Preparation and Characterization of Graphene Oxide for Pb(II) and Zn(II) Ions Adsorption from Aqueous Solution: Experimental, Thermodynamic and Kinetic Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
Synthesis of Graphene Oxide
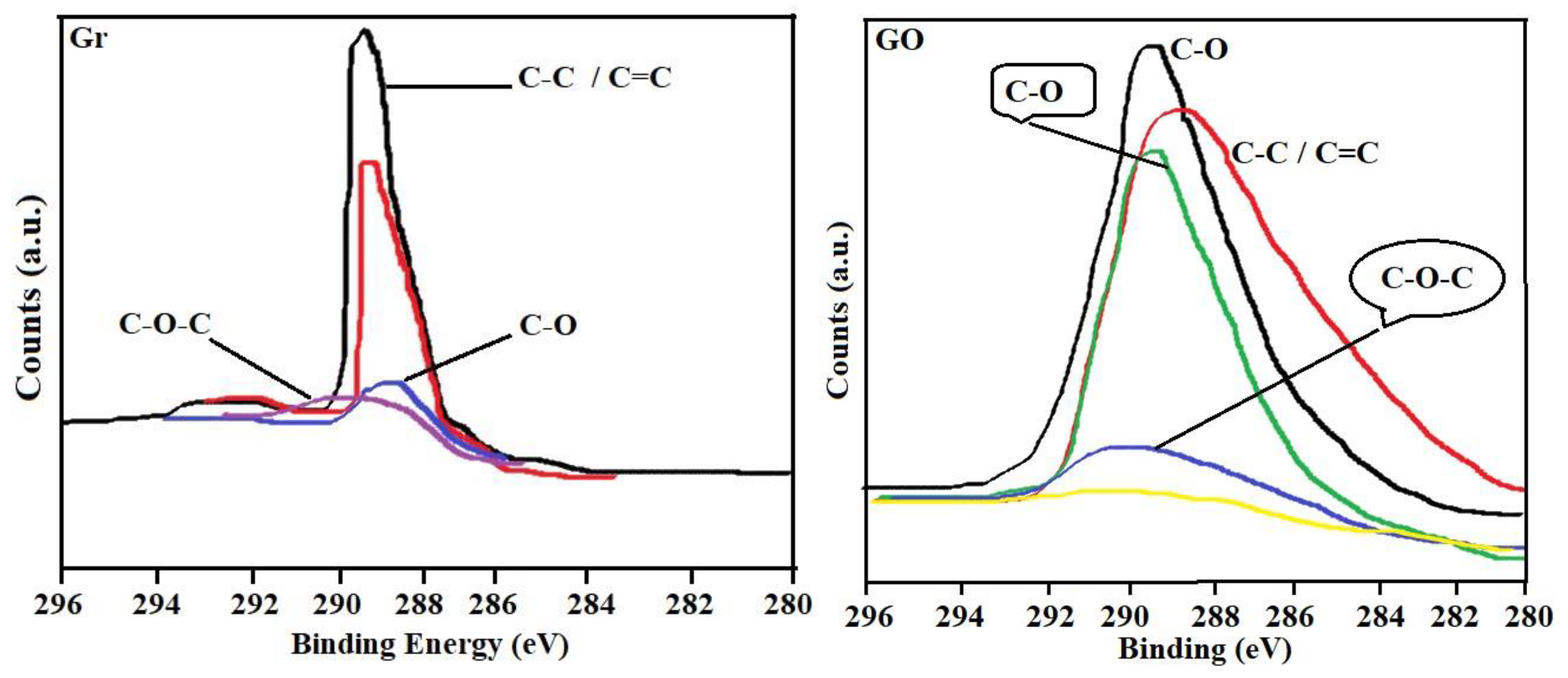
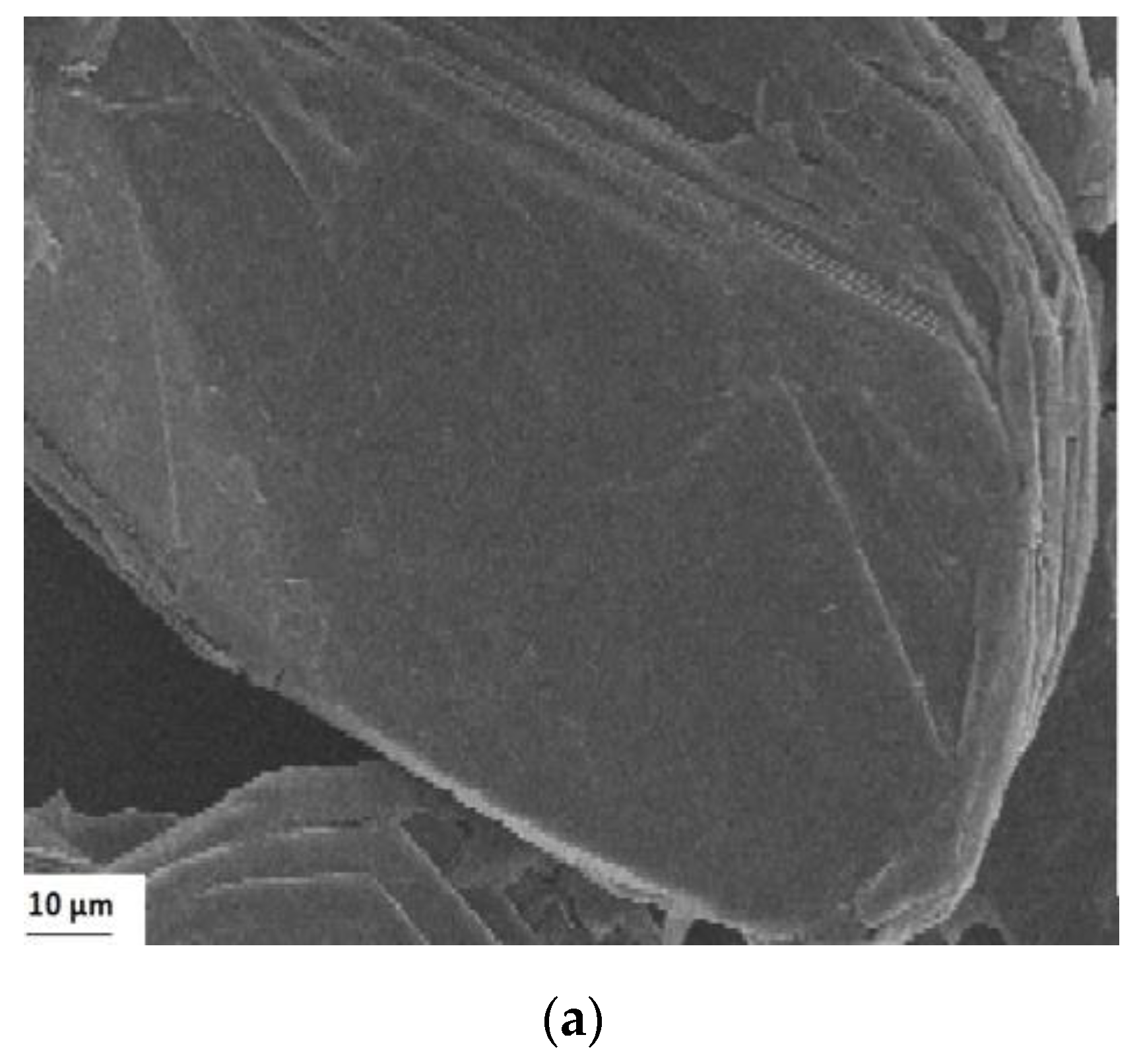
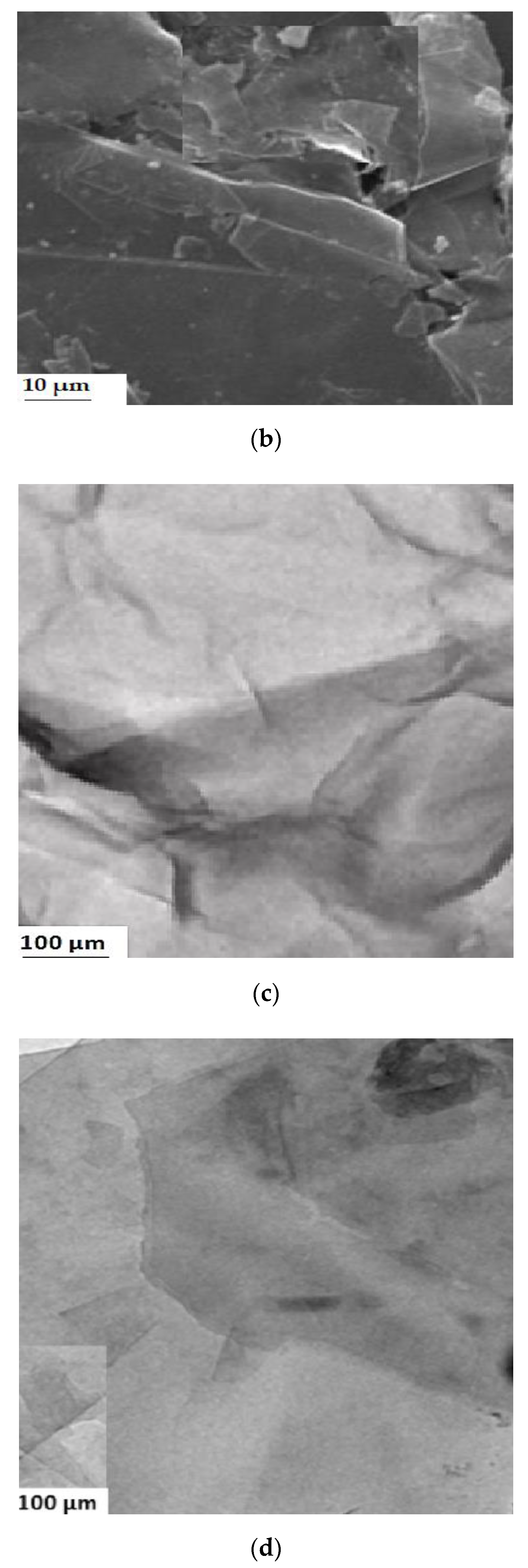
2.2. Graphene Oxide Characterization
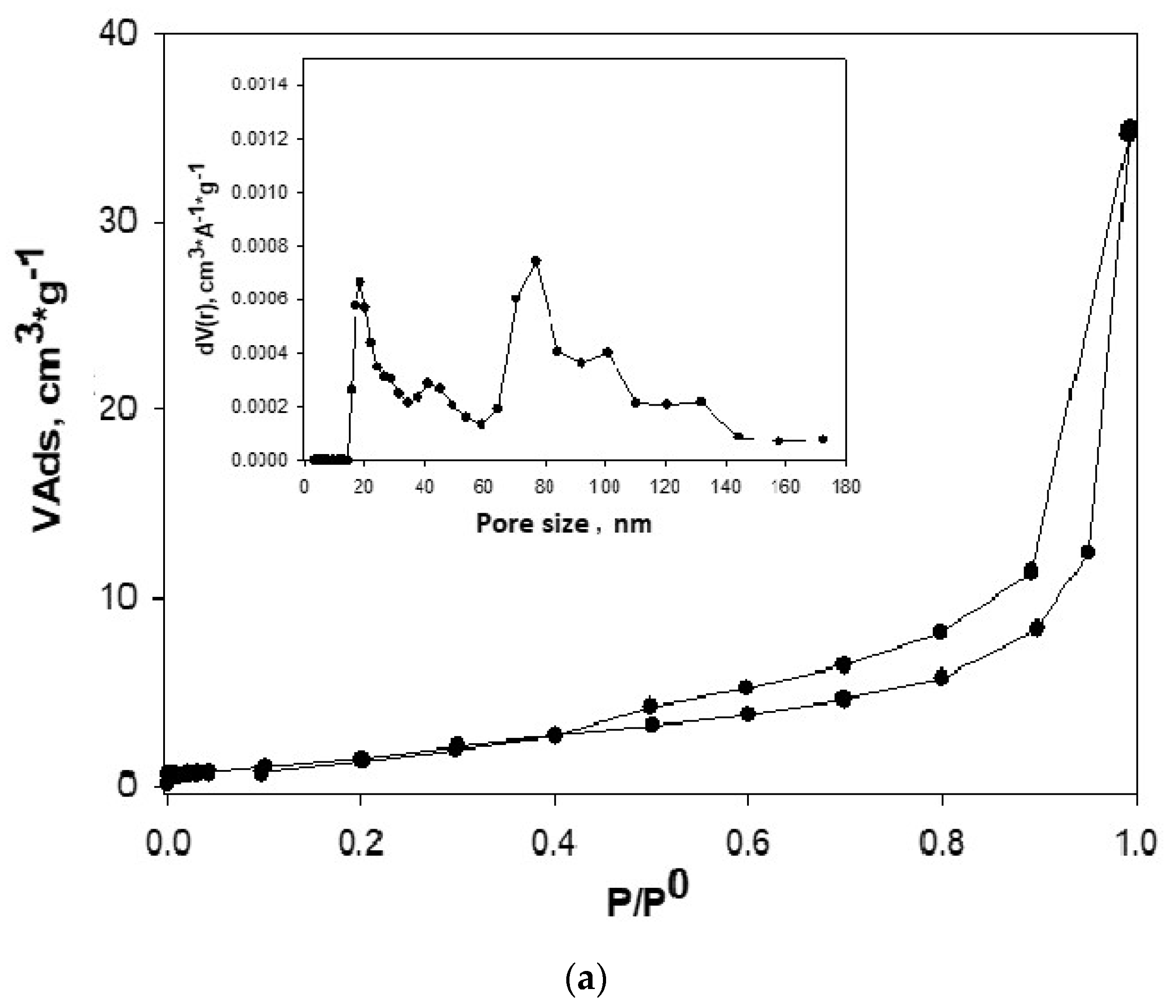
2.3. Analysis of N2 Adsorption Isotherms at 77 K
2.4. Adsorbate–Adsorbent Interaction Studies
2.4.1. Zn (II) and Pb (II) Ions Adsorption Capacity in Batch on GO
2.4.2. Kinetic and Thermodynamic Study of the Adsorption of Zn(II) and Pb(II) Ions on Graphene Oxide
3. Results
3.1. Characterization of Adsorbates
3.2. Analysis of N2 Adsorption Isotherms at 77 K
3.3. Analysis of the Adsorption Studies of Zn (II) and Pb (II) Ions on GO
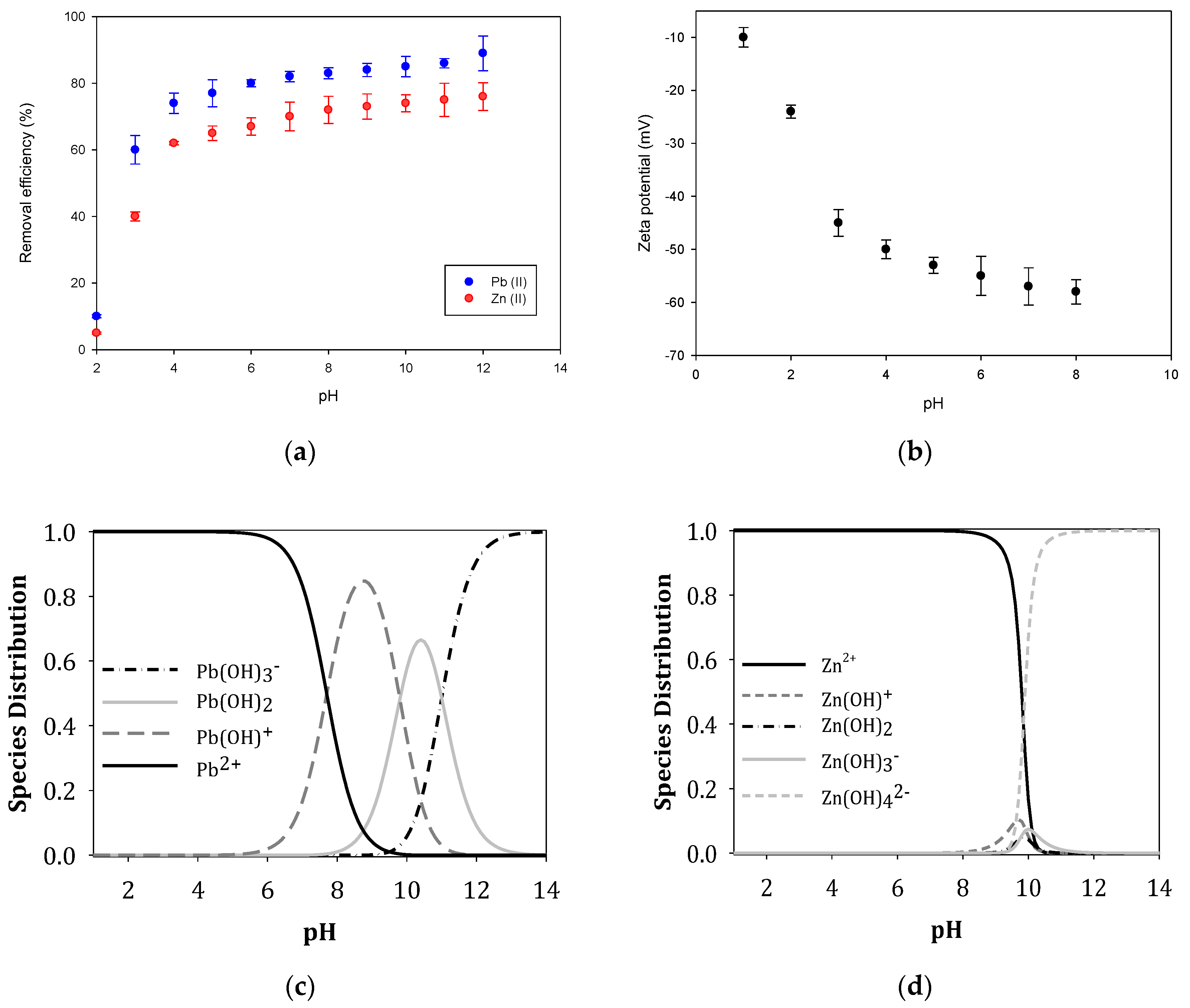
3.3.1. Effect of pH of Pb (II) and Zn (II) Ions on GO Surface
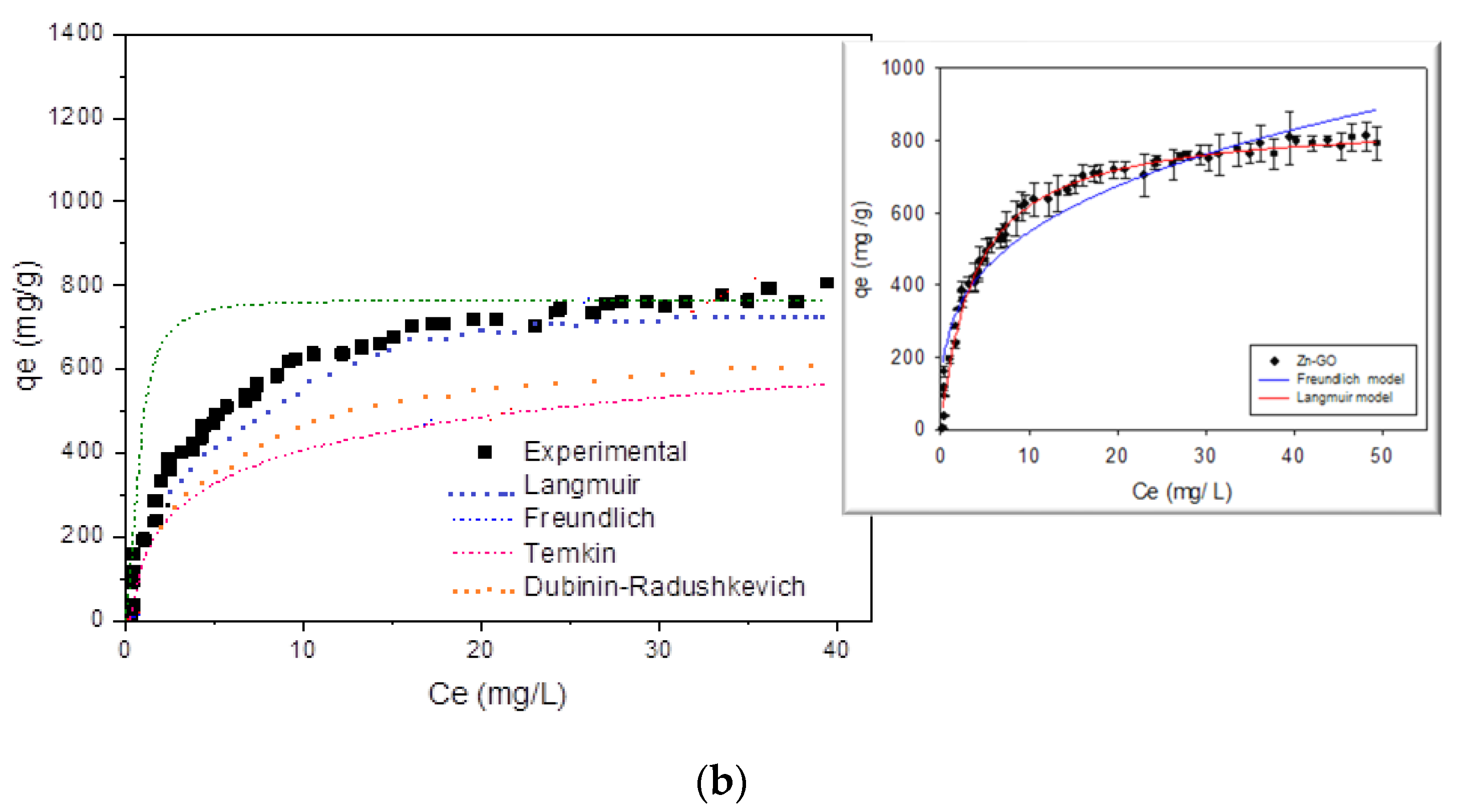
3.3.2. Analysis of Results of the Adsorption Isotherms for Pb(II) and Zn(II) Ions on GO
Langmuir Model
Freundlich Model
Dubinin–Radushkevich (DR) Isotherm Model
Temkin Model
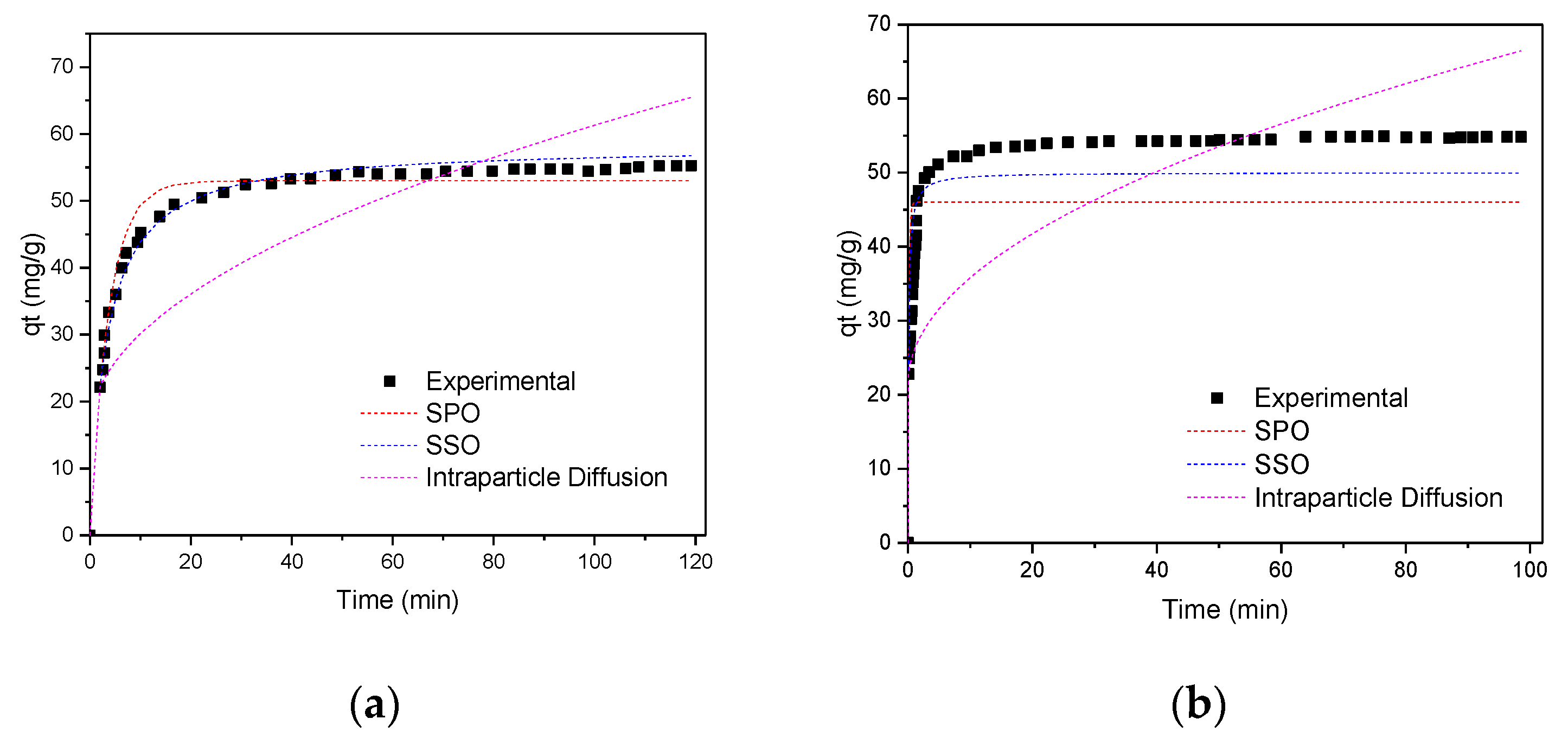
3.3.3. Studies of the Adsorption Kinetics of Pb (II) and Zn (II) Ions on GO
3.3.4. Analysis of Thermodynamic Parameters in the Adsorbate–Adsorbent System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kong, Q.; Preis, S.; Li, L.; Luo, P.; Wei, C.; Li, Z.; Hu, Y.; Wei, C. Relations between metal ion characteristics and adsorption performance of graphene oxide: A comprehensive experimental and theoretical study. Sep. Purif. Technol. 2020, 232, 115956. [Google Scholar] [CrossRef]
- Radu, V.M.; Ionescu, P.; Diacu, E.; Ivanov, A.A. Ivanov Removal of heavy metals from aquatic environments using water hyacinth and water lettuce. Rev. Chim. 2017, 68, 2765–2767. [Google Scholar] [CrossRef]
- Kong, Q.; Li, Z.; Zhao, Y.; Wei, C.; Qiu, G.; Wei, C. Investigation of the fate of heavy metals based on process regulation-chemical reaction-phase distribution in an A-O1-H-O2 biological coking wastewater treatment system. J. Environ. Manag. 2019, 247, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, C.; Chen, Y.; Li, J.; Wang, D.; Wang, X.; Hu, W. Competitive adsorption of PbII, NiII, and SrII ions on graphene oxides: A combined experimental and theoretical study. ChemPlusChem 2015, 80, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, L.; Wang, L.; Fan, Q.; Pan, D.; Li, J.; Chi, F.; Xie, Y.; Yu, S.; Xiao, C.; et al. Synthesis of novel nanomaterials and their application in efficient removal of radionuclides. Sci. China Chem. 2019, 62, 933–967. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Katsnelson, M.I. Modeling of graphite oxide. J. Am. Chem. Soc. 2008, 130, 10697–10701. [Google Scholar] [CrossRef]
- Casabianca, L.B.; Shaibat, M.A.; Cai, W.W.; Park, S.; Piner, R.; Ruoff, R.S.; Ishii, Y. NMR-based structural modeling of graphite oxide using multidimensional 13C solid-state NMR and ab initio chemical shift calculations. J. Am. Chem. Soc. 2010, 132, 5672–5676. [Google Scholar] [CrossRef]
- Guerrero-Contreras, J.; Caballero-Briones, F. Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method. Mater. Chem. Phys. 2015, 153, 209–220. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon N. Y. 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Allahbakhsh, A.; Haghighi, A.; Sheydaei, M. Poly(ethylene trisulfide)/graphene oxide nanocomposites. J. Therm. Anal. Calorim. 2017, 128, 427–442. [Google Scholar] [CrossRef]
- Justh, N.; Berke, B.; László, K.; Szilágyi, I.M. Thermal analysis of the improved Hummers’ synthesis of graphene oxide. J. Therm. Anal. Calorim. 2018, 131, 2267–2272. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Pang, H.; Yu, S.; Ai, Y.; Ma, X.; Song, G.; Hayat, T.; Alsaedi, A.; Wang, X. Effect of graphene oxide surface modification on the elimination of Co(II) from aqueous solutions. Chem. Eng. J. 2018, 344, 380–390. [Google Scholar] [CrossRef]
- Kong, Q.; Wei, J.; Hu, Y.; Wei, C. Fabrication of terminal amino hyperbranched polymer modified graphene oxide and its prominent adsorption performance towards Cr(VI). J. Hazard. Mater. 2019, 363, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Wei, C.; Preis, S.; Hu, Y.; Wang, F. Facile preparation of nitrogen and sulfur co-doped graphene-based aerogel for simultaneous removal of Cd2+ and organic dyes. Environ. Sci. Pollut. Res. 2018, 25, 21164–21175. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, X.; Yao, W.; Wang, J.; Ji, Y.; Ai, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Macroscopic, Spectroscopic, and Theoretical Investigation for the Interaction of Phenol and Naphthol on Reduced Graphene Oxide. Environ. Sci. Technol. 2017, 51, 3278–3286. [Google Scholar] [CrossRef]
- Moon, H.S.; Lee, J.H.; Kwon, S.; Kim, I.T.; Lee, S.G. Mechanisms of na adsorption on graphene and graphene oxide: Density functional theory approach. Carbon Lett. 2015, 16, 116–120. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef]
- Yang, S.T.; Chang, Y.; Wang, H.; Liu, G.; Chen, S.; Wang, Y.; Liu, Y.; Cao, A. Folding/aggregation of graphene oxide and its application in Cu2+ removal. J. Colloid Interface Sci. 2010, 351, 122–127. [Google Scholar] [CrossRef]
- Sitko, R.; Turek, E.; Zawisza, B.; Malicka, E.; Talik, E.; Heimann, J.; Gagor, A.; Feist, B.; Wrzalik, R. Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalt. Trans. 2013, 42, 5682–5689. [Google Scholar] [CrossRef]
- Zhao, G.; Ren, X.; Gao, X.; Tan, X.; Li, J.; Chen, C.; Huang, Y.; Wang, X. Removal of Pb(ii) ions from aqueous solutions on few-layered graphene oxide nanosheets. Dalt. Trans. 2011, 40, 10945–10952. [Google Scholar] [CrossRef]
- Oliveira, M.M.; Zardin, G.J. Nanoestrutura de Carbono (Nanotubos, Grafeno): Quo Vadis? Quim. Nova 2013, 36, 1533–1539. [Google Scholar]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Cheng, H.M. The reduction of graphene oxide. Carbon N. Y. 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhen, Z.; Zhu, H. Graphene: Fundamental research and potential applications. FlatChem 2017, 4, 20–32. [Google Scholar] [CrossRef]
- Khan, Z.U.; Kausar, A.; Ullah, H.; Badshah, A.; Khan, W.U. A review of graphene oxide, graphene buckypaper, and polymer/graphene composites: Properties and fabrication techniques. J. Plast. Film Sheeting 2016, 32, 336–379. [Google Scholar] [CrossRef]
- de Jesus, K.A.; Freire, E.; Guimarães, M.J. Grafeno: Aplicações e Tendências Tecnológicas. Dep. Process. Orgânicos 2012, 737, 14–19. [Google Scholar]
- Mehl, H.; Matos, C.F.; Neiva, E.G.C.; Domingues, S.H.; Zarbin, A.J.G. Efeito da variacão de parâmetros reacionais na preparacão de grafeno via oxidacão e reducão do grafite. Quim. Nova 2014, 37, 1639–1645. [Google Scholar] [CrossRef]
- Moo, J.G.S.; Khezri, B.; Webster, R.D.; Pumera, M. Graphene Oxides Prepared by Hummers’, Hofmann’s, and Staudenmaier’s Methods: Dramatic Influences on Heavy-Metal-Ion Adsorption. ChemPhysChem 2014, 15, 2922–2929. [Google Scholar] [CrossRef]
- López, M.D.P.L.; Palomino, J.L.V.; Silva, M.L.S.; Izquierdo, A.R. Optimization of the Synthesis Procedures of Graphene and Graphite Oxide. Recent Adv. Graphene Res. 2016, 12, 113–133. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef]
- Chen, T.; Zeng, B.; Liu, J.L.; Dong, J.H.; Liu, X.Q.; Wu, Z.; Yang, X.Z.; Li, Z.M. High throughput exfoliation of graphene oxide from expanded graphite with assistance of strong oxidant in modified Hummers method. J. Phys. Conf. Ser. 2009, 188, 12051. [Google Scholar] [CrossRef]
- Subrahmanyam, K.S.; Vivekchand, S.R.C.; Govindaraj, A.; Rao, C.N.R. A study of graphenes prepared by different methods: Characterization, properties and solubilization. J. Mater. Chem. 2008, 18, 1517–1523. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Sood, A.K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene: The new two-dimensional nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yan, L.; Bangal, P.R. Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon N. Y. 2010, 48, 1146–1152. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Hou, W.C.; Chowdhury, I.; Goodwin, D.G.; Henderson, W.M.; Fairbrother, D.H.; Bouchard, D.; Zepp, R.G. Photochemical transformation of graphene oxide in sunlight. Environ. Sci. Technol. 2015, 49, 3435–3443. [Google Scholar] [CrossRef]
- Li, H.; Bubeck, C. Photoreduction processes of graphene oxide and related applications. Macromol. Res. 2013, 21, 290–297. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Gallegos-Pérez, W.R.; Reynosa-Martínez, A.C.; Soto-Ortiz, C.; Angélica Álvarez-Lemus, M.; Barroso-Flores, J.; García Montalvo, V.; López-Honorato, E. Effect of UV radiation on the structure of graphene oxide in water and its impact on cytotoxicity and As(III) adsorption. Chemosphere 2020, 249, 126160. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Emmett, P.H.; Brunauer, S. The Use of Low Temperature van der Waals Adsorption Isotherms in Determining the Surface Area of Iron Synthetic Ammonia Catalysts. J. Am. Chem. Soc. 1937, 57, 1754–1755. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H. The Use of Low Temperature van der Waals Adsorption Isotherms in Determining the Surface Areas of Various Adsorbents. J. Am. Chem. Soc. 1937, 59, 2682–2689. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Carrott, P.J.M.; Ribeiro Carrott, M.M.L. Suhas Comparison of the Dubinin-Radushkevich and Quenched Solid Density Functional Theory approaches for the characterisation of narrow microporosity in activated carbons obtained by chemical activation with KOH or NaOH of Kraft and hydrolytic lignins. Carbon N. Y. 2010, 48, 4162–4169. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Neimark, A.V. Density functional theory model of adsorption on amorphous and microporous silica materials. Langmuir 2006, 22, 11171–11179. [Google Scholar] [CrossRef]
- Neimark, A.V.; Lin, Y.; Ravikovitch, P.I.; Thommes, M. Quenched solid density functional theory and pore size analysis of micro-mesoporous carbons. Carbon N. Y. 2009, 47, 1617–1628. [Google Scholar] [CrossRef]
- Olivier, J.P. Improving the models used for calculating the size distribution of micropore volume of activated carbons from adsorption data. Carbon N. Y. 1998, 36, 1469–1472. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Vishnyakov, A.; Russo, R.; Neimark, A.V. Unified approach to pore size characterization of microporous carbonaceous materials from N2, Ar, and CO2 adsorption isotherms. Langmuir 2000, 16, 2311–2319. [Google Scholar] [CrossRef]
- Stoeckli, F.; Guillot, A.; Slasli, A.M.; Hugi-Cleary, D. Microporosity in carbon blacks. Carbon N. Y. 2002, 40, 211–215. [Google Scholar] [CrossRef]
- Stoeckli, F.; Slasli, A.; Hugi-Cleary, D.; Guillot, A. The characterization of microporosity in carbons with molecular sieve effects. Microporous Mesoporous Mater. 2002, 51, 197–202. [Google Scholar] [CrossRef]
- Stoeckli, H.F.; Rebstein, P.; Ballerini, L. On the assessment of microporosity in active carbons, a comparison of theoretical and experimental data. Carbon N. Y. 1990, 28, 907–909. [Google Scholar] [CrossRef]
- Gegg, S.J.; Sing, K.S.W. Adsorption Surface Area and Porosity; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Seifi, A.; Bahramian, A.R.; Sharif, A. Correlation between structure and oxidation behavior of carbon aerogels. J. Energy Storage 2016, 7, 195–203. [Google Scholar] [CrossRef]
- Thommes, M.; Cychosz, K.A.; Neimark, A.V. Advanced Physical Adsorption Characterization of Nanoporous Carbons. In Novel Carbon Adsorbents; Tascon, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 107–145. ISBN 9780080977447. [Google Scholar]
- Yari, M.; Rajabi, M.; Moradi, O.; Yari, A.; Asif, M.; Agarwal, S.; Gupta, V.K. Kinetics of the adsorption of Pb(II) ions from aqueous solutions by graphene oxide and thiol functionalized graphene oxide. J. Mol. Liq. 2015, 209, 50–57. [Google Scholar] [CrossRef]
- Vuković, G.D.; Marinković, A.D.; Škapin, S.D.; Ristić, M.T.; Aleksić, R.; Perić-Grujić, A.A.; Uskoković, P.S. Removal of lead from water by amino modified multi-walled carbon nanotubes. Chem. Eng. J. 2011, 173, 855–865. [Google Scholar] [CrossRef]
- Moradi, O.; Norouzi, M.; Fakhri, A.; Naddafi, K. Interaction of removal Ethidium Bromide with Carbon Nanotube: Equilibrium and Isotherm studies. J. Environ. Health Sci. Eng. 2014, 12, 17–26. [Google Scholar] [CrossRef]
- Ren, X.; Shao, D.; Yang, S.; Hu, J.; Sheng, G.; Tan, X.; Wang, X. Comparative study of Pb(II) sorption on XC-72 carbon and multi-walled carbon nanotubes from aqueous solutions. Chem. Eng. J. 2011, 170, 170–177. [Google Scholar] [CrossRef]
- Algothmi, W.M.; Bandaru, N.M.; Yu, Y.; Shapter, J.G.; Ellis, A.V. Alginate-graphene oxide hybrid gel beads: An efficient copper adsorbent material. J. Colloid Interface Sci. 2013, 397, 32–38. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Y.; Shi, M.; Lu, X.; Qin, C.; Li, C.; Ye, M. Fast and facile preparation of graphene oxide and reduced graphene oxide nanoplatelets. Chem. Mater. 2009, 21, 3514–3520. [Google Scholar] [CrossRef]
- Yoo, E.J.; Kim, J.; Hosono, E.; Zhou, H.S.; Kudo, T.; Honma, I. Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Fernández-Merino, M.J.; Guardia, L.; Paredes, J.I.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascón, J.M.D. Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; De Los Santos, V.L.; Seo, J.W.; Felix, L.L.; Bustamante, D.A.; Cole, J.M.; Barnes, C.H. The structure of graphite oxide: Investigation of its surface chemical groups. J. Phys. Chem. B 2010, 114, 5723–5728. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Meng, L.; Gao, R.; Wang, Y.; Chai, J.; Yang, Z.; Kong, E.S.W.; Zhang, Y. A facile route for the large scale fabrication of graphene oxide papers and their mechanical enhancement by cross-linking with glutaraldehyde. Nano-Micro Lett. 2011, 3, 215–222. [Google Scholar] [CrossRef]
- Muniyalakshmi, M.; Sethuraman, K.; Silambarasan, D. Synthesis and characterization of graphene oxide nanosheets. Mater. Today Proc. 2020, 21, 408–410. [Google Scholar] [CrossRef]
- Awasthi, K.; Singh, D.P.; Singh, S. Attachment of biomolecules (protein and DNA) to amino-functionalized carbon nanotubes. New Carbon Mater. 2009, 24, 301–306. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, H.; Zhang, K.; Zhang, S.; Wang, J. An improved DNA biosensor built by layer-by-layer covalent attachment of multi-walled carbon nanotubes and gold nanoparticles. Electrochim. Acta 2009, 54, 2385–2391. [Google Scholar] [CrossRef]
- Fierro, V.; Torné-Fernández, V.; Montané, D.; Celzard, A. Adsorption of phenol onto activated carbons having different textural and surface properties. Microporous Mesoporous Mater. 2008, 111, 276–284. [Google Scholar] [CrossRef]
- Weber, W. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Barnakov, C.N.; Khokhlova, G.P.; Malysheva, V.Y.; Popova, A.N.; Ismagilov, Z.R. X-ray diffraction analysis of the crystal structures of different graphites. Solid Fuel Chem. 2015, 49, 25–29. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, C.; Santamaría, R.; Granda, M.; Gutiérrez, M.D.; Rodríguez-Reinoso, F.; Menéndez, R. Critical temperatures in the synthesis of graphene-like materials by thermal exfoliation-reduction of graphite oxide. Carbon N. Y. 2013, 52, 476–485. [Google Scholar] [CrossRef]
- Ma, J.; Zhu, Z.; Chen, B.; Yang, M.; Zhou, H.; Li, C.; Yu, F.; Chen, J. One-pot, large-scale synthesis of magnetic activated carbon nanotubes and their applications for arsenic removal. J. Mater. Chem. A 2013, 1, 4662. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401–187404. [Google Scholar] [CrossRef]
- Hollander, J.M.; Jolly, W.L. X-ray Photoelectron Spectroscopy. Acc. Chem. Res. 1970, 3, 193–200. [Google Scholar] [CrossRef]
- Vargas Astudillo, D.R. Síntesis de Óxido de Grafeno Reducido y Aminado Químicamente y su Influencia en las Propiedades Eléctricas y Mecánicas de Nanocompósitos a Base de Caucho Natural. Bachelor’s Thesis, Universidad de Chile, San Diego, Chile, 2017. [Google Scholar]
- Wang, G.; Shen, X.; Yao, J.; Park, J. Graphene nanosheets for enhanced lithium storage in lithium ion batteries. Carbon N. Y. 2009, 47, 2049–2053. [Google Scholar] [CrossRef]
- Huang, X.; Pan, M. The highly efficient adsorption of Pb(II) on graphene oxides: A process combined by batch experiments and modeling techniques. J. Mol. Liq. 2016, 215, 410–416. [Google Scholar] [CrossRef]
- Liao, B.; Sun, W.Y.; Guo, N.; Ding, S.L.; Su, S.J. Equilibriums and kinetics studies for adsorption of Ni(II) ion on chitosan and its triethylenetetramine derivative. Colloids Surf. A Physicochem. Eng. Asp. 2016, 501, 32–41. [Google Scholar] [CrossRef]
- Wei, B.; Cheng, X.; Wang, G.; Li, H.; Song, X.; Dai, L. Graphene Oxide Adsorption Enhanced by Attapulgite to Remove Pb (II) from Aqueous Solution. Appl. Sci. 2019, 9, 1390. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Chen, C.; Tan, X.; Wang, X. Interaction between Eu(III) and graphene oxide nanosheets investigated by batch and extended X-ray absorption fine structure spectroscopy and by modeling techniques. Environ. Sci. Technol. 2012, 46, 6020–6027. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wen, T.; Yang, X.; Yang, S.; Liao, J.; Hu, J.; Shao, D.; Wang, X. Preconcentration of U(vi) ions on few-layered graphene oxide nanosheets from aqueous solutions. Dalt. Trans. 2012, 41, 6182–6188. [Google Scholar] [CrossRef] [PubMed]
- Visual MINTEQ Software for the Calculation of Metal Speciation. Available online: https://vminteq.lwr.kth.se/ (accessed on 7 May 2020).
- Xu, D.; Tan, X.; Chen, C.; Wang, X. Removal of Pb(II) from aqueous solution by oxidized multiwalled carbon nanotubes. J. Hazard. Mater. 2008, 154, 407–416. [Google Scholar] [CrossRef]
- Depci, T.; Kul, A.R.; Önal, Y. Competitive adsorption of lead and zinc from aqueous solution on activated carbon prepared from Van apple pulp: Study in single- and multi-solute systems. Chem. Eng. J. 2012, 200–202, 224–236. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry; Mc Graw Hill, Inc.: New York, NY, USA, 1999; ISBN 0-07-016384-7. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die Adsorption in Lösungen. Z. Phys. Chem. 1906, 57U, 385–470. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the characteristic curve of activated charcoal. Proc. Acad. Sci. USSR Phys. Chem. Sect. 1947, 55, 331. [Google Scholar] [CrossRef]
- Tan, L.; Wang, S.; Du, W.; Hu, T. Effect of water chemistries on adsorption of Cs(I) onto graphene oxide investigated by batch and modeling techniques. Chem. Eng. J. 2016, 292, 92–97. [Google Scholar] [CrossRef]
- Dąbrowski, A. Adsorption—From theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Freundlich, H. Kapillarchemie, Eine Darstellung der Chemie der Kolloide und Verwandter Gebiete; Freundlich, H., Ed.; Verlag der Akademischen Verlagsgesellschaft: Leipzig, Germany, 1909. [Google Scholar]
- Tempkin, M.J.; Pyzhev, V. Recent modification to Langmuir isotherms. Acta Physiochem. USSR 1940, 12, 217–222. [Google Scholar] [CrossRef]
- Venkata Ramana, D.K.; Yu, J.S.; Seshaiah, K. Silver nanoparticles deposited multiwalled carbon nanotubes for removal of Cu(II) and Cd(II) from water: Surface, kinetic, equilibrium, and thermal adsorption properties. Chem. Eng. J. 2013, 223, 806–815. [Google Scholar] [CrossRef]
- Najafi, F. Removal of zinc(II) ion by graphene oxide (GO) and functionalized graphene oxide–glycine (GO–G) as adsorbents from aqueous solution: Kinetics studies. Int. Nano Lett. 2015, 5, 171–178. [Google Scholar] [CrossRef]
- Danish, M.; Sulaiman, O.; Rafatullah, M.; Hashim, R.; Ahmad, A. Kinetics for the removal of paraquat dichloride from aqueous solution by activated date (phoenix dactylifera) stone carbon. J. Dispers. Sci. Technol. 2010, 31, 248–259. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der Sogenannten Adsorption Gelöster Stoffe. Handlingar 1898, 24, 1–39. [Google Scholar]
- Jin, Y.; Zeng, C.; Lü, Q.F.; Yu, Y. Efficient adsorption of methylene blue and lead ions in aqueous solutions by 5-sulfosalicylic acid modified lignin. Int. J. Biol. Macromol. 2019, 123, 50–58. [Google Scholar] [CrossRef]
- Georgescu, A.M.; Nardou, F.; Zichil, V.; Nistor, I.D. Adsorption of lead(II) ions from aqueous solutions onto Cr-pillared clays. Appl. Clay Sci. 2018, 152, 44–50. [Google Scholar] [CrossRef]
- Adebisi, G.A.; Chowdhury, Z.Z.; Alaba, P.A. Equilibrium, kinetic, and thermodynamic studies of lead ion and zinc ion adsorption from aqueous solution onto activated carbon prepared from palm oil mill effluent. J. Clean. Prod. 2017, 148, 958–968. [Google Scholar] [CrossRef]
- Chen, J.L.; Gao, L.; Shi, C.L.; Wang, Y.Z.; Qi, D.W.; Hong, Y.; Shen, W.J.; Wang, Y.; Zhu, J.H. New versatile zincic sorbent for tobacco specific nitrosamines and lead ion capture. J. Hazard. Mater. 2020, 383, 121188. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, R.; Esmaeili, H.; Rishehri, S.D.; Sadeghzadeh, F.; Mirahmadi, S.; Kosarifard, M.; Ramavandi, B. Zinc, nickel, and cobalt ions removal from aqueous solution and plating plant wastewater by modified Aspergillus flavus biomass: A dataset. Data Br. 2017, 12, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, O.; Amin, N.K.; El-Ashtoukhy, E.S.Z. Removal of zinc ions from aqueous solution using a cation exchange resin. Chem. Eng. Res. Des. 2013, 91, 165–173. [Google Scholar] [CrossRef]
- Gedam, A.H.; Dongre, R.S.; Bansiwal, A.K. Synthesis and characterization of graphite doped chitosan composite for batch adsorption of lead (II) ions from aqueous solution. Adv. Mater. Lett. 2015, 6, 59–67. [Google Scholar] [CrossRef]
- Hur, J.; Shin, J.; Yoo, J.; Seo, Y.S. Competitive adsorption of metals onto magnetic graphene oxide: Comparison with other carbonaceous adsorbents. Sci. World J. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Lu, C.; Chiu, H. Adsorption of zinc(II) from water with purified carbon nanotubes. Chem. Eng. Sci. 2006, 61, 1138–1145. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Huang, H.; Zeng, G.; Liu, Y.; Wang, X.; Lin, N.; Qi, Y. Adsorption characteristics and behaviors of graphene oxide for Zn(II) removal from aqueous solution. Appl. Surf. Sci. 2013, 279, 432–440. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Duttagupta, S.P.; Chatterjee, A.K.; Mukherji, S. Potential of carbon nanomaterials for removal of heavy metals from water. Desalination 2008, 232, 145–156. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Roh, H.; Choi, Y.L.; Chang, Y.Y.; Yang, J.K. Adsorption removal of Co(II) from waste-water using graphene oxide. Hydrometallurgy 2016, 165, 90–96. [Google Scholar] [CrossRef]
- Rudzinski, W.; Plazinski, W. Theoretical description of the kinetics of solute adsorption at heterogeneous solid/solution interfaces. On the possibility of distinguishing between the diffusional and the surface reaction kinetics models. Appl. Surf. Sci. 2007, 253, 5827–5840. [Google Scholar] [CrossRef]
- Rudzinski, W.; Plazinski, W. Kinetics of dyes adsorption at the solid-solution interfaces: A theoretical description based on the two-step kinetic model. Environ. Sci. Technol. 2008, 42, 2470–2475. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S. Pseudo-Isotherms using a second order kinetic expression constant. Adsorption 2004, 10, 151–158. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The Exchange Adsorption of Ions from Aqueous Solutions by Organic Zeolites. II. Kinetics. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Roginsky, S.; Zeldovich, Y.B. The catalytic oxidation of carbon monoxide on manganese dioxide. Acta Phys. Chem. 1934, 1, 554. [Google Scholar]
- Kaveeshwar, A.R.; Ponnusamy, S.K.; Revellame, E.D.; Gang, D.D.; Zappi, M.E.; Subramaniam, R. Pecan shell based activated carbon for removal of iron(II) from fracking wastewater: Adsorption kinetics, isotherm and thermodynamic studies. Process. Saf. Environ. Prot. 2018, 114, 107–122. [Google Scholar] [CrossRef]
- O’Shannessy, D.J.; Winzor, D.J. Interpretation of deviations from pseudo-first-order kinetic behavior in the characterization of ligand binding by biosensor technology. Anal. Biochem. 1996, 236, 275–283. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Blachnio, M.; Marczewski, A.W.; Seczkowska, M.; Tarasiuk, B. Phenoxyacid pesticide adsorption on activated carbon—Equilibrium and kinetics. Chemosphere 2019, 214, 349–360. [Google Scholar] [CrossRef]
- Cui, L.; Wang, Y.; Gao, L.; Hu, L.; Yan, L.; Wei, Q.; Du, B. EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu(II) in water treatment: Adsorption mechanism and separation property. Chem. Eng. J. 2015, 281, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, C.; Zhao, J.; Ma, L.; Sun, S.; Zhao, C. Polyethersulfone enwrapped graphene oxide porous particles for water treatment. Chem. Eng. J. 2013, 215, 72–81. [Google Scholar] [CrossRef]
- Leng, Y.; Guo, W.; Su, S.; Yi, C.; Xing, L. Removal of antimony(III) from aqueous solution by graphene as an adsorbent. Chem. Eng. J. 2012, 211, 406–411. [Google Scholar] [CrossRef]
- Chaabane, L.; Beyou, E.; El Ghali, A.; Baouab, M.H.V. Comparative studies on the adsorption of metal ions from aqueous solutions using various functionalized graphene oxide sheets as supported adsorbents. J. Hazard. Mater. 2020, 389, 121839. [Google Scholar] [CrossRef]
- Niwas, R.; Gupta, U.; Khan, A.A.; Varshney, K.G. The adsorption of phosphamidon on the surface of styrene supported zirconium (IV) tungstophosphate: A thermodynamic study. Colloids Surf. A Physicochem. Eng. Asp. 2000, 164, 115–119. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, X.; Liu, M.; Yang, L.; Wu, Z.; Xia, S.; Zhao, J. Adsorption of Pb(II) in aqueous solutions by bamboo charcoal modified with KMnO4 via microwave irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2012, 414, 1–8. [Google Scholar] [CrossRef]
- Li, Y.H.; Di, Z.; Ding, J.; Wu, D.; Luan, Z.; Zhu, Y. Adsorption thermodynamic, kinetic and desorption studies of Pb2+ on carbon nanotubes. Water Res. 2005, 39, 605–609. [Google Scholar] [CrossRef] [PubMed]

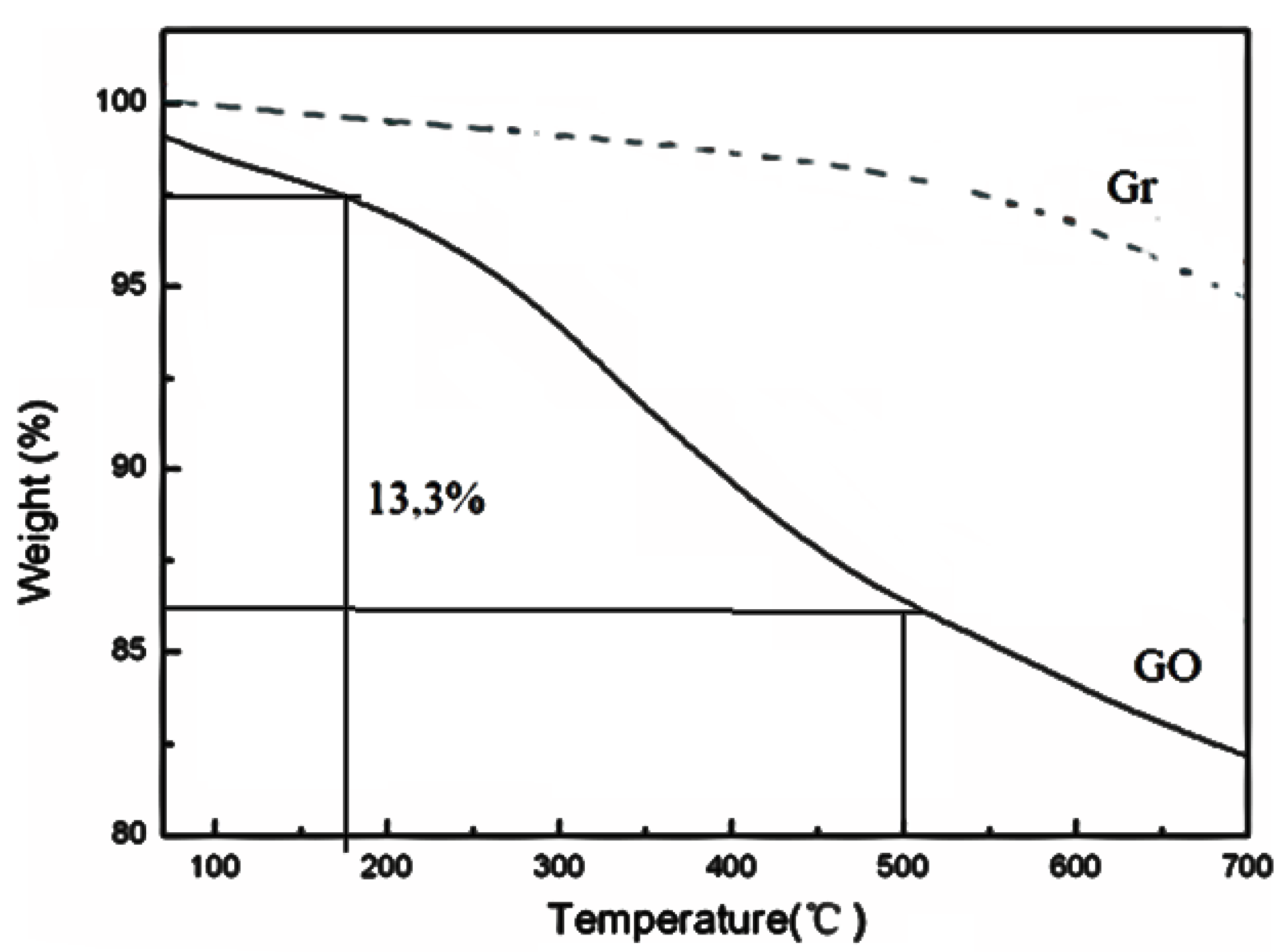
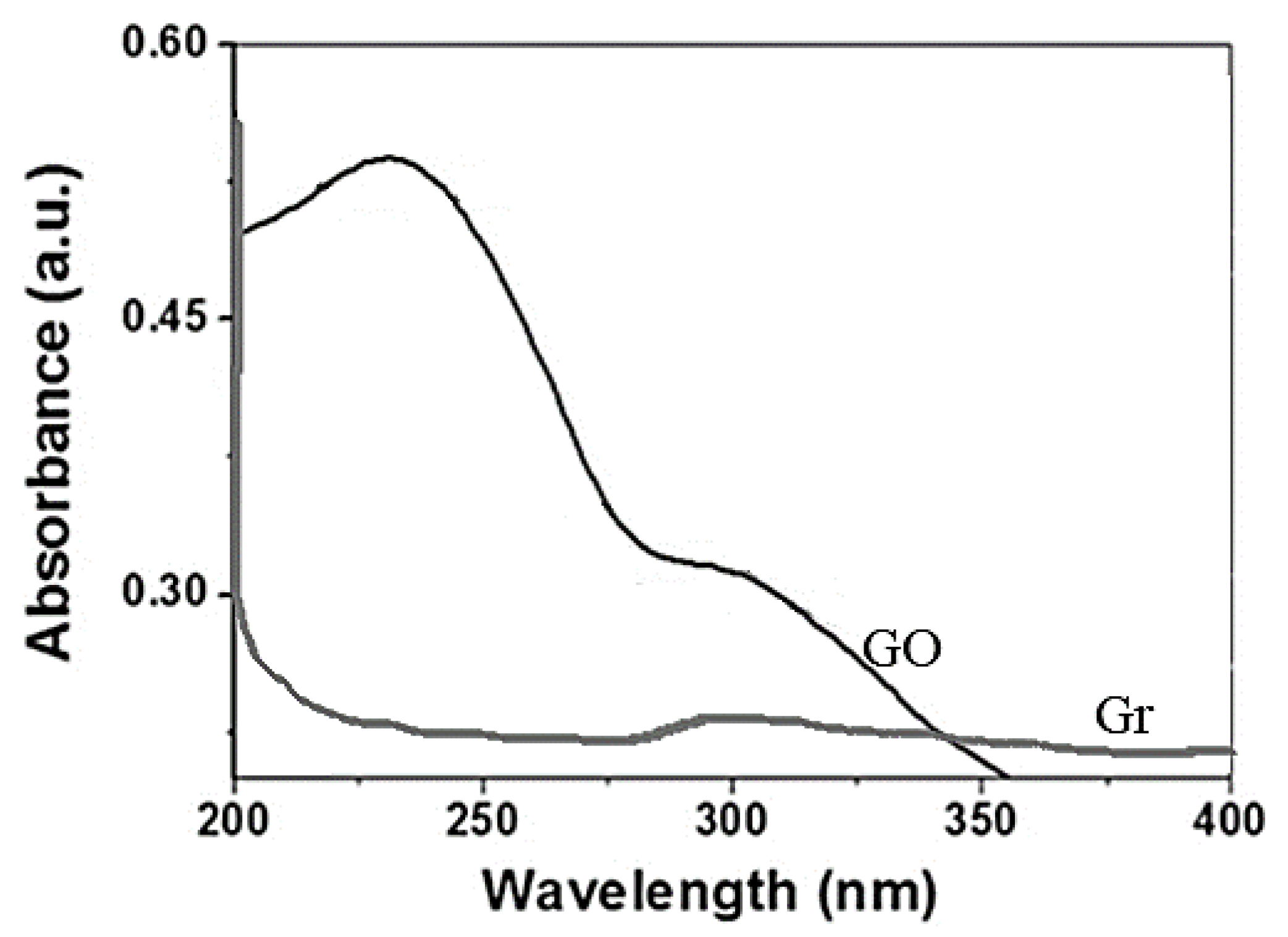

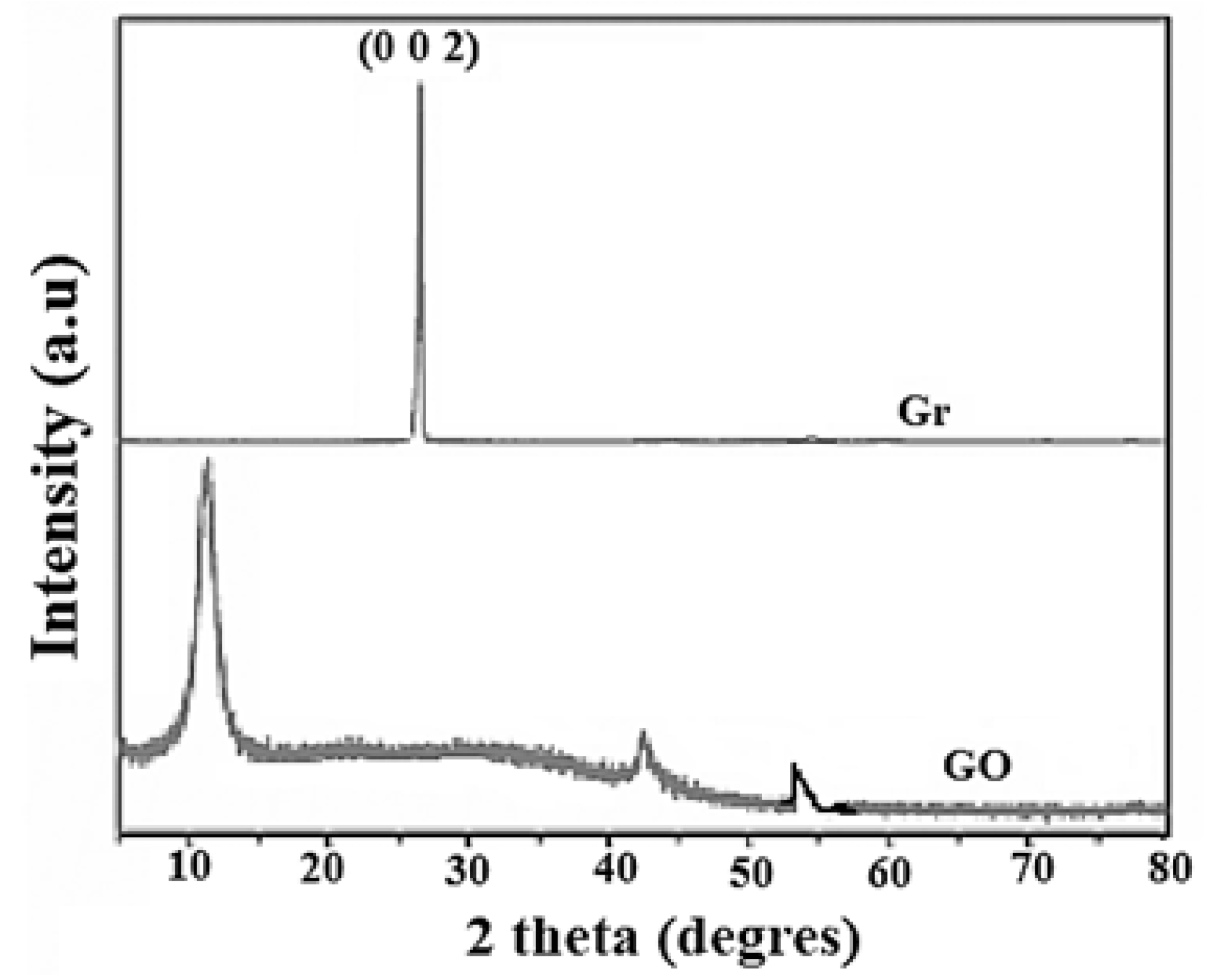
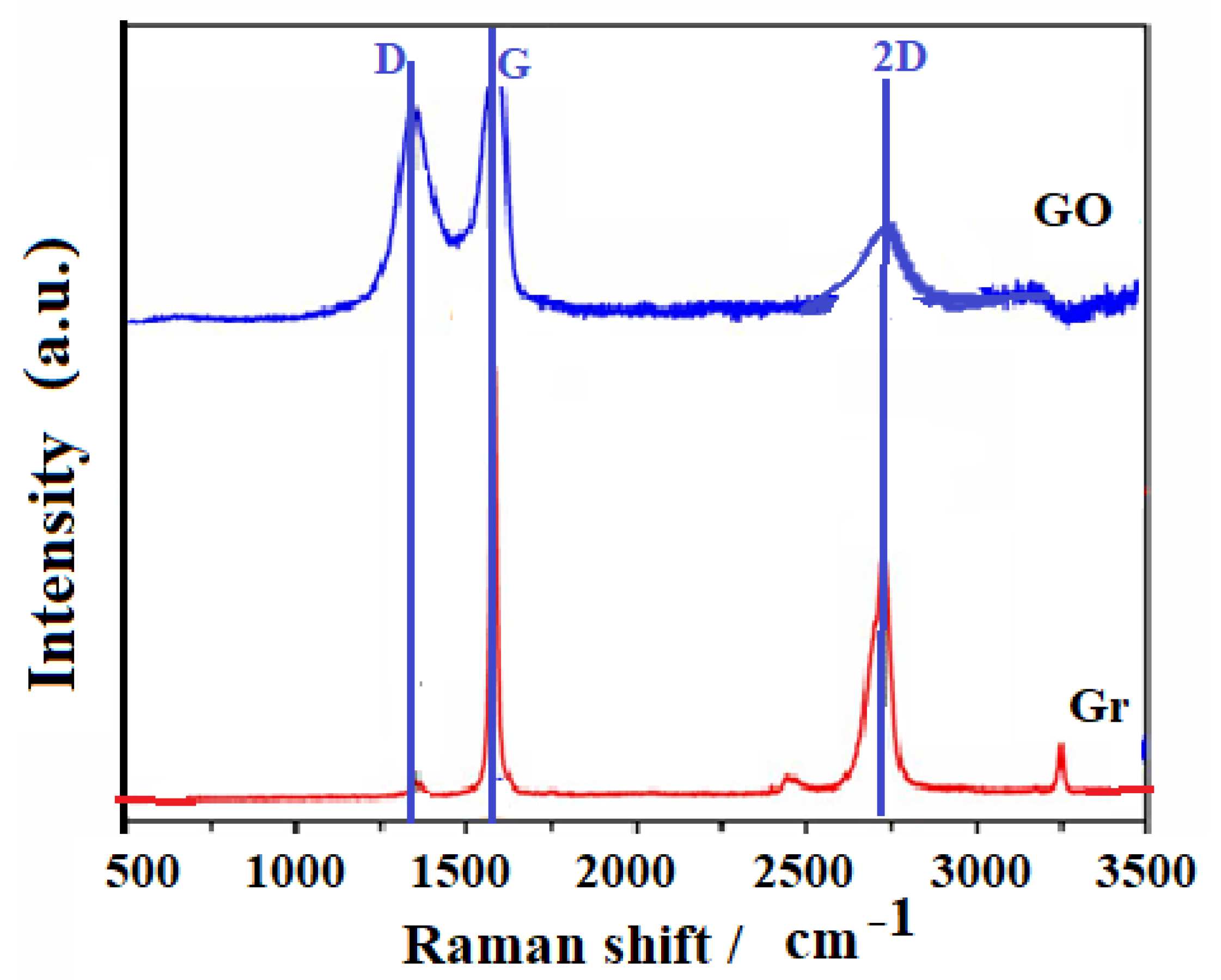


| Samples | SBET [m2·g−1] | DR (P/P° < 0.1) | DFT (P/P° 10−7 − 1) | ||||
|---|---|---|---|---|---|---|---|
| Vmic [cm3·g−1] | Eo [Kj·mol−1] | n | Pore Radius [Å] | VP [cm3·g−1] | Half Pore Width [nm] | ||
| Graphite (Gr) | 5.2 | 0.010 | 7.250 | 3.4 | 7.4 | 0.04 | 7.80 |
| Oxide Graphene (GO) | 47.5 | 0.154 | 18.60 | 5.4 | 9.3 | 0.18 | 8.75 |
| NLDFT | QSDFT | |||||
|---|---|---|---|---|---|---|
| Sample | Fitting Error (Slit Pore) [%] | Fitting Error (Cyl. Pore) [%] | Fitting Error (Cyl.-Slit) [%] | Fitting Error (Slit Pore) [%] | Fitting Error (Cyl. Pore) [%] | Fitting Error (Cyl.-Silt) [%] |
| Graphite (Gr) | 5.296 | 6.819 | 6.819 | 4.364 | 7.510 | 7.910 |
| Graphene Oxide (GO) | 2.345 | 3.576 | 4.765 | 1.354 | 2.546 | 3.087 |
| Adsorption Parameters | Metals Ions | |
|---|---|---|
| Pb (II) | Zn (II) | |
| Langmuir model | ||
| Qm (mg·g−1) | 987.3 | 313.4 |
| KL (L·mg−1) | 0.1237 | 0.1035 |
| R2 | 0.9968 | 0.9989 |
| Freundlich model | ||
| KF (mg·g−1) (L·mg−1)1/n | 290.5 | 96.56 |
| n | 4.1672 | |
| R2 | 0.9647 | |
| Dubinin–Radushkevich model | ||
| Qm (g·g−1) | 123.4 | 95.32 |
| E (kJ·mol−1) | 6.5301 | 4.3021 |
| R2 | 0.9461 | 0.9570 |
| Temkin model | ||
| KT (L·mol−1) | 0.9651 | 0.3722 |
| b (J·g·mol−2) | 645.3 | 321.3 |
| R2 | 0.8310 | 0.7622 |
| Adsorbent | Pb(II) mg·g−1 | Zn(II) mg·g−1 | Ref. |
|---|---|---|---|
| Aloji clay | 39. 30 | [107] | |
| Pillared clays | 222.22 | [108] | |
| Activated carbon prepared from palm oil mill effluent New | 69.44 | 59.88 | [109] |
| carbon-doped ferric zinc oxide | 150.0 | [110] | |
| Aspergillus flavus biomass | 27.855 | [111] | |
| Purolite C100-MH resin | 64.10 | [112] | |
| Graphite doped chitosan composite | 6.711 | [113] | |
| Magnetic graphite | 38.5 | [114] | |
| Carbon nanotubes | 32.68 | [115] | |
| Graphene Oxide | 246.0 | [116] | |
| Nanoporous carbon | 130.76 | [117] | |
| Graphene oxide | 250.0 | [118] | |
| Few-layered graphene oxide | 842.0 | [119] | |
| Graphene oxide | 246.0 | [120] | |
| Graphene oxide | 345.0 | [121] | |
| Graphene oxide | 987.33 | 313.43 | Present study |
| Model | Parameters | Adsorbate | |
|---|---|---|---|
| Pb (II) | Zn (II) | ||
| Pseudo-first order | qe (mg/g) | 196.7 | 169.6 |
| K1 × 104 (1/min) | 4.5406 | 2.3402 | |
| R2 | 0.9560 | 0.9341 | |
| Pseudo-second order | qe (mg/g) | 220.5 | 199.6 |
| K2 (g/mg min) | 0.2450 | 0.1351 | |
| R2 | 0.9998 | 0.9998 | |
| Elovich | α (mmol·g−1·min−1) | 189.3 | 152.1 |
| β × 104 (mmol·g−1) | 4.0232 | 2.1005 | |
| R2 | 0.9878 | 0.9768 | |
| Intraparticle diffusion | C (mg/g) | 28.84 | 23.82 |
| KI (mg g min1/2) | 0.4532 | 0.4151 | |
| R2 | 0.9375 | 0.9436 | |
| Metal Ions | Temperature (K) | (ΔGo) (kJ mol−1) | (ΔSo) (JK−1 mol−1) | (ΔHo) (kJ mol−1) | R2 |
|---|---|---|---|---|---|
| Pb (II) | 298 | −6787 | 45.32 | 37.54 | 0.995 |
| 308 | −7165 | ||||
| 318 | −7784 | ||||
| Zn (II) | 298 | −5497 | 32.48 | 23.12 | 0.997 |
| 308 | −5871 | ||||
| 318 | −6187 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Fajardo, C.A.; Giraldo, L.; Moreno-Piraján, J.C. Preparation and Characterization of Graphene Oxide for Pb(II) and Zn(II) Ions Adsorption from Aqueous Solution: Experimental, Thermodynamic and Kinetic Study. Nanomaterials 2020, 10, 1022. https://doi.org/10.3390/nano10061022
Guerrero-Fajardo CA, Giraldo L, Moreno-Piraján JC. Preparation and Characterization of Graphene Oxide for Pb(II) and Zn(II) Ions Adsorption from Aqueous Solution: Experimental, Thermodynamic and Kinetic Study. Nanomaterials. 2020; 10(6):1022. https://doi.org/10.3390/nano10061022
Chicago/Turabian StyleGuerrero-Fajardo, Carlos A., Liliana Giraldo, and Juan Carlos Moreno-Piraján. 2020. "Preparation and Characterization of Graphene Oxide for Pb(II) and Zn(II) Ions Adsorption from Aqueous Solution: Experimental, Thermodynamic and Kinetic Study" Nanomaterials 10, no. 6: 1022. https://doi.org/10.3390/nano10061022
APA StyleGuerrero-Fajardo, C. A., Giraldo, L., & Moreno-Piraján, J. C. (2020). Preparation and Characterization of Graphene Oxide for Pb(II) and Zn(II) Ions Adsorption from Aqueous Solution: Experimental, Thermodynamic and Kinetic Study. Nanomaterials, 10(6), 1022. https://doi.org/10.3390/nano10061022






