Fabrication and Characterization of a Novel Composite Magnetic Photocatalyst β-Bi2O3/BiVO4/MnxZn1−xFe2O4 for Rhodamine B Degradation under Visible Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of BV/MZF
2.2. Characterization
2.3. Photocatalytic Tests
3. Results and Discussion
3.1. Synthesis Optimization
3.2. Characterization on the Structure and Specific Surface Property
3.3. Magnetic Properties
3.4. UV-vis DRS
3.5. Stability and Reusability
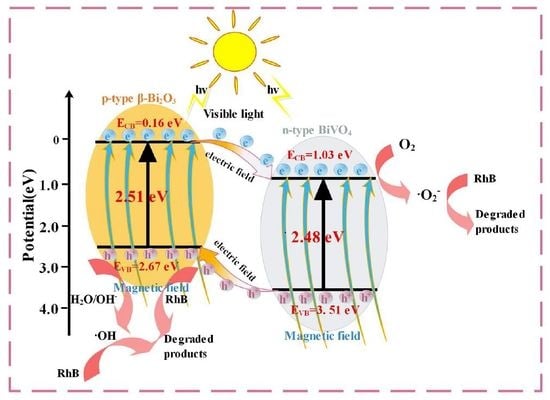
3.6. Degradation Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cui, M.-H.; Gao, L.; Lee, H.-S.; Wang, A.-J. Mixed dye wastewater treatment in a bioelectrochemical system-centered process. Bioresour. Technol. 2020, 297, 122420. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Hu, Y.; Xu, G.; Li, M.; Zhu, Y.; Jiang, L.; Tu, Y.; Zhu, X.; Xie, X.; Li, A. Green synthesis of a magnetic β-cyclodextrin polymer for rapid removal of organic micro-pollutants and heavy metals from dyeing wastewater. Environ. Res. 2020, 180, 108796. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Yuan, L.; Zhang, N.; Liu, D.; Meng, Y.; Peng, X. High-efficiency adsorption of methylene blue dye from wastewater by a thiosemicarbazide functionalized graphene oxide composite. Diam. Relat. Mater. 2020, 101, 107604. [Google Scholar] [CrossRef]
- Alias, S.S.; Harun, Z.; Azhar, F.H.; Ibrahim, S.A.; Johar, B. Comparison between commercial and synthesised nano flower-like rutile TiO2 immobilised on green super adsorbent towards dye wastewater treatment. J. Clean. Prod. 2020, 251, 119448. [Google Scholar] [CrossRef]
- Yang, S.; Feng, Y.; Liu, N.; Zhao, Y.; Wang, X.; Zhang, Z.; Chen, H.; Yu, Y. Enhancement on the removal of Rhodamine B (RhB) by means of the Enlarged Anode Electric Biological (EAEB) reactor. Chemosphere 2020, 245, 125566. [Google Scholar] [CrossRef]
- Feng, L.; Li, X.-y.; Gan, L.-h.; Xu, J. Synergistic effects of electricity and biofilm on Rhodamine B (RhB) degradation in three-dimensional biofilm electrode reactors (3D-BERs). Electrochim. Acta 2018, 290, 165–175. [Google Scholar] [CrossRef]
- Frindy, S.; Sillanpaa, M. Synthesis and application of novel α-Fe2O3/graphene for visible-light enhanced photocatalytic degradation of RhB. Mater. Des. 2019, 188, 108461. [Google Scholar] [CrossRef]
- Han, X.; Yang, X.; Liu, G.; Li, Z.; Shao, L. Boosting visible light photocatalytic activity via impregnation-induced RhB-sensitized MIL-125(Ti). Chem. Eng. Res. Des. 2019, 143, 90–99. [Google Scholar] [CrossRef]
- Cheng, X.; Shang, Y.; Cui, Y.; Shi, R.; Zhu, Y.; Yang, P. Enhanced photoelectrochemical and photocatalytic properties of anatase-TiO2(B) nanobelts decorated with CdS nanoparticles. Solid State Sci. 2020, 99, 106075. [Google Scholar] [CrossRef]
- Hashim, F.S.; Alkaim, A.F.; Mahdi, S.M.; Omran Alkhayatt, A.H. Photocatalytic degradation of GRL dye from aqueous solutions in the presence of ZnO/Fe2O3 nanocomposites. Compos. Commun. 2019, 16, 111–116. [Google Scholar] [CrossRef]
- Lei, X.; Xu, T.; Yao, W.; Wu, Q.; Zou, R. Hollow hydroxyapatite microspheres modified by CdS nanoparticles for efficiently photocatalytic degradation of tetracycline. J. Taiwan Inst. Chem. Eng. 2019, 106, 148–158. [Google Scholar] [CrossRef]
- Kiama, N.; Ponchio, C. Photoelectrocatalytic performance improvement of BiVO4 thin film fabrication via effecting of calcination temperature strategy. Surf. Coat. Technol. 2020, 383, 125257. [Google Scholar] [CrossRef]
- Guo, R.; Yan, A.; Xu, J.; Xu, B.; Li, T.; Liu, X.; Yi, T.; Luo, S. Effects of morphology on the visible-light-driven photocatalytic and bactericidal properties of BiVO4/CdS heterojunctions: A discussion on photocatalysis mechanism. J. Alloys Compd. 2020, 817, 153246. [Google Scholar] [CrossRef]
- Xie, T.; Li, H.; Liu, C.; Xu, L. Facile Synthesis of Magnetic Photocatalyst Ag/BiVO4/Mn1-xZnxFe2O4 and Its Highly Visible-Light-Driven Photocatalytic Activity. Materials 2018, 11, 810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samran, B.; Lunput, S.; Tonnonchiang, S.; Chaiwichian, S. BiFeO3/BiVO4 nanocomposite photocatalysts with highly enhanced photocatalytic activity for rhodamine B degradation under visible light irradiation. Phys. B Condens. Matter 2019, 561, 23–28. [Google Scholar] [CrossRef]
- Baral, B.; Reddy, K.H.; Parida, K.M. Construction of M-BiVO4/T-BiVO4 isotype heterojunction for enhanced photocatalytic degradation of Norfloxacine and Oxygen evolution reaction. J. Colloid Interface Sci. 2019, 554, 278–295. [Google Scholar] [CrossRef]
- Fakhrul Ridhwan Samsudin, M.; Sufian, S.; Bashiri, R.; Muti Mohamed, N.; Tau Siang, L.; Mahirah Ramli, R. Optimization of photodegradation of methylene blue over modified TiO2/BiVO4 photocatalysts: Effects of total TiO2 loading and different type of co-catalyst. Mater. Today Proc. 2018, 5, 21710–21717. [Google Scholar] [CrossRef]
- Feng, S.; Du, H.; Xie, T.; Xu, L.; Wang, Y. Preparation and photocatalytic activity of BiOI/MnxZn1-xFe2O4 magnetic photocatalyst. Ceram. Int. 2019, 45, 10468–10474. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, L.; Liu, C. Preparation and characterization of composite magnetic photocatalyst MnxZn1-xFe2O4/β-Bi2O3. RSC Adv. 2015, 5, 79997–80004. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, L.; Liu, C.; Zhang, Z. Synthesis and photocatalytic activity of composite magnetic photocatalyst MnxZn1-xFe2O4/α-Bi2O3. Mater. Technol. 2018, 34, 301–311. [Google Scholar] [CrossRef]
- Feng, S.; Xu, L.; Liu, C.; Du, H.; Xie, T.; Zhu, Q. Preparation and property of magnetic photocatalyst BiOCl/MnxZn1-xFe2O4. J. Nanopart. Res. 2017, 19, 33. [Google Scholar] [CrossRef]
- Xiang, W.; Long-jun, X.; Cheng-lun, L. Preparation and Properties of BiVO4-MnO2 Composite Photocatalysts. Fine Chem. 2019, 36, 1916–1922. [Google Scholar] [CrossRef]
- Wu, T.; Wang, W.; Xu, L.; Liu, C.; Xiang, W.; Lu, Y.; Wang, H.; Feng, Q.; Chen, H. MnxZn1-xFe2O4 coated with β-Bi2O3 and β-MnO2 as a highly efficient and magnetically recyclable photocatalyst for water treatment. Mater. Technol. 2019, 35, 317–325. [Google Scholar] [CrossRef]
- Wang, H.; Xu, L.; Liu, C.; Lu, Y.; Feng, Q.; Wu, T.; Wang, R. Composite Magnetic Photocatalyst Bi5O7I/MnxZn1−xFe2O4: Hydrothermal-Roasting Preparation and Excellent Photocatalytic Activity. Nanomaterials 2019, 9, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolić, S.D.; Jovanović, D.J.; Smits, K.; Babić, B.; Marinović-Cincović, M.; Porobić, S.; Dramićanin, M.D. A comparative study of photocatalytically active nanocrystalline tetragonal zyrcon-type and monoclinic scheelite-type bismuth vanadate. Ceram. Int. 2018, 44, 17953–17961. [Google Scholar] [CrossRef]
- Xie, T.; Liu, C.; Xu, L.; Li, H. New Insights into Mn1-xZnxFe2O4 via Fabricating Magnetic Photocatalyst Material BiVO4/Mn1-xZnxFe2O4. Materials 2018, 11, 335. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Xu, W.; Fang, J.; Xu, X.; Wu, S.; Zhu, X.; Chen, Z. Decoration of BiOI quantum size nanoparticles with reduced graphene oxide in enhanced visible-light-driven photocatalytic studies. Appl. Surf. Sci. 2012, 259, 441–447. [Google Scholar] [CrossRef]
- Xie, T.; Li, H.; Liu, C.; Yang, J.; Xiao, T.; Xu, L. Magnetic Photocatalyst BiVO4/Mn-Zn ferrite/Reduced Graphene Oxide: Synthesis Strategy and Its Highly Photocatalytic Activity. Nanomaterials 2018, 8, 380. [Google Scholar] [CrossRef] [Green Version]
- Xie, T.; Liu, C.; Xu, L.; Yang, J.; Zhou, W. Novel Heterojunction Bi2O3/SrFe12O19 Magnetic Photocatalyst with Highly Enhanced Photocatalytic Activity. J. Phys. Chem. C 2013, 117, 24601–24610. [Google Scholar] [CrossRef]
- Yuejun, L.I.; Cao, T.; Zhang, J. Synthesis Characterization and Photocatalysis of the Bi2O3 Nanofibers. J. Hebei Norm. Univ. 2011, 35, 598. [Google Scholar] [CrossRef]
- Rezaei, A.; Saffari, J.; Nabiyouni, G.; Ghanbari, D. Magnetic and photo-catalyst BaFe12O19-ZnO: Hydrothermal preparation of barium ferrite nanoparticles and hexagonal zinc oxide nanostructures. J. Mater. Sci. Mater. Electron. 2017, 28, 6607–6618. [Google Scholar] [CrossRef]
- Lutz, A.; Malet, L.; Dille, J.; de Almeida, L.H.; Lapeire, L.; Verbeken, K.; Godet, S.; Terryn, H.; De Graeve, I. Effect of Zn on the grain boundary precipitates and resulting alkaline etching of recycled Al-Mg-Si-Cu alloys. J. Alloys Compd. 2019, 794, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Xie, T.; Xu, L.; Liu, C.; Wang, Y. Magnetic composite ZnFe2O4/SrFe12O19: Preparation, characterization, and photocatalytic activity under visible light. Appl. Surf. Sci. 2013, 273, 684–691. [Google Scholar] [CrossRef]
- Lu, J.; Jin, H.; Dai, Y.; Yang, K.; Huang, B. Effect of Electronegativity and Charge Balance on the Visible-Light-Responsive Photocatalytic Activity of Nonmetal Doped Anatase TiO2. Int. J. Photoenergy 2012, 2012, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mao, M.M.; Chen, F.; Zheng, C.C.; Ning, J.Q.; Zhong, Y.J.; Hu, Y. Facile synthesis of porous Bi2O3-BiVO4 p-n heterojunction composite microrods with highly efficient photocatalytic degradation of phenol. J. Alloys Compd. 2016, 688, 1080–1087. [Google Scholar] [CrossRef]
- Li, W.; Cui, X.; Wang, P.; Shao, Y.; Li, D.; Teng, F. Enhanced photosensitized degradation of rhodamine B on CdS/TiO2 nanocomposites under visible light irradiation. Mater. Res. Bull. 2013, 48, 3025–3031. [Google Scholar] [CrossRef]












| Samples | BET Surface/m2∙g−1 | Langmuir Surface Area/m2∙g−1 | Pore Size/nm |
|---|---|---|---|
| BiVO4 | 6.00 | 4.35 | 6.09 |
| β-Bi2O3 | 11.81 | 11.34 | 6.29 |
| MZF | 53.42 | 55.60 | 15.77 |
| BV/MZF | 17.84 | 17.39 | 9.49 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Yang, Y.; Jiang, Z.; Xu, L.; Liu, C. Fabrication and Characterization of a Novel Composite Magnetic Photocatalyst β-Bi2O3/BiVO4/MnxZn1−xFe2O4 for Rhodamine B Degradation under Visible Light. Nanomaterials 2020, 10, 797. https://doi.org/10.3390/nano10040797
Cheng Y, Yang Y, Jiang Z, Xu L, Liu C. Fabrication and Characterization of a Novel Composite Magnetic Photocatalyst β-Bi2O3/BiVO4/MnxZn1−xFe2O4 for Rhodamine B Degradation under Visible Light. Nanomaterials. 2020; 10(4):797. https://doi.org/10.3390/nano10040797
Chicago/Turabian StyleCheng, Yong, Yahan Yang, Zao Jiang, Longjun Xu, and Chenglun Liu. 2020. "Fabrication and Characterization of a Novel Composite Magnetic Photocatalyst β-Bi2O3/BiVO4/MnxZn1−xFe2O4 for Rhodamine B Degradation under Visible Light" Nanomaterials 10, no. 4: 797. https://doi.org/10.3390/nano10040797