparative analysis. Mater. Res. Express 2018, 5, 075012. [Google Scholar] [CrossRef]
- Kefallinou, D.; Ellinas, K.; Speliotis, T.; Stamatakis, K.; Gogolides, E.; Tserepi, A. Optimization of antibacterial properties of “hybrid” metal-sputtered superhydrophobic surfaces. Coatings 2019, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Petkova, P.; Francesko, A.; Perelshtein, I.; Gedanken, A.; Tzanov, T. Simultaneous sonochemical-enzymatic coating of medical textiles with antibacterial ZnO nanoparticles. Ultrason. Sonochem. 2016, 29, 244–250. [Google Scholar] [CrossRef]
- Dubas, S.T.; Wacharanad, S.; Potiyaraj, P. Tunning of the antimicrobial activity of surgical sutures coated with silver nanoparticles. Coll. Surf. A Physicochem. Eng. Asp. 2011, 380, 25–28. [Google Scholar] [CrossRef]
- Adlhart, C.; Verran, J.; Azevedo, N.F.; Olmez, H.; Keinänen-Toivola, M.M.; Gouveia, I.; Melo, L.F.; Crijns, F. Surface modifications for antimicrobial effects in the healthcare setting: A critical overview. J. Hosp. Infect. 2018, 99, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Rajaboopathi, S.; Thambidurai, S. Evaluation of UPF and antibacterial activity of cotton fabric coated with colloidal seaweed extract functionalized silver nanoparticles. J. Photochem. Photobiol. B Biol. 2018, 183, 75–87. [Google Scholar] [CrossRef]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Pareek, V.; Gupta, R.; Panwar, J. Do physico-chemical properties of silver nanoparticles decide their interaction with biological media and bactericidal action? A review. Mater. Sci. Eng. C 2018, 90, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Stebounova, L.V.; Guio, E.; Grassian, V.H. Silver nanoparticles in simulated biological media: A study of aggregation, sedimentation, and dissolution. J. Nanopart. Res. 2010, 13, 233–244. [Google Scholar] [CrossRef]
- Ajitha, B.; Kumar Reddy, Y.A.; Reddy, P.S.; Jeon, H.-J.; Ahn, C.W. Role of capping agents in controlling silver nanoparticles size, antibacterial activity and potential application as optical hydrogen peroxide sensor. RSC Adv. 2016, 6, 36171–36179. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Gharibshahi, L.; Saion, E.; Gharibshahi, E.; Shaari, A.H.; Matori, K.A. Influence of Poly(vinylpyrrolidone) concentration on properties of silver nanoparticles manufactured by modified thermal treatment method. PLoS ONE 2017, 12, e0186094. [Google Scholar] [CrossRef] [Green Version]
- Mitrano, D.M.; Lombi, E.; Dasilva, Y.A.R.; Nowack, B. Unraveling the complexity in the aging of nanoenhanced textiles: A comprehensive sequential study on the effects of sunlight and washing on silver nanoparticles. Environ. Sci. Technol. 2016, 50, 5790–5799. [Google Scholar] [CrossRef]
- Du, J.; Tang, J.; Xu, S.; Ge, J.; Dong, Y.; Li, H.; Jin, M. A review on silver nanoparticles-induced ecotoxicity and the underlying toxicity mechanisms. Regul. Toxicol. Pharmacol. 2018, 98, 231–239. [Google Scholar] [CrossRef]
- Hadrup, N.; Sharma, A.K.; Loeschner, K. Toxicity of silver ions, metallic silver, and silver nanoparticle materials after in vivo dermal and mucosal surface exposure: A review. Regul. Toxicol. Pharmacol. 2018, 98, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Stensberg, M.C.; Wei, Q.; McLamore, E.S.; Porterfield, D.M.; Wei, A.; Sepúlveda, M.S. Toxicological studies on silver nanoparticles: Challenges and opportunities in assessment, monitoring and imaging. Nanomedicine 2011, 6, 879–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, Z.; Xiong, Z.; Shu, X.; Liu, X.; Zhang, H.; Yin, X.; Zhou, Y.; Liu, M.; Zhang, M.; Xu, W.; et al. Multifunctionalization of cotton fabrics with polyvinylsilsesquioxane/ZnO composite coatings. Carbohydr. Polym. 2018, 199, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, D.; Brzeziński, S.; Kamińska, I. Multifunctional bioactive and improving the performance durability nanocoatings for finishing PET/CO woven fabrics by the sol–gel method. J. Alloy Compd. 2015, 649, 387–393. [Google Scholar] [CrossRef]
- Rehan, M.; Barhoum, A.; Van Assche, G.; Dufresne, A.; Gätjen, L.; Wilken, R. Towards multifunctional cellulosic fabric: UV photo-reduction and in-situ synthesis of silver nanoparticles into cellulose fabrics. Int. J. Biol. Macromol. 2017, 98, 877–886. [Google Scholar] [CrossRef]
- Peng, L.; Guo, R.; Lan, J.; Jiang, S.; Lin, S. Microwave-assisted deposition of silver nanoparticles on bamboo pulp fabric through dopamine functionalization. Appl. Surf. Sci. 2016, 386, 151–159. [Google Scholar] [CrossRef]
- Spielman-Sun, E.; Zaikova, T.; Dankovich, T.; Yun, J.; Ryan, M.; Hutchison, J.E.; Lowry, G.V. Effect of silver concentration and chemical transformations on release and antibacterial efficacy in silver-containing textiles. NanoImpact 2018, 11, 51–57. [Google Scholar] [CrossRef]
- Ren, Y.; Ding, Z.; Wang, C.; Zang, C.; Zhang, Y.; Xu, L. Influence of DBD plasma pretreatment on the deposition of chitosan onto UHMWPE fiber surfaces for improvement of adhesion and dyeing properties. Appl. Surf. Sci. 2017, 396, 1571–1579. [Google Scholar] [CrossRef]
- Borris, J.; Dohse, A.; Hinze, A.; Thomas, M.; Klages, C.-P.; Möbius, A.; Elbick, D.; Weidlich, E.-R. Improvement of the Adhesion of a Galvanic Metallization of Polymers by Surface Functionalization Using Dielectric Barrier Discharges at Atmospheric Pressure. Plasma Process. Polym. 2009, 6, S258–S263. [Google Scholar] [CrossRef]
- Sun, X.; DenHartog, E.; Zhang, X.; McCord, M. Study of poly(N-isopropylacrylamide) grafted cotton fabrics initiated by atmospheric pressure plasma. Appl. Surf. Sci. 2018, 453, 182–191. [Google Scholar] [CrossRef]
- Vinisha Rani, K.; Sarma, B.; Sarma, A. Plasma treatment on cotton fabrics to enhance the adhesion of Reduced Graphene Oxide for electro-conductive properties. Diam. Relat. Mater. 2018, 84, 77–85. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Souto, A.P.; Carneiro, N.; Nascimento, J.H.O. Surface Modification on Polyamide 6.6 with Double Barrier Discharge (DBD) Plasma to Optimise Dyeing Process by Direct Dyes. Mater. Sci. Forum 2010, 636, 846–852. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Woodhead Publishing: Cambridge, UK, 2017; pp. 43–58. [Google Scholar]
- Farrokhi-Rad, M.; Shahrabi, T. Effect of suspension medium on the electrophoretic deposition of hydroxyapatite nanoparticles and properties of obtained coatings. Ceram. Int. 2014, 40, 3031–3039. [Google Scholar] [CrossRef]
- Guan, Q.; Xia, C.; Li, W. Bio-friendly controllable synthesis of silver nanoparticles and their enhanced antibacterial property. Catal. Today 2019, 327, 196–202. [Google Scholar] [CrossRef]
- Yoon, H.J.; Jo, Y.; Jeong, S.; Lim, J.W.; Lee, S.-Y. Colored and semitransparent silver nanoparticle layers deposited by spin coating of silver nanoink. Appl. Phys. Express 2018, 11, 052302. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Senturk, D.; Silva, K.K.; Modic, M.; Cvelbar, U.; Dinescu, G.; Mitu, B.; Nikiforov, A.; Leys, C.; Kuchakova, I.; et al. Antimicrobial efficacy of low concentration PVP-silver nanoparticles deposited on DBD plasma-treated polyamide 6,6 fabric. Coatings 2019, 9, 581. [Google Scholar] [CrossRef] [Green Version]
- Zille, A.; Fernandes, M.M.; Francesko, A.; Tzanov, T.; Fernandes, M.; Oliveira, F.R.; Almeida, L.; Amorim, T.; Carneiro, N.; Esteves, M.F.; et al. Size and aging effects on antimicrobial efficiency of silver nanoparticles coated on polyamide fabrics activated by atmospheric DBD plasma. ACS Appl. Mater. Inter. 2015, 7, 13731–13744. [Google Scholar] [CrossRef]
- Bhatia, D.; Mittal, A.; Malik, D.K. Antimicrobial activity of PVP coated silver nanoparticles synthesized by Lysinibacillus varians. 3 Biotech 2016, 6, 196. [Google Scholar] [CrossRef] [Green Version]
- Takke, V.; Behary, N.; Perwuelz, A.; Campagne, C. Studies on the atmospheric air-plasma treatment of PET (polyethylene terephtalate) woven fabrics: Effect of process parameters and of aging. J. Appl. Polym. Sci. 2009, 114, 348–357. [Google Scholar] [CrossRef]
- Montazer, M.; Shamei, A.; Alimohammadi, F. Synthesizing and stabilizing silver nanoparticles on polyamide fabric using silver-ammonia/PVP/UVC. Prog. Org. Coat. 2012, 75, 379–385. [Google Scholar] [CrossRef]
- Ballal, D.; Chapman, W.G. Hydrophobic and hydrophilic interactions in aqueous mixtures of alcohols at a hydrophobic surface. J. Chem. Phys. 2013, 139, 114706. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Kim, K.; Nam, J.; Shim, S.E. Synthesis of silica-coated graphite by enolization of polyvinylpyrrolidone and its thermal and electrical conductivity in polymer composites. Carbon 2013, 60, 254–265. [Google Scholar] [CrossRef]
- Zanini, S.; Citterio, A.; Leonardi, G.; Riccardi, C. Characterization of atmospheric pressure plasma treated wool/cashmere textiles: Treatment in nitrogen. Appl. Surf. Sci. 2018, 427, 90–96. [Google Scholar] [CrossRef]
- Gorjanc, M.; Gorensek, M.; Jovancic, P.; Mozetic, M. Multifunctional Textiles—Modification by Plasma, Dyeing and Nanoparticles. In Eco-Friendly Textile Dyeing and Finishing; BoD–Books on Demand: Norderstedt, Germany, 2013. [Google Scholar]
- Liu, Y.; Chen, S.; Zhong, L.; Wu, G. Preparation of high-stable silver nanoparticle dispersion by using sodium alginate as a stabilizer under gamma radiation. Radiat. Phys. Chem. 2009, 78, 251–255. [Google Scholar] [CrossRef]
- Zhao, X.; Xia, Y.; Li, Q.; Ma, X.; Quan, F.; Geng, C.; Han, Z. Microwave-assisted synthesis of silver nanoparticles using sodium alginate and their antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 180–188. [Google Scholar] [CrossRef]
- Liu, C.Z.; Wu, J.Q.; Ren, L.Q.; Tong, J.; Li, J.Q.; Cui, N.; Brown, N.M.D.; Meenan, B.J. Comparative study on the effect of RF and DBD plasma treatment on PTFE surface modification. Mater. Chem. Phys. 2004, 85, 340–346. [Google Scholar] [CrossRef]
- Kohli, R. Methods for Monitoring and Measuring Cleanliness of Surfaces. In Developments in Surface Contamination and Cleaning; William Andrew: Pukekohe, New Zealand, 2012; pp. 107–178. [Google Scholar]
- Manakhov, A.; Michlíček, M.; Felten, A.; Pireaux, J.-J.; Nečas, D.; Zajíčková, L. XPS depth profiling of derivatized amine and anhydride plasma polymers: Evidence of limitations of the derivatization approach. Appl. Surf. Sci. 2017, 394, 578–585. [Google Scholar] [CrossRef]
- Montarsolo, A.; Varesano, A.; Mossotti, R.; Rombaldoni, F.; Periolatto, M.; Mazzuchetti, G.; Tonin, C. Enhanced adhesion of conductive coating on plasma-treated polyester fabric: A study on the ageing effect. J. Appl. Polym. Sci. 2012, 126, 1385–1393. [Google Scholar] [CrossRef]
- Khorshidi, B.; Thundat, T.; Fleck, B.A.; Sadrzadeh, M. A novel approach toward fabrication of high performance thin film composite polyamide membranes. Sci. Rep. 2016, 6, 22069. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Li, Z.; Chen, J.; Zhong, Y.; Ren, L.; Pu, Y.; Dong, Z.; Wu, H. Influence of blending zwitterionic functionalized titanium nanotubes on flux and anti-fouling performance of polyamide nanofiltration membranes. J. Mater. Sci. 2018, 53, 10499–10512. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. Characterization and optical studies of PVP-capped silver nanoparticles. J. Nanostruct. Chem. 2016, 7, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Pagliai, M.; Muniz-Miranda, F.; Schettino, V.; Muniz-Miranda, M. Competitive Solvation and Chemisorption in Silver Colloidal Suspensions. In UK Colloids 2011; Springer: Berlin/Heidelberg, Germany, 2012; pp. 39–44. [Google Scholar]
- Chahar, V.; Sharma, B.; Shukla, G.; Srivastava, A.; Bhatnagar, A. Study of antimicrobial activity of silver nanoparticles synthesized using green and chemical approach. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 149–155. [Google Scholar] [CrossRef]
- Barras, F.; Aussel, L.; Ezraty, B. Silver and antibiotic, new facts to an old story. Antibiotics 2018, 7, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, A.-L.; Capjak, I.; Vrček, I.V.; Bondarenko, O.M.; Kurvet, I.; Vija, H.; Ivask, A.; Kasemets, K.; Kahru, A. Antimicrobial potency of differently coated 10 and 50 nm silver nanoparticles against clinically relevant bacteria Escherichia coli and Staphylococcus aureus. Coll. Surf. B Biointerfaces 2018, 170, 401–410. [Google Scholar] [CrossRef] [PubMed]
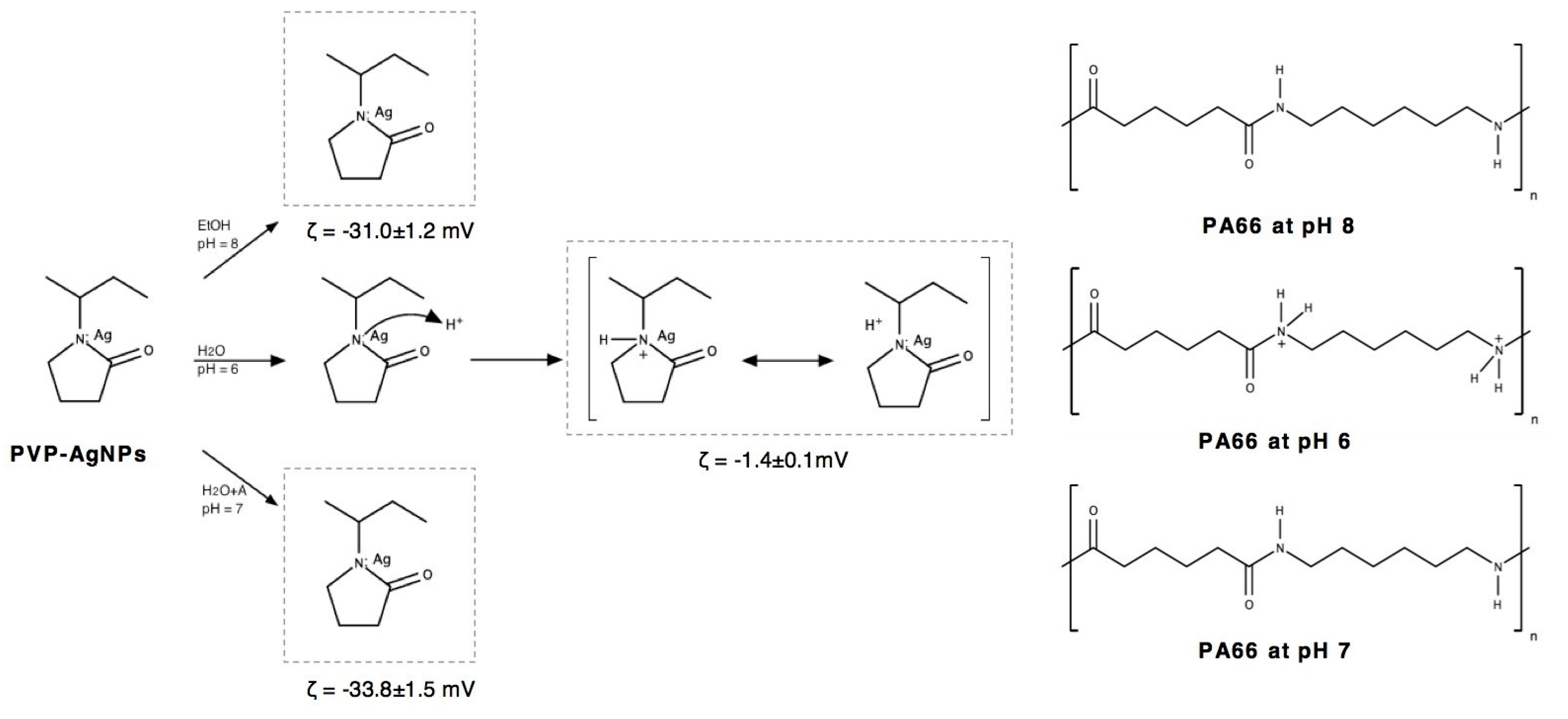


| PVP-AgNPs Dispersion | Size (d.nm) | ζ (mV) | PdI |
|---|---|---|---|
| Ethanol | 281.0 ± 1.5 | −31.0 ± 1.2 | 0.10 ± 0.02 |
| Water | 188.0 ± 0.7 | −1.40 ± 0.1 | 0.30 ± 0.02 |
| Water/Alginate | 156.6 ± 0.5 | −33.8 ± 1.5 | 0.40 ± 0.01 |
| Solvent | Samples | C (At%) | O (At%) | N (At%) | Ag (At%) | O/C ratio | N/C ratio |
|---|---|---|---|---|---|---|---|
| Water | Sp UT | 81.98 | 9.20 | 8.19 | 0.68 | 0.11 | 0.10 |
| Sp DBD | 81.05 | 10.64 | 8.03 | 0.28 | 0.13 | 0.10 | |
| Ex UT | 81.13 | 9.35 | 8.12 | 1.40 | 0.12 | 0.10 | |
| Ex DBD | 80.58 | 11.29 | 6.59 | 1.54 | 0.14 | 0.08 | |
| Ethanol | Sp UT | 82.41 | 9.05 | 8.31 | 0.23 | 0.11 | 0.10 |
| Sp DBD | 80.71 | 11.18 | 7.85 | 0.26 | 0.14 | 0.10 | |
| Ex UT | 84.48 | 6.83 | 5.20 | 3.49 | 0.08 | 0.06 | |
| Ex DBD | 81.60 | 10.19 | 5.91 | 2.30 | 0.12 | 0.07 |
| Solvent | Sample | C1s | O1s | N1s | Ag3d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 368.3 eV | 286.2 eV | 287.8 eV | 289.1 eV | 531.6 eV | 533.1 eV | 399.7 eV | 401.3 eV | 368.3 eV | 369.6 eV | 374.3 eV | 375.7 eV | ||
| Water | Sp UT | 48.6 | 16.9 | 14.3 | - | 75.6 | 24.4 | 91.7 | 8.3 | 48.6 | 10.8 | 33.3 | 7.3 |
| Sp DBD | 47.6 | 18.0 | 15.2 | 1.3 | 67.3 | 32.7 | 89.3 | 10.7 | 47.6 | 9.6 | 33.9 | 8.9 | |
| Ex UT | 49.4 | 15.3 | 13.9 | - | 77.1 | 22.9 | 44.6 | 55.4 | 49.4 | 10.5 | 32.9 | 7.3 | |
| Ex DBD | 45.1 | 22.5 | 14.9 | 3.3 | 65.8 | 34.2 | 84.4 | 15.6 | 45.1 | 14.3 | 33.2 | 7.4 | |
| Ethanol | Sp UT | 52.1 | 19.3 | 13.0 | - | 82.3 | 17.7 | 89.1 | 10.9 | 52.1 | 11.9 | 28.1 | 7.9 |
| Sp DBD | 47.9 | 16.8 | 13.6 | 1.7 | 75.0 | 25.0 | 85.2 | 14.8 | 47.9 | 14.2 | 25.0 | 13.0 | |
| Ex UT | 54.8 | 13.2 | 14.8 | - | 84.9 | 15.1 | 83.9 | 16.1 | 54.8 | 6.8 | 33.0 | 5.4 | |
| Ex DBD | 44.4 | 15.0 | 13.1 | - | 72.8 | 27.2 | 85.7 | 14.3 | 44.4 | 14.6 | 31.1 | 9.9 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.I.; Modic, M.; Cvelbar, U.; Dinescu, G.; Mitu, B.; Nikiforov, A.; Leys, C.; Kuchakova, I.; De Vrieze, M.; Felgueiras, H.P.; et al. Effect of Dispersion Solvent on the Deposition of PVP-Silver Nanoparticles onto DBD Plasma-Treated Polyamide 6,6 Fabric and Its Antimicrobial Efficiency. Nanomaterials 2020, 10, 607. https://doi.org/10.3390/nano10040607
Ribeiro AI, Modic M, Cvelbar U, Dinescu G, Mitu B, Nikiforov A, Leys C, Kuchakova I, De Vrieze M, Felgueiras HP, et al. Effect of Dispersion Solvent on the Deposition of PVP-Silver Nanoparticles onto DBD Plasma-Treated Polyamide 6,6 Fabric and Its Antimicrobial Efficiency. Nanomaterials. 2020; 10(4):607. https://doi.org/10.3390/nano10040607
Chicago/Turabian StyleRibeiro, Ana I., Martina Modic, Uros Cvelbar, Gheorghe Dinescu, Bogdana Mitu, Anton Nikiforov, Christophe Leys, Iryna Kuchakova, Mike De Vrieze, Helena P. Felgueiras, and et al. 2020. "Effect of Dispersion Solvent on the Deposition of PVP-Silver Nanoparticles onto DBD Plasma-Treated Polyamide 6,6 Fabric and Its Antimicrobial Efficiency" Nanomaterials 10, no. 4: 607. https://doi.org/10.3390/nano10040607
APA StyleRibeiro, A. I., Modic, M., Cvelbar, U., Dinescu, G., Mitu, B., Nikiforov, A., Leys, C., Kuchakova, I., De Vrieze, M., Felgueiras, H. P., Souto, A. P., & Zille, A. (2020). Effect of Dispersion Solvent on the Deposition of PVP-Silver Nanoparticles onto DBD Plasma-Treated Polyamide 6,6 Fabric and Its Antimicrobial Efficiency. Nanomaterials, 10(4), 607. https://doi.org/10.3390/nano10040607









