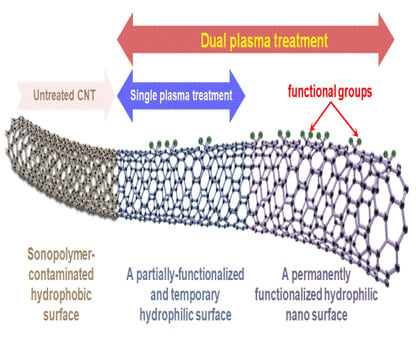
Enhanced Stability and Amplified Signal Output of Single-Wall Carbon Nanotube-Based NH3-Sensitive Electrode after Dual Plasma Treatment
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
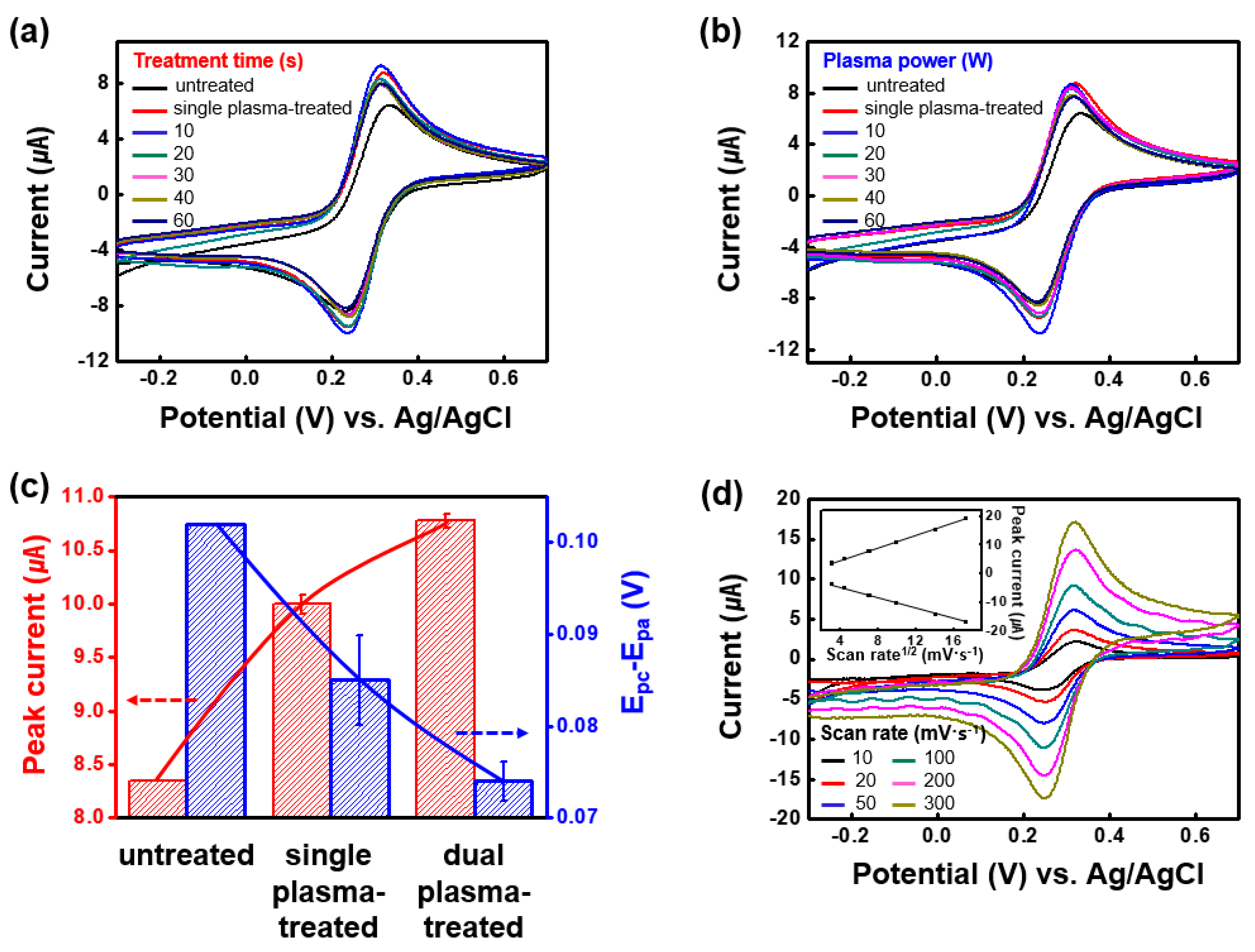
3.1. Electrochemical Characteristics of the DPT-SWCNT Electrode
3.2. Structural Analyses by Raman Spectra of the DPT-SWCNT Surface
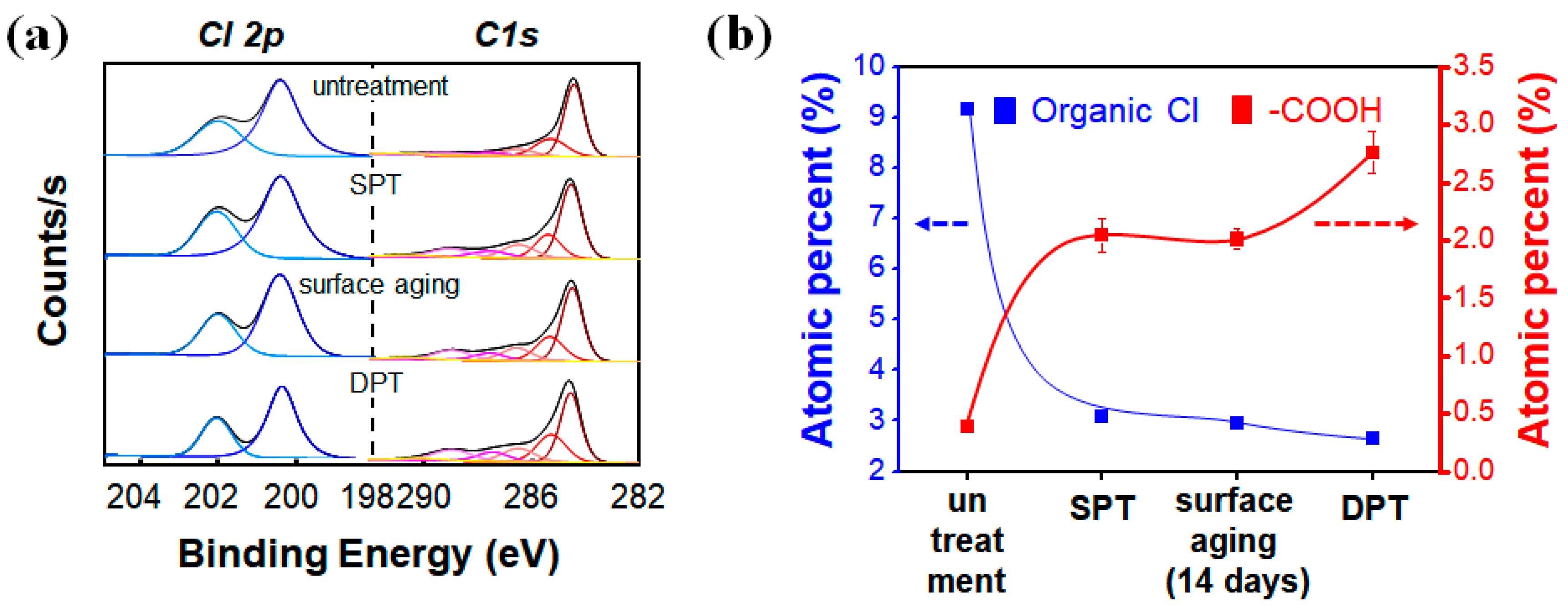
3.3. Composition Analyses by X-ray Photoelectron Spectra of the DPT-SWCNT Surface
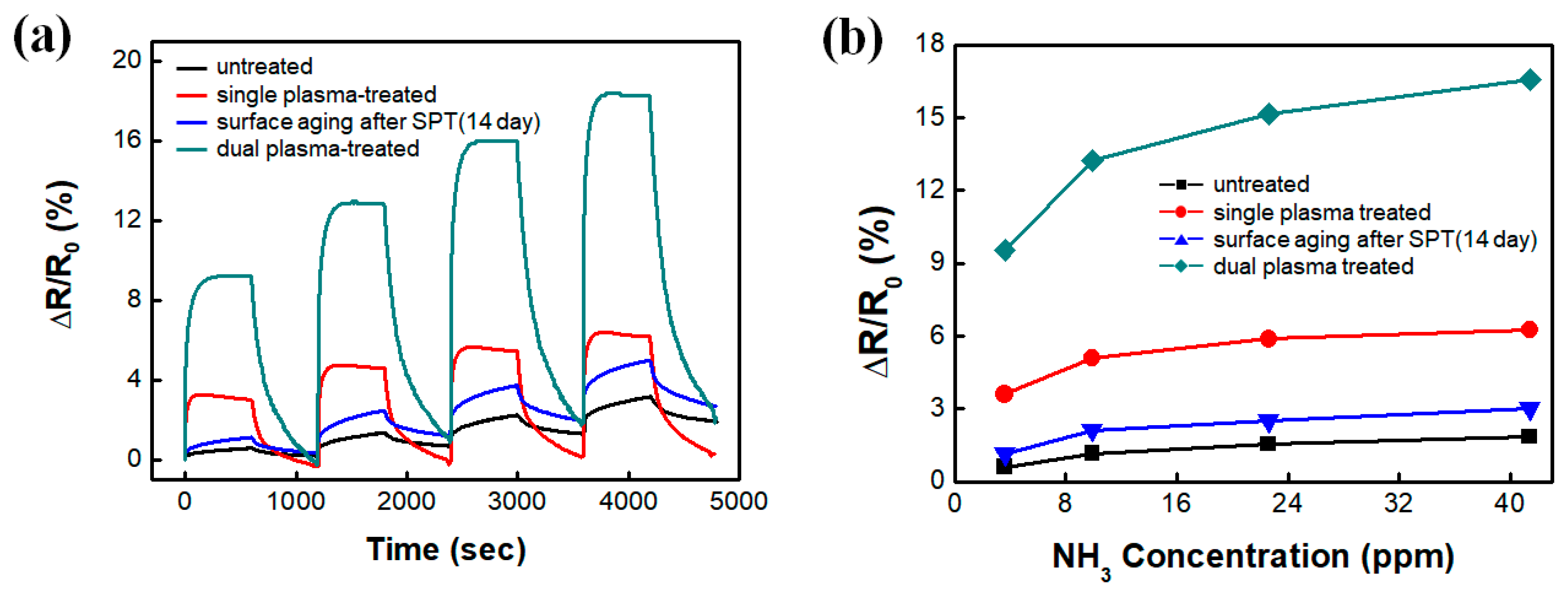
3.4. Ammonia Gas Detection–Response Characteristics of the DPT-SWCNT Electrode
3.5. Aging Pattern of the DPT-SWCNT Network Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hong, H.P.; Kim, J.H.; Lee, C.J.; Min, N.K. In-plane impedancemetric ammonia sensing of solution-deposited, highly semiconductor-enriched single-wall carbon nanotube submonolayer network gas sensors. Sens. Actuat. B Chem. 2015, 220, 27–32. [Google Scholar] [CrossRef]
- Rhee, Y.H.; Lee, C.J.; Ko, M.J.; Jin, J.H.; Min, N.K. Enhancing the Efficiency of Electron Conduction in Spray-Coated Anode of Photoelectrochemical Cell Using Oxygenated Multi-Walled Carbon Nanotubes. J. Phys. Chem. C 2015, 119, 9085–9091. [Google Scholar] [CrossRef]
- Lee, D.W.; Lee, J.H.; Min, N.K.; Jin, J.H. Buckling Structured Stretchable Pseudocapacitor Yarn. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, Y.H.; Ahn, D.J.; Ko, M.J.; Jin, H.Y.; Jin, J.H.; Min, N.K. Enhanced electrocatalytic activity of plasma functionalized multiwalled carbon nanotube-entrapped poly(3,4-ethylendioxythiophene): Poly(styrene sulfonate) photocathode. Electrochim. Acta 2014, 146, 68–72. [Google Scholar] [CrossRef]
- Jin, J.H.; Kim, J.; Jeon, T.; Shin, S.K.; Sohn, J.R.; Yi, H.; Lee, B.Y. Real-time selective monitoring of allergenic Aspergillus molds using pentameric antibody-immobilized single-walled carbon nanotube-field effect transistors. RSC Adv. 2015, 5, 15728–15735. [Google Scholar] [CrossRef]
- Azimi, S.; Farahani, A.; Sereshti, H. Plasma-functionalized Highly Aligned CNT-based Biosensor for Point of Care Determination of Glucose in Human Blood Plasma. Electroanal 2020, 32, 394–403. [Google Scholar] [CrossRef]
- Adusei, P.K.; Gbordzoe, S.; Kanakaraj, S.N.; Hsieh, Y.-Y.; Alvarez, N.T.; Fang, Y.; Johnson, K.; McConnell, C.; Shanov, V. Fabrication and study of supercapacitor electrodes based on oxygen plasma functionalized carbon nanotube fibers. J. Energy Chem. 2020, 40, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.W.; Burghard, M.; Kern, K. Chemical defect decoration of carbon nanotubes. Adv. Mater. 2002, 14, 130–133. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Popova, I.; Yates, J.T.; Bronikowski, M.J.; Huffman, C.B.; Liu, J.; Smalley, R.E.; Hwu, H.H.; Chen, J.G.G. Oxygen-containing functional groups on single-wall carbon nanotubes: NEXAFS and vibrational spectroscopic studies. J. Am. Chem. Soc. 2001, 123, 10699–10704. [Google Scholar] [CrossRef]
- Fung, A.O.; Tsiokos, C.; Paydar, O.; Chen, L.H.; Jin, S.G.; Wang, Y.B.; Judy, J.W. Electrochemical Properties and Myocyte Interaction of Carbon Nanotube Microelectrodes. Nano Lett. 2010, 10, 4321–4327. [Google Scholar] [CrossRef] [PubMed]
- Zandi, A.; Gilani, A.; Fard, H.G.; Koohsorkhi, J. An optimized resistive CNT-based gas sensor with a novel configuration by top electrical contact. Diam. Relat. Mater. 2019, 93, 224–232. [Google Scholar] [CrossRef]
- Moreira, C.M.; Scala-Benuzzi, M.L.; Takara, E.A.; Pereira, S.V.; Regiart, M.; Soler-Illia, G.J.A.A.; Raba, J.; Messina, G.A. Paper surface modification strategies employing N-SBA-15/polymer composites in bioanalytical sensor design. Talanta 2019, 200, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Belaidi, F.S.; Farouil, L.; Salvagnac, L.; Temple-Boyer, P.; Seguy, I.; Heully, J.L.; Alary, F.; Bedel-Pereira, E.; Launay, J. Towards integrated multi-sensor platform using dual electrochemical and optical detection for on-site pollutant detection in water. Biosens. Bioelectron. 2019, 132, 90–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.T.; Yu, S.Y.; Zan, H.W.; Yeh, P.H.; Lu, C.J.; Meng, H.F.; Luo, C.W.; Soppera, O. Photo-assisted recovery in ammonia sensor based on organic vertical diode. Org. Electron. 2019, 67, 272–278. [Google Scholar] [CrossRef]
- Kim, J.H.; Song, M.J.; Lee, C.J.; Lee, J.H.; Kim, J.H.; Min, N.K. A comparative study of electrochemical and biointerfacial properties of acid- and plasma-treated single-walled carbon-nanotube-film electrode systems for use in biosensors. Carbon 2013, 52, 398–407. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Fan, S.Q.; Chen, Y.; Feng, Z.H.; Zhang, H.; Pang, W.; Zhang, D.H.; Zhang, M.L. Oxygen Plasma-Treated Graphene Oxide Surface Functionalization for Sensitivity Enhancement of Thin-Film Piezoelectric Acoustic Gas Sensors. ACS Appl. Mater. Inter. 2017, 9, 40774–40781. [Google Scholar] [CrossRef]
- Shin, S.; Kim, S.W.; Jang, J.H.; Kim, J.B. A simple maskless process for the fabrication of vertically aligned high density hematite and graphene/magnetite nanowires. J. Mater. Chem. C 2017, 5, 1313–1320. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, C.Y.; An, H.R.; Kim, H.; Lee, Y.C.; Park, E.C.; Chun, H.S.; Yang, H.Y.; Choi, S.H.; Kim, H.S.; et al. Advanced carbon dots via plasma-induced surface functionalization for fluorescent and bio-medical applications. Nanoscale 2017, 9, 9210–9217. [Google Scholar] [CrossRef] [Green Version]
- Gokus, T.; Nair, R.R.; Bonetti, A.; Bohmler, M.; Lombardo, A.; Novoselov, K.S.; Geim, A.K.; Ferrari, A.C.; Hartschuh, A. Making Graphene Luminescent by Oxygen Plasma Treatment. ACS Nano 2009, 3, 3963–3968. [Google Scholar] [CrossRef] [Green Version]
- Meyyappan, M. Plasma nanotechnology: Past, present and future. J. Phys. D Appl. Phys. 2011, 44, 174002. [Google Scholar] [CrossRef]
- Xu, W.; Yang, T.T.; Qin, F.; Gong, D.D.; Du, Y.J.; Dai, G. A Sprayed Graphene Pattern-Based Flexible Strain Sensor with High Sensitivity and Fast Response. Sensors 2019, 19, 1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chetty, R.; Maniam, K.K.; Schuhmann, W.; Muhler, M. Oxygen-Plasma-Functionalized Carbon Nanotubes as Supports for Platinum-Ruthenium Catalysts Applied in Electrochemical Methanol Oxidation. Chempluschem 2015, 80, 130–135. [Google Scholar] [CrossRef]
- Ueda, A.; Kato, D.; Sekioka, N.; Hirono, S.; Niwa, O. Local Imaging of an Electrochemical Active/Inactive Region on a Conductive Carbon Surface by Using Scanning Electrochemical Microscopy. Anal. Sci. 2009, 25, 645–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, S.; Kim, J.; Park, C.; Jin, J.H.; Min, N.K. Long-term stability of superhydrophilic oxygen plasma-modified single-walled carbon nanotube network surfaces and the influence on ammonia gas detection. Appl. Surf. Sci. 2017, 410, 105–110. [Google Scholar] [CrossRef]
- Bodas, D.; Khan-Malek, C. Hydrophilization and hydrophobic recovery of PDMS by oxygen plasma and chemical treatment—An SEM investigation. Sens. Actuat. B Chem. 2007, 123, 368–373. [Google Scholar] [CrossRef]
- Eddington, D.T.; Puccinelli, J.P.; Beebe, D.J. Thermal aging and reduced hydrophobic recovery of polydimethylsiloxane. Sens. Actuat. B Chem. 2006, 114, 170–172. [Google Scholar] [CrossRef]
- Garbassi, F.; Morra, M.; Ochiello, E. Polymer surfaces: From physics to technology. Polym. Int. 2002, 36, 300. [Google Scholar]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef]
- Gao, C.; Guo, Z.; Liu, J.H.; Huang, X.J. The new age of carbon nanotubes: An updated review of functionalized carbon nanotubes in electrochemical sensors. Nanoscale 2012, 4, 1948–1963. [Google Scholar] [CrossRef]
- Yang, W.R.; Ratinac, K.R.; Ringer, S.P.; Thordarson, P.; Gooding, J.J.; Braet, F. Carbon Nanomaterials in Biosensors: Should You Use Nanotubes or Graphene? Angew. Chem. Int. Edit. 2010, 49, 2114–2138. [Google Scholar] [CrossRef]
- Kim, D.S.; Nepal, D.; Geckeler, K.E. Individualization of single-walled carbon nanotubes: Is the solvent important? Small 2005, 1, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Kuroda, S.; Osawa, Z. Dynamics of polymeric solid surfaces treated with oxygen plasma: Effect of aging media after plasma treatment. J. Colloid. Interf. Sci. 1998, 202, 37–44. [Google Scholar] [CrossRef]
- Yung, C.S.; Tomlin, N.A.; Heuerman, K.; Keller, M.W.; White, M.G.; Stephens, M.; Lehman, J.H. Plasma modification of vertically aligned carbon nanotubes: Superhydrophobic surfaces with ultra-low reflectance. Carbon 2018, 127, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, V.S.; Miranda, A.N.; Nunes, W.G.; Costa, L.H.; Pascon, A.M.; Rodella, C.B.; Zanin, H.; Doubek, G. Radially ordered carbon nanotubes performance for Li-O2 batteries: Pre-treatment influence on capacity and discharge products. Catal. Today 2019, 348, 299–306. [Google Scholar] [CrossRef]
- Chirila, V.; Marginean, G.; Brandl, W. Effect of the oxygen plasma treatment parameters on the carbon nanotubes surface properties. Surf. Coat. Technol. 2005, 200, 548–551. [Google Scholar] [CrossRef]
- Song, M.J.; Kim, S.; Min, N.K.; Jin, J.H. Electrochemical serotonin monitoring of poly (ethylenedioxythiophene): Poly (sodium 4-styrenesulfonate)-modified fluorine-doped tin oxide by predeposition of self-assembled 4-pyridylporphyrin. Biosens. Bioelectron. 2014, 52, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Utegulov, Z.N.; Mast, D.B.; He, P.; Shi, D.L.; Gilland, R.F. Functionalization of single-walled carbon nanotubes using isotropic plasma treatment: Resonant Raman spectroscopy study. J. Appl. Phys. 2005, 97, 104324. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.A.; Seong, D.G.; Kang, T.J.; Youn, J.R. Effects of surface modification on rheological and mechanical properties of CNT/epoxy composites. Carbon 2006, 44, 1898–1905. [Google Scholar] [CrossRef]
- Ionescu, R.; Espinosa, E.H.; Sotter, E.; Llobet, E.; Vilanova, X.; Correig, X.; Felten, A.; Bittencourt, C.; van Lier, G.; Charlier, J.C.; et al. Oxygen functionalisation of MWNT and their use as gas sensitive thick-film layers. Sens. Actuat. B Chem. 2006, 113, 36–46. [Google Scholar] [CrossRef]
- Yu, X.Y.; Prevot, M.S.; Sivula, K. Multiflake Thin Film Electronic Devices of Solution Processed 2D MoS2 Enabled by Sonopolymer Assisted Exfoliation and Surface Modification. Chem. Mater. 2014, 26, 5892–5899. [Google Scholar] [CrossRef]
- Kim, J.H.; Song, M.J.; Kim, K.B.; Jin, J.H.; Min, N.K. Evaluation of Surface Cleaning Procedures in Terms of Gas Sensing Properties of Spray-Deposited CNT Film: Thermal-and O-2 Plasma Treatments. Sensors 2017, 17, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Lee, M.J.; Park, E.J.; Lee, J.Y.; Lee, C.J.; Min, N.K. Plasma Processing: Technology for the Batch Fabrication of Carbon Nanotube Film Electrodes for Biointerfaces. Plasma Process. Polym. 2012, 9, 873–883. [Google Scholar] [CrossRef]
- Struzzi, C.; Scardamaglia, M.; Hemberg, A.; Petaccia, L.; Colomer, J.F.; Snyders, R.; Bittencourt, C. Plasma fluorination of vertically aligned carbon nanotubes: Functionalization and thermal stability. Beilstein J. Nanotech. 2015, 6, 2263–2271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
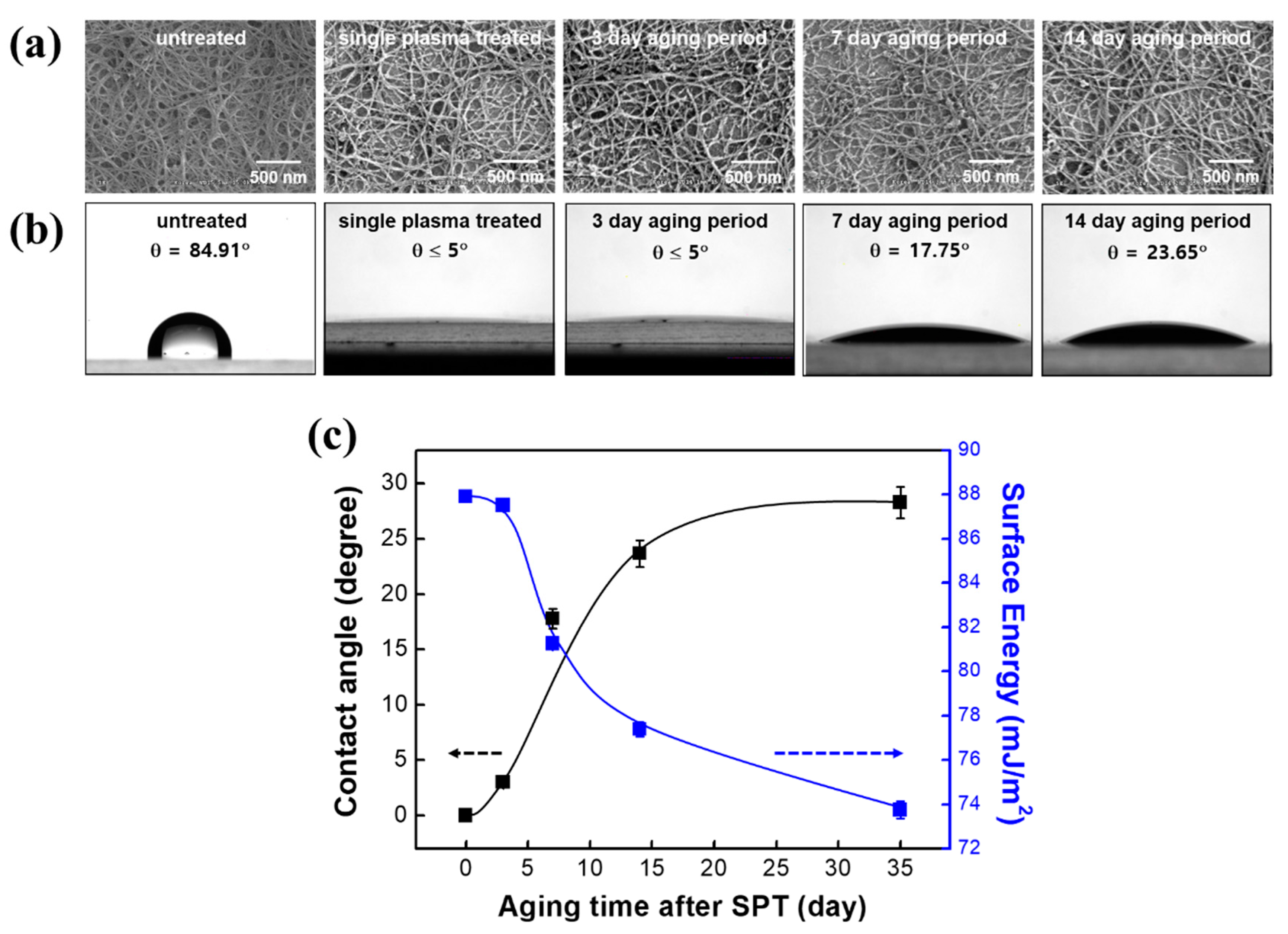


| Samples | mJ m−2 | mJ m−2 | mJ m−2 |
|---|---|---|---|
| Untreated | 34.6 | 2.91 | 37.5 |
| As-prepared (SPT) | 1.96 | 85.9 | 87.9 |
| 3-day aging (SPT) | 2.00 | 85.5 | 87.5 |
| 7-day aging (SPT) | 2.80 | 78.5 | 81.3 |
| 14-day aging (SPT) | 3.07 | 74.3 | 77.4 |
| 35-day aging (SPT) | 3.34 | 70.4 | 73.7 |
| Organic Cl (%) | C sp2 (%) | C sp3 (%) | C–O (%) | C=O (%) | –COO (%) | π-π* (%) | COx/C (Total) (%) | |
|---|---|---|---|---|---|---|---|---|
| Untreated | 3.14 | 69.0 | 16.8 | 6.70 | 3.17 | 2.89 | 1.41 | 12.8 |
| SPT (20W/20s) | 0.47 | 57.9 | 18.6 | 10.3 | 5.14 | 6.68 | 1.37 | 22.1 |
| Aged 14 days | 0.42 | 57.8 | 18.8 | 9.79 | 5.54 | 6.59 | 1.55 | 21.9 |
| DPT (10W/10s) | 0.28 | 52.5 | 20.6 | 9.98 | 6.93 | 8.31 | 1.63 | 25.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Jin, J.-H.; Min, N.K. Enhanced Stability and Amplified Signal Output of Single-Wall Carbon Nanotube-Based NH3-Sensitive Electrode after Dual Plasma Treatment. Nanomaterials 2020, 10, 1026. https://doi.org/10.3390/nano10061026
Kim JH, Jin J-H, Min NK. Enhanced Stability and Amplified Signal Output of Single-Wall Carbon Nanotube-Based NH3-Sensitive Electrode after Dual Plasma Treatment. Nanomaterials. 2020; 10(6):1026. https://doi.org/10.3390/nano10061026
Chicago/Turabian StyleKim, Joon Hyub, Joon-Hyung Jin, and Nam Ki Min. 2020. "Enhanced Stability and Amplified Signal Output of Single-Wall Carbon Nanotube-Based NH3-Sensitive Electrode after Dual Plasma Treatment" Nanomaterials 10, no. 6: 1026. https://doi.org/10.3390/nano10061026