Effects of Nanoparticle Size and Radiation Energy on Copper-Cysteamine Nanoparticles for X-ray Induced Photodynamic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Copper-Cysteamine (Cu-Cy) Nanoparticle Synthesis and Conjugation with pH-Low Insertion Peptide
2.3. Nanoparticle Characterization
2.4. Reactive Oxygen Species (ROS) Detection
2.5. Tumor Size Experiment
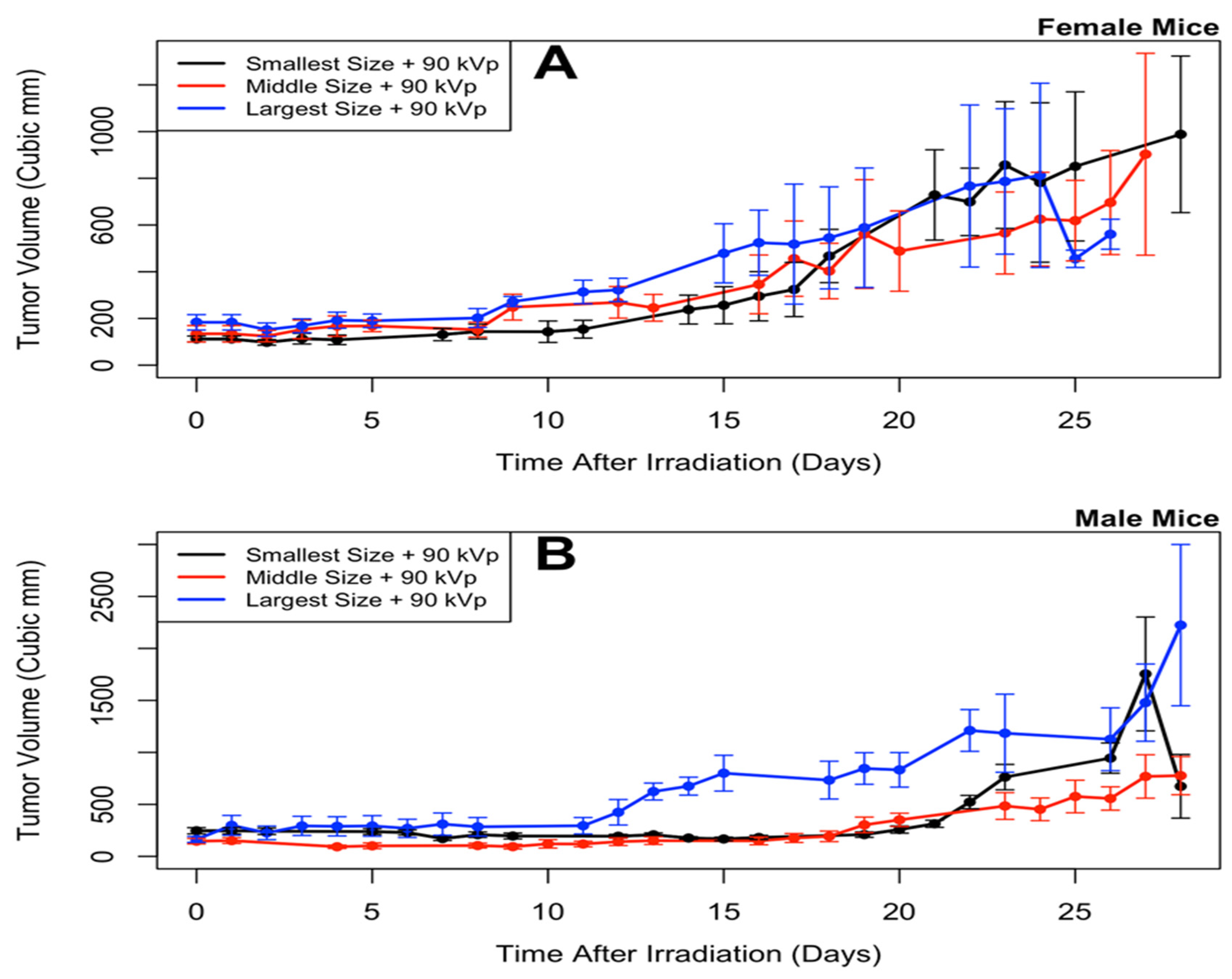
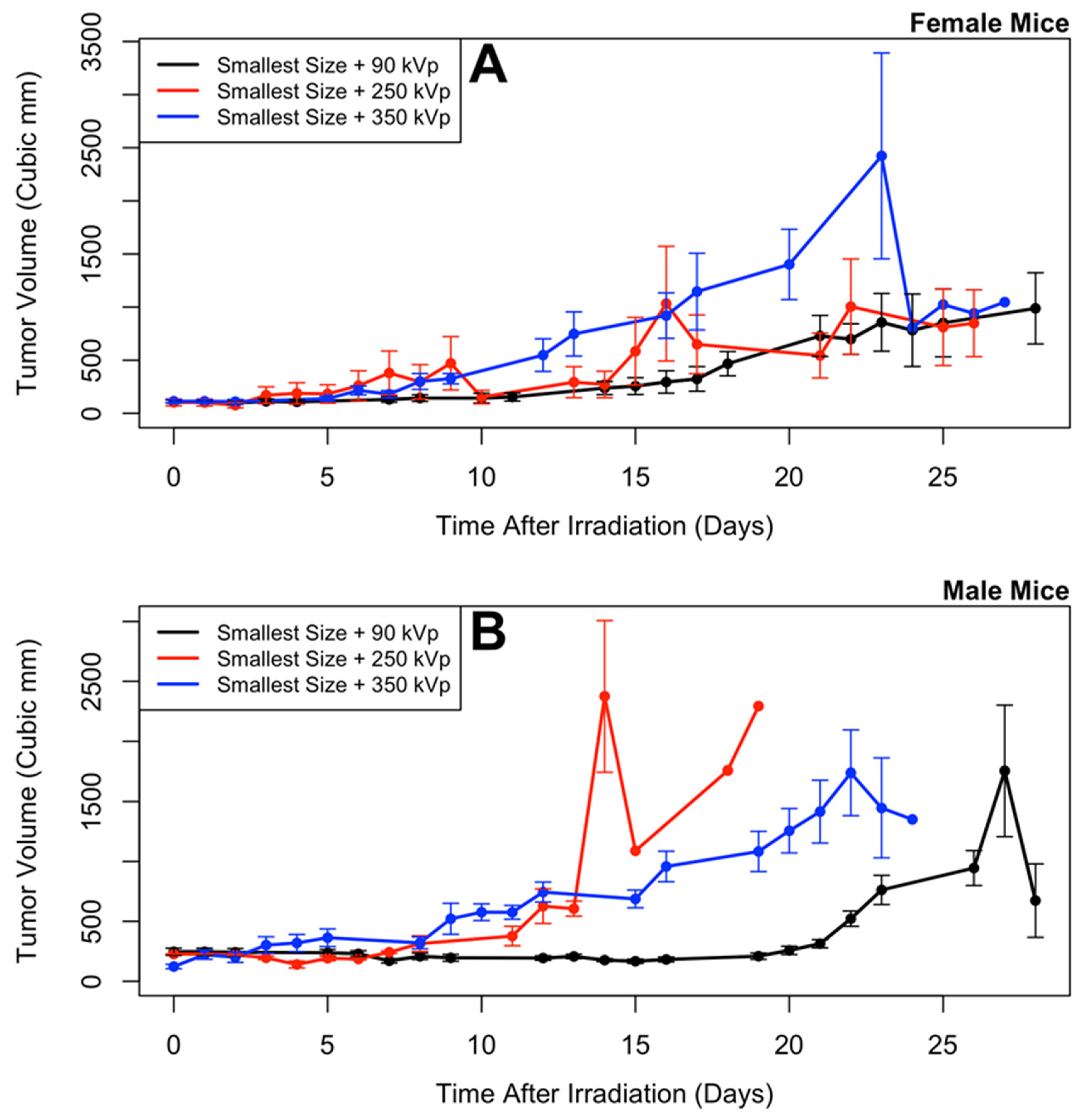
- (Smallest nanoparticles + 90 kVp) (Note: this is the same group as in Shrestha et al. [20])
- (Smallest nanoparticles + 250 kVp)
- (Smallest nanoparticles + 350 kVp)
- (Middle nanoparticles + 90 kVp)
- (Largest nanoparticles + 90 kVp)
2.6. Tumor Size Analysis
3. Results
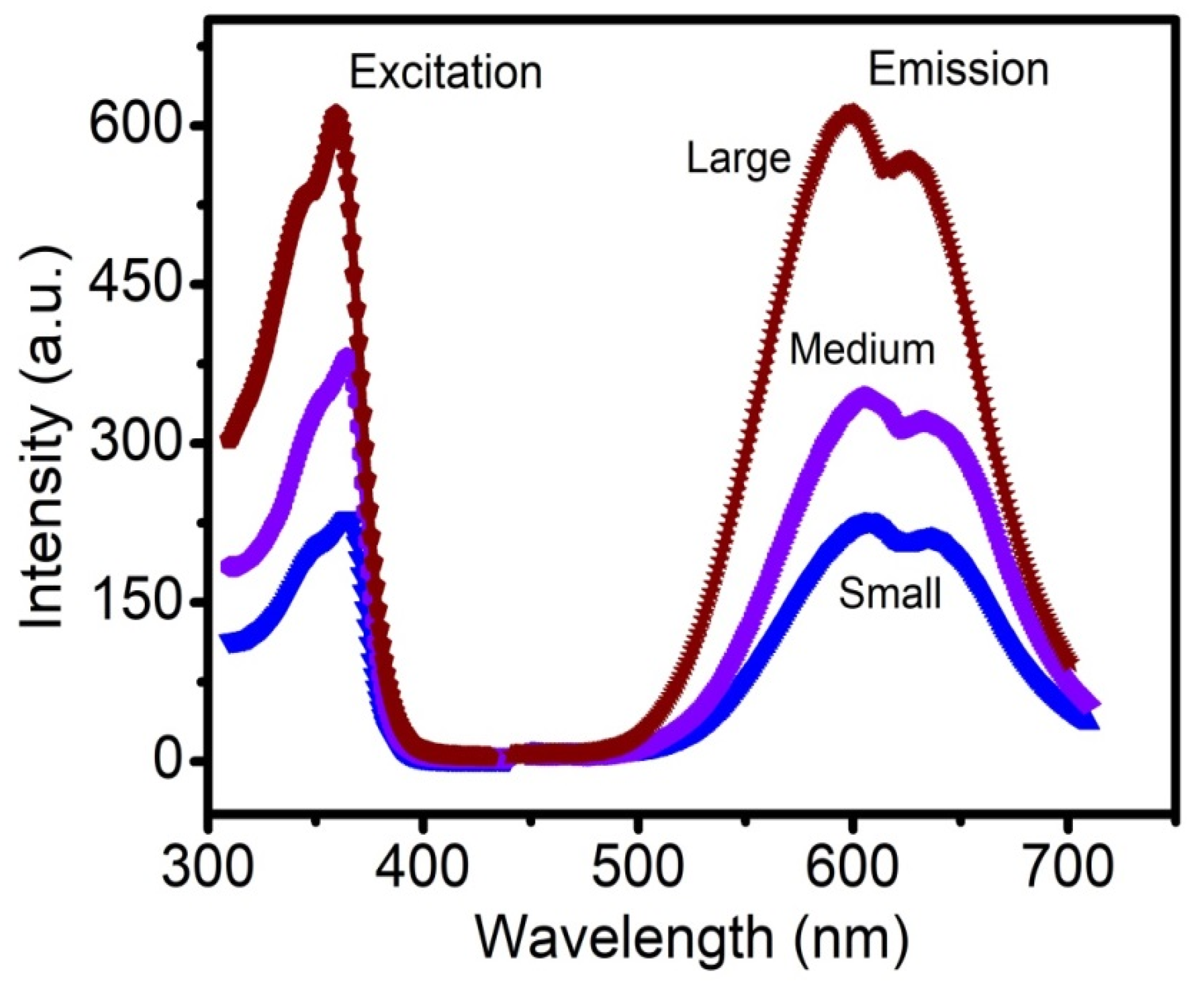
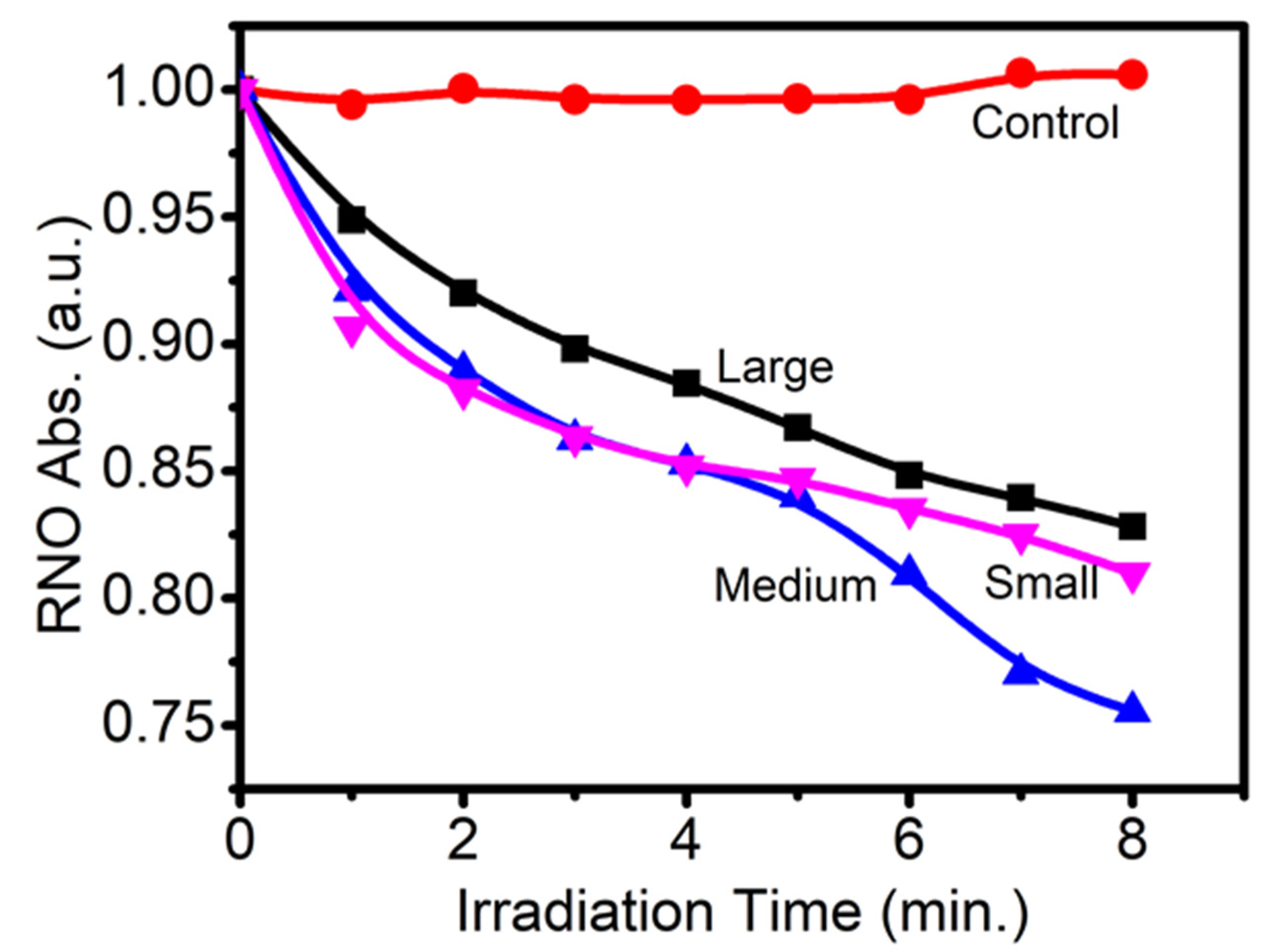
3.1. Study of Size Distribution, Photoluminescence (PL) Spectra, and ROS Production
3.2. Longitudinal Model of Tumor Size Response
3.3. Missing Data Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Puolakkainen, P.; Schröder, T. Photodynamic therapy. Ann. Chir. Gynaecol. Suppl. 1990, 4, 240–243. [Google Scholar]
- Tamai, K.; Mizushima, T.; Wu, X.; Inoue, A.; Ota, M.; Yokoyama, Y.; Miyoshi, N.; Haraguchi, N.; Takahashi, H.; Nishimura, J. Photodynamic therapy using indocyanine green loaded on super carbonate apatite as minimally invasive cancer treatment. Mol. Cancer Ther. 2018, 17, 1613–1622. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Masamune, K. Flexible laser endoscope for minimally invasive photodynamic diagnosis (PDD) and therapy (PDT) toward efficient tumor removal. Opt. Express 2017, 25, 16795–16812. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rashidi, L.H.; Yao, M.; Ma, L.; Chen, L.; Zhang, J.; Zhang, Y.; Chen, W. CuS nanoagents for photodynamic and photothermal therapies: Phenomena and possible mechanisms, photodiagnosis and photodynamic therapy. Photodign. Photody. Ther. 2017, 19, 5–14. [Google Scholar] [CrossRef]
- Chudal, L.; Pandey, N.K.; Phan, J.; Johnson, O.; Li, X.; Chen, W. Investigation of PPIX-Lipo-MnO2 to enhance photodynamic therapy by improving tumor hypoxia. Mater. Sci. Eng. C 2019, 104, 109979. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zou, X.; Chen, W. A new X-ray activated nanoparticle photosensitizer for cancer treatment. J. Biomed. Nanotechnol. 2014, 10, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Qian, H.; Idris, N.M.; Zhang, Y. Singlet oxygen-induced apoptosis of cancer cells using upconversion fluorescent nanoparticles as a carrier of photosensitizer. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 486–495. [Google Scholar] [CrossRef]
- Wang, C.; Tao, H.; Cheng, L.; Liu, Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials 2011, 32, 6145–6154. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J. Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. J. Nanosci. Nanotechnol. 2006, 6, 1159–1166. [Google Scholar] [CrossRef]
- Chen, W. Nanoparticle self-lighting photodynamic therapy for cancer treatment. J. Biomed. Nanotechnol. 2008, 4, 369–376. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Wang, S.; Joly, A.G. Investigation of water-soluble X-ray luminescence nanoparticles for photodynamic activation. Appl. Phys. Lett. 2008, 92, 043901. [Google Scholar] [CrossRef]
- Zou, X.; Yao, M.; Ma, L.; Hossu, M.; Han, X.; Juzenas, P.; Chen, W. X-ray-induced nanoparticle-based photodynamic therapy of cancer. Nanomedicine 2014, 9, 2339–2351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, X.; Shen, Y.; Shi, F.; Song, C.; Liu, T.; Gao, P.; Lan, B.; Liu, M.; Wang, S. Ultra-high FRET efficiency NaGdF4: Tb3+-Rose Bengal biocompatible nanocomposite for X-ray excited photodynamic therapy application. Biomaterials 2018, 184, 31–40. [Google Scholar] [CrossRef]
- Ma, L.; Chen, W.; Schatte, G.; Wang, W.; Joly, A.G.; Huang, Y.; Sammynaiken, R.; Hossu, M. A new Cu-cysteamine complex: Structure and optical properties. J. Mater. Chem. C 2014, 2, 4239–4246. [Google Scholar] [CrossRef]
- Huang, X.; Wan, F.; Ma, L.; Phan, J.B.; Lim, R.X.; Li, C.; Chen, J.; Deng, J.; Li, Y.; Chen, W.; et al. Investigation of copper-cysteamine nanoparticles as a new photosensitizer for anti-hepatocellular carcinoma. Cancer Biol. Ther. 2019, 20, 812–825. [Google Scholar] [CrossRef]
- Yao, M.; Ma, L.; Li, L.; Zhang, J.; Lim, R.X.; Chen, W.; Zhang, Y. A new modality for cancer treatment—Nanoparticle mediated microwave induced photodynamic therapy. J. Biomed. Nanotechnol. 2016, 12, 1835–1851. [Google Scholar] [CrossRef]
- Pandey, N.K.; Chudal, L.; Phan, J.; Lin, L.; Johnson, O.; Xing, M.; Liu, P.; Li, H.; Huang, X.; Shu, Y.; et al. A facile method for synthesis of copper-cysteamine nanoparticles and study of ROS production for cancer treatment. J. Mater. Chem. B 2019, 7, 6630–6642. [Google Scholar] [CrossRef]
- Liu, Z.; Xiong, L.; Ouyang, G.; Ma, L.; Sahi, S.; Wang, K.; Lin, L.; Huang, H.; Miao, X.; Chen, W. Investigation of copper cysteamine nanoparticles as a new type of radiosensitiers for colorectal carcinoma treatment. Sci. Rep. 2017, 7, 9290. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, S.; Wu, J.; Sah, B.; Vanasse, A.; Cooper, L.N.; Ma, L.; Li, G.; Zheng, H.; Chen, W.; Antosh, M.P. X-ray induced photodynamic therapy with pH-low insertion peptide targeted copper-cysteamine nanoparticles in mice. Proc. Natl. Acad. Sci. USA 2019, 116, 16823–16828. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Wang, X.; Ma, L.; Sahi, S.; Li, L.; Wang, X.; Wang, Q.; Chen, Y.; Chen, W.; Liu, Q. Nanosonosensitization by using copper–cysteamine nanoparticles augmented sonodynamic cancer treatment. Part. Part. Syst. Charact. 2018, 35, 1700378. [Google Scholar] [CrossRef]
- Chudal, L.; Pandey, N.K.; Phan, J.; Johnson, O.; Lin, L.; Yu, H.; Shu, Y.; Huang, Z.; Xing, M.; Liu, J.P.; et al. Copper-cysteamine nanoparticles as a heterogeneous fenton-like catalyst for highly selective cancer treatment. ACS Appl. Biomater. 2020, 3, 1804–1814. [Google Scholar] [CrossRef]
- Zhen, X.; Chudal, L.; Pandey, N.K.; Phan, J.; Ran, X.; Amador, E.; Huang, X.; Johnson, O.; Ran, Y.; Chen, W.; et al. A powerful combination of copper-cysteamine nanoparticles with potassium iodide for bacterial destruction. Mater. Sci. Eng. C 2020, 110, 110659. [Google Scholar] [CrossRef] [PubMed]
- Johns, H.E.; Cunningham, J.R. The Physics of Radiology, 4th ed.; Charles, C., Ed.; Thomas: Springfield, IL, USA, 1983; p. 796. [Google Scholar]
- McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Coulter, J.A.; Jain, S.; Butterworth, K.T.; Schettino, G.; Dickson, G.R.; Hounsell, A.R.; O‘Sullivan, J.M.; et al. Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci. Rep. 2011, 1, 18. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Ma, H.; Liu, J.; Huo, S.; Kumar, A.; Wei, T.; Zhang, X.; Jin, S.; Gan, Y.; Wang, P.C.; et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano 2012, 6, 4483–4493. [Google Scholar] [CrossRef] [Green Version]
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009, 9, 1909–1915. [Google Scholar] [CrossRef]
- Sah, B.; Antosh, M. Effect of size on gold nanoparticles in radiation therapy: Uptake and survival effects. J. Nano Med. 2019, 2, 1013. [Google Scholar]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf. B Biointerfaces 2008, 66, 274–280. [Google Scholar] [CrossRef]
- Andreev, O.A.; Engelman, D.M.; Reshetnyak, Y.K. Targeting acidic diseased tissue: New technology based on use of the pH (Low) Insertion Peptide (pHLIP). Chim. Oggi 2009, 27, 34–37. [Google Scholar]
- Kraljić, I.; Mohsni, S.E. A new method for the detection of singlet oxygen in aqueous solutions. Photochem. Photobiol. 1978, 28, 577–581. [Google Scholar] [CrossRef]
- Yao, L.; Daniels, J.; Moshnikova, A.; Kuznetsov, S.; Ahmed, A.; Engelman, D.M.; Reshetnyak, Y.K.; Andreev, O.A. pHLIP peptide targets nanogold particles to tumors. Proc. Natl. Acad. Sci. USA 2013, 110, 465–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sah, B.; Shrestha, S.; Wu, J.; Vanasse, A.; Cooper, L.N.; Antosh, M. Gold nanoparticles enhance radiation therapy at low concentrations, and remain in tumors for days. J. Biomed. Nanotechnol. 2019, 15, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Rofstad, E.K.; Brustad, T. Tumour growth delay following single dose irradiation of human melanoma xenografts. Correlations with tumour growth parameters, vascular structure and cellular radiosensitivity. Br. J. Cancer 1985, 51, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubin, D.B. Inference and missing data. Biometrika 1976, 63, 581–592. [Google Scholar] [CrossRef]
- Robins, J.M.; Rotnitzky, A. Semiparametric efficiency in multivariate regression models with missing data. J. Am. Stat. Assoc. 1995, 90, 122–129. [Google Scholar] [CrossRef]
- Spiegelhalter, D.J.; Best, N.G.; Carlin, B.P.; van der Linde, A. Bayesian measures of model complexity and fit (with discussion). J. R. Stat. Soc. Ser. B 2002, 64, 583–639. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.Y.; Lee, C.H.; Kang, Y.C. Effect of surface area and crystallite size on luminescent intensity of Y2O3: Eu phosphor prepared by spray pyrolysis. Mater. Lett. 2005, 59, 2451–2456. [Google Scholar] [CrossRef]
- Wang, W.-N.; Widiyastuti, W.; Ogi, T.; Lenggoro, I.W.; Okuyama, K. Correlations between crystallite/particle size and photoluminescence properties of submicrometer phosphors. Chem. Mater. 2007, 19, 1723–1730. [Google Scholar] [CrossRef]
- Chen, W.; Sammynaiken, R.; Huang, Y.N.; Malm, J.O.; Wallenberg, R.; Bovin, J.O.; Zwiller, V.; Kotov, N.A. Crystal field, phonon coupling and emission shift of Mn2+ in ZnS:Mn nanoparticles. J. Appl. Phys. 2001, 89, 1120–1129. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.G.; Lin, Z.J.; Lin, L.Y. Thermoluminescence of ZnS Nanoparticles. Appl. Phys. Lett. 1997, 70, 1465–1467. [Google Scholar] [CrossRef]
- Chen, W. Nanoparticle fluorescence based technology for biological applications. J. Nanosci. Nanotechnol. 2008, 8, 1019–1051. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.-H.; Childs, C.J.; Sibata, C.H. Photosensitizers in clinical PDT. Photodiagnosis Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, W.F.; Althuis, M.D.; Brinton, L.A.; Devesa, S.S. Is male breast cancer similar or different than female breast cancer? Breast Cancer Res. Treat. 2004, 83, 77–86. [Google Scholar] [CrossRef]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist, 7th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Kramer, C.Y. Extension of multiple range tests to group means with unequal numbers of replications. Biometrics 1956, 12, 307–310. [Google Scholar] [CrossRef]
- Shi, L.; Liu, P.; Wu, J.; Ma, L.; Zheng, H.; Antosh, M.P.; Zhang, H.; Wang, B.; Chen, W.; Wang, X. The effectiveness and safety of copper-cysteamine nanoparticle mediated X-PDT for cutaneous squamous cell carcinoma and melanoma. Nanomed. Future Med. 2019, 14, 2027–2043. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, X.; Cheng, Y.; Chudal, L.; Pandey, N.K.; Zhang, J.; Ma, L.; Xi, Q.; Yang, G.; Chen, Y.; et al. Use of copper-cysteamine nanoparticles to simultaneously enable radiotherapy, oxidative therapy and immunotherapy for melanoma treatment. Signal. Transduct. Target. Ther. Nat. 2020, 5, 58. [Google Scholar] [CrossRef]

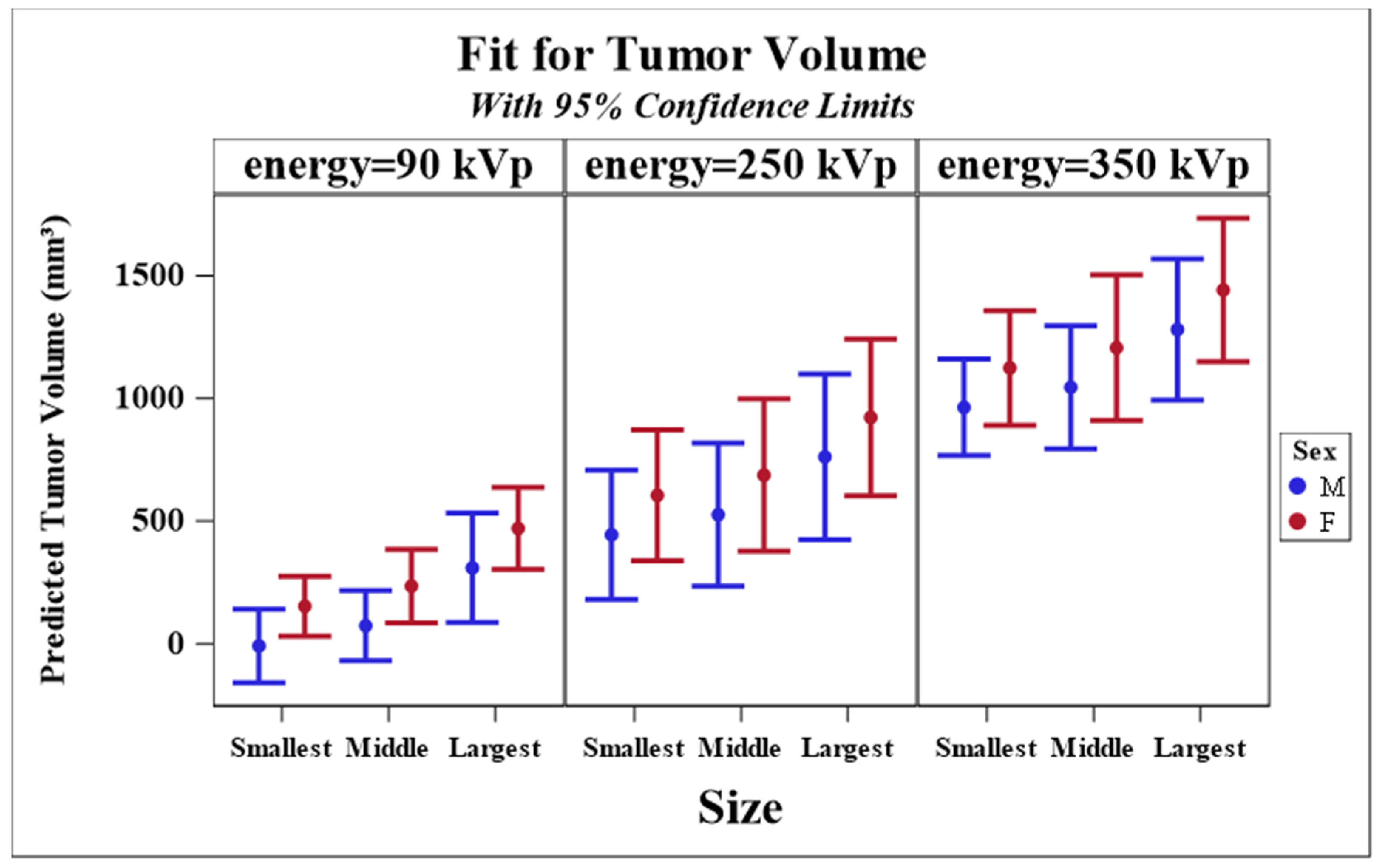
| Parameter Estimates for Response Model | ||||
|---|---|---|---|---|
| Parameter | Estimate | Standard Error | p Value | |
| Intercept | −0.8984 | 0.1490 | <0.0001 | |
| Log(time) | −1.5387 | 0.1143 | <0.0001 | |
| Log(time)2 | 0.6422 | 0.0446 | <0.0001 | |
| Sex | F | 0.3136 | 0.1465 | 0.0323 |
| Sex | M | 0 | - | - |
| Age at Irradiation | −0.2552 | 0.0545 | <0.0001 | |
| Tumor Volume at Irradiation | 0.3751 | 0.0814 | <0.0001 | |
| Energy | 250 kVp | 0.8810 | 0.2854 | 0.0020 |
| Energy | 350 kVp | 1.8928 | 0.2220 | <0.0001 |
| Energy | 90 kVp | 0 | - | - |
| Size | Middle | 0.1599 | 0.1678 | 0.3407 |
| Size | Largest | 0.6183 | 0.1864 | 0.0009 |
| Size | Smallest | 0 | - | - |
| Size Comparisons | |||||
|---|---|---|---|---|---|
| Size 1 | Size 2 | Estimate | Standard Error | p Value | Adjusted p Value |
| Middle | Largest | −0.4584 | 0.2063 | 0.0263 | 0.0675 |
| Middle | Smallest | 0.1599 | 0.1678 | 0.3407 | 0.6068 |
| Largest | Smallest | 0.6183 | 0.1864 | 0.0009 | 0.0026 |
| Energy Comparisons | |||||
| Energy 1 | Energy 2 | Estimate | Standard Error | p Value | Adjusted p Value |
| 250 kVp | 350 kVp | −1.0118 | 0.3534 | 0.0042 | 0.0117 |
| 250 kVp | 90 kVp | 0.8810 | 0.2854 | 0.0020 | 0.0057 |
| 350 kVp | 90 kVp | 1.8928 | 0.2220 | <0.0001 | <0.0001 |
| Parameter Estimates for Missing Data Model | ||||
|---|---|---|---|---|
| Parameter | Estimate | Standard Error | p Value | |
| Intercept | 3.6207 | 3.9875 | 0.3639 | |
| Log(time) | 5.4121 | 3.1964 | 0.0904 | |
| Log(time)2 | −1.6934 | 0.6434 | 0.0085 | |
| Tumor Volume at Previous Day | −0.6733 | 0.1315 | <0.0001 | |
| Sex | F | −0.6169 | 0.3835 | 0.1077 |
| Sex | M | 0 | - | - |
| Age at Irradiation | −0.1016 | 0.2423 | 0.6750 | |
| Tumor Volume at Irradiation | −0.0135 | 0.2244 | 0.9520 | |
| Energy | 250 kVp | −1.4274 | 0.6902 | 0.0386 |
| Energy | 350 kVp | −0.5345 | 0.6811 | 0.4326 |
| Energy | 90 kVp | 0 | - | - |
| Size | Middle | −0.0749 | 0.6110 | 0.9025 |
| Size | Largest | −0.4060 | 0.5793 | 0.4834 |
| Size | Smallest | 0 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sah, B.; Wu, J.; Vanasse, A.; Pandey, N.K.; Chudal, L.; Huang, Z.; Song, W.; Yu, H.; Ma, L.; Chen, W.; et al. Effects of Nanoparticle Size and Radiation Energy on Copper-Cysteamine Nanoparticles for X-ray Induced Photodynamic Therapy. Nanomaterials 2020, 10, 1087. https://doi.org/10.3390/nano10061087
Sah B, Wu J, Vanasse A, Pandey NK, Chudal L, Huang Z, Song W, Yu H, Ma L, Chen W, et al. Effects of Nanoparticle Size and Radiation Energy on Copper-Cysteamine Nanoparticles for X-ray Induced Photodynamic Therapy. Nanomaterials. 2020; 10(6):1087. https://doi.org/10.3390/nano10061087
Chicago/Turabian StyleSah, Bindeshwar, Jing Wu, Adam Vanasse, Nil Kanatha Pandey, Lalit Chudal, Zhenzhen Huang, Wenzhi Song, Hongmei Yu, Lun Ma, Wei Chen, and et al. 2020. "Effects of Nanoparticle Size and Radiation Energy on Copper-Cysteamine Nanoparticles for X-ray Induced Photodynamic Therapy" Nanomaterials 10, no. 6: 1087. https://doi.org/10.3390/nano10061087
APA StyleSah, B., Wu, J., Vanasse, A., Pandey, N. K., Chudal, L., Huang, Z., Song, W., Yu, H., Ma, L., Chen, W., & Antosh, M. P. (2020). Effects of Nanoparticle Size and Radiation Energy on Copper-Cysteamine Nanoparticles for X-ray Induced Photodynamic Therapy. Nanomaterials, 10(6), 1087. https://doi.org/10.3390/nano10061087





