Titanium Dioxide Microscale and Macroscale Structures: A Mini-Review
Abstract
:1. Introduction
2. TiO2 Microscale Structures
2.1. TiO2 Solid Microscale Structures
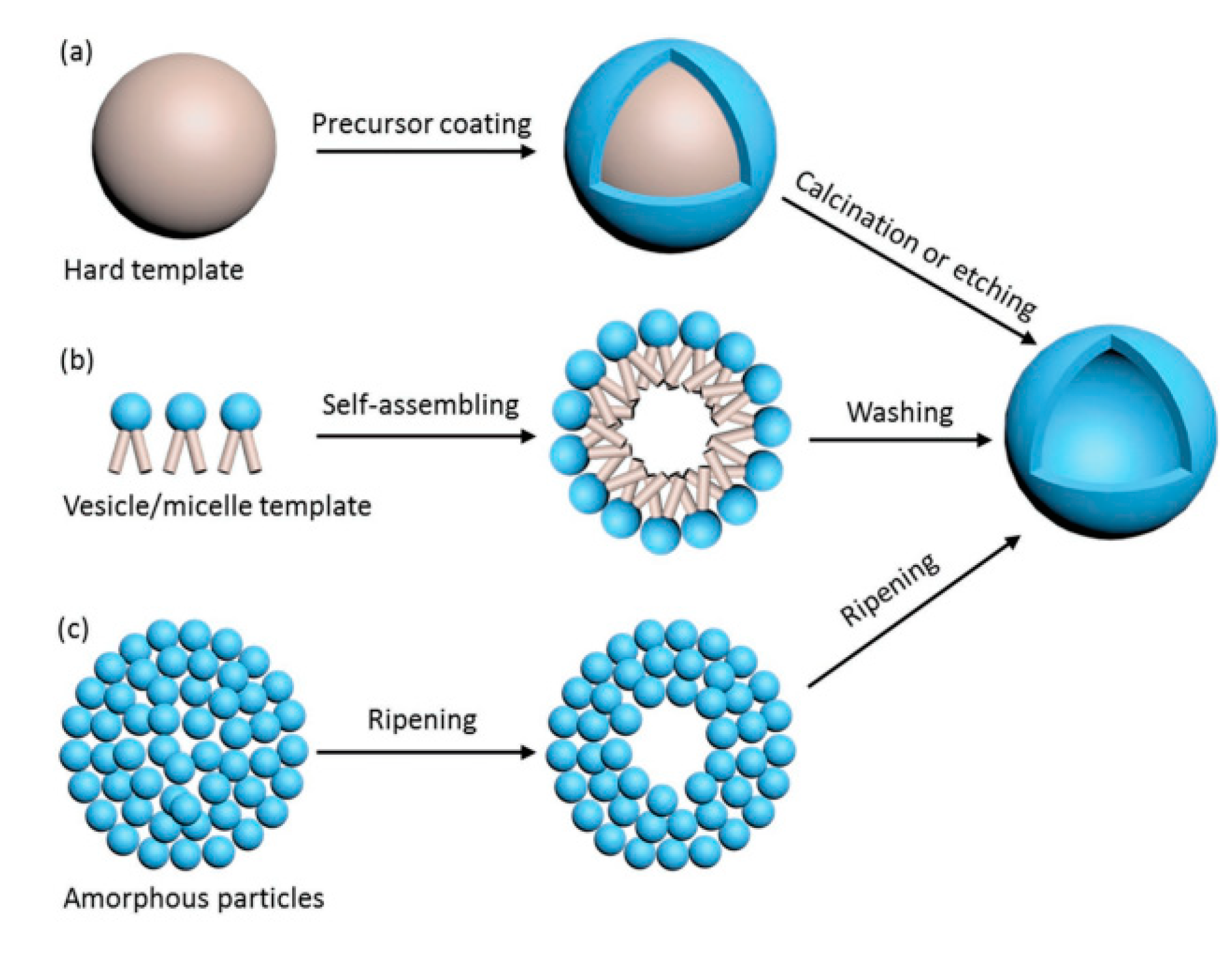
2.2. TiO2 Hollow Microscale Structures
3. TiO2 Macroscale Structures
3.1. Pure TiO2 Pellets
3.2. TiO2 Composite Pellets
3.3. Immobilized TiO2 Macroscale Structure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Erol, M.; Ertugrul, O. HIPed TiO2 dense pellets with improved photocatalytic performance. Ceram. Int. 2018, 44, 2991–2999. [Google Scholar] [CrossRef]
- Mai, N.X.D.; Bae, J.; Kim, I.T.; Park, S.H.; Lee, G.-W.; Kim, J.H.; Lee, D.; Son, H.B.; Lee, Y.-C.; Hur, J. A recyclable, recoverable, and reformable hydrogel-based smart photocatalyst. Environ. Sci. Nano 2017, 4, 955–966. [Google Scholar] [CrossRef]
- Kadam, A.N.; Salunkhe, T.T.; Kim, H.; Lee, S.W. Biogenic synthesis of mesoporous N–S–C tri-doped TiO2 photocatalyst via ultrasonic-assisted derivatization of biotemplate from expired egg white protein. Appl. Surf. Sci. 2020, 518, 146194. [Google Scholar] [CrossRef]
- Scrimieri, L.; Serra, A.; Manno, D.; Alifano, P.; Tredici, S.M.; Calcagnile, M.; Calcagnile, L. TiO2 films by sol-gel spin-coating deposition with microbial antiadhesion properties. Surf. Interface Anal. 2019, 51, 1351–1358. [Google Scholar] [CrossRef]
- Wisitsoraat, A.; Tuantranont, A.; Comini, E.; Sberveglieri, G.; Wlodarski, W. Characterization of n-type and p-type semiconductor gas sensors based on NiOx doped TiO2 thin films. Thin Solid Films 2009, 517, 2775–2780. [Google Scholar] [CrossRef]
- Miyagi, T.; Kamei, M.; Mitsuhashi, T.; Ishigaki, T.; Yamazaki, A. Charge separation at the rutile/anatase interface: A dominant factor of photocatalytic activity. Chem. Phys. Lett. 2004, 390, 399–402. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Park, D.; Lee, Y.-C. Chitosan combined with ZnO, TiO2 and Ag nanoparticles for antimicrobial wound healing applications: A mini review on the research trends. Polymers 2017, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Asahi, R.; Taga, Y.; Mannstadt, W.; Freeman, A.J. Electronic and optical properties of anatase TiO2. Phys. Rev. B 2000, 61, 7459–7465. [Google Scholar] [CrossRef]
- Koelsch, M.; Cassaignon, S.; Ta Thanh Minh, C.; Guillemoles, J.F.; Jolivet, J.P. Electrochemical comparative study of titania (anatase, brookite and rutile) nanoparticles synthesized in aqueous medium. Thin Solid Films 2004, 451, 86–92. [Google Scholar] [CrossRef]
- Arias, L.M.F.; Duran, A.A.; Cardona, D.; Camps, E.; Gomez, M.E.; Zambrano, G. Effect of annealing treatment on the photocatalytic activity of TiO2 thin films deposited by DC reactive magnetron sputtering. J. Phys. Conf. Ser. 2015, 614, 012008. [Google Scholar] [CrossRef]
- Zachariah, A.; Baiju, K.V.; Shukla, S.; Deepa, K.S.; James, J.; Warner, K.G.K. Synergistic effect in photocatalysis as observed for mixed-phase nanocrystalline titania processed via sol-gel solvent mixing and calcination. J. Phys. Chem. C 2008, 112, 11345–11356. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile? - model studies on epitaxial TiO2 films. Sci. Rep. 2015, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuangpho, N.; Le, S.T.T.; Treerujiraphapong, T.; Khanitchaidecha, W.; Nakaruk, A. Enhanced photocatalytic performance of TiO2 particles via effect of anatase-rutile ratio. Phys. E 2015, 67, 18–22. [Google Scholar] [CrossRef]
- Shahi, S.K.; Kaur, N.; Singh, V. Fabrication of phase and morphology controlled pure rutile and rutile/anatase TiO2 nanostructures in functional ionic liquid/water. Appl. Surf. Sci. 2016, 360, 953–960. [Google Scholar] [CrossRef]
- Patra, A.K.; Das, S.K.; Bhaumik, A. Self-assembled mesporous TiO2 spherical nanoparticles by a new templating pathways and its enhanced photoconductivity in the presence of an organic dye. J. Mater. Chem. 2011, 21, 3925–3930. [Google Scholar] [CrossRef]
- Velardi, L.; Scrimieri, L.; Serra, A.; Manno, D.; Calcagnile, L. The synergistic role of pH and calcination temperature in sol–gel titanium dioxide powders. Appl. Phys. A-Mater. 2019, 125, 1–7. [Google Scholar] [CrossRef]
- Yan, X.; Chen, X. Titanium dioxide nanomaterials. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–38. [Google Scholar]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Lazar, M.; Varghese, S.; Nair, S. Photocatalytic water treatment by titanium dioxide: Recent updates. Catalysts 2012, 2, 572–601. [Google Scholar] [CrossRef] [Green Version]
- Xiao, M.; Wang, Z.; Lyu, M.; Luo, B.; Wang, S.; Liu, G.; Cheng, H.M.; Wang, L. Hollow nanostructures for photocatalysis: Advantages and challenges. Adv. Mater. 2018, 31, 1801369. [Google Scholar] [CrossRef]
- MiarAlipour, S.; Friedmann, D.; Scott, J.; Amal, R. TiO2/porous adsorbents: Recent advances and novel applications. J. Hazard. Mater. 2018, 341, 404–423. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hwang, H.M.; Wang, L.; Kim, I.; Yoon, Y.; Lee, H. Solar-light photocatalytic disinfection using crystalline/amorphous low energy bandgap reduced TiO2. Sci. Rep. 2016, 6, 25212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, D.L.; Kuznetsov, A.; Achete, C.A.; Machado, A.E.d.H.; Marques, M. Immobilized TiO2 on glass spheres applied to heterogeneous photocatalysis: Photoactivity, leaching and regeneration process. PeerJ 2018, 6, e4464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhang, M.; Guan, Z.; Li, Q.; He, C.; Yang, J. Synergistic effect of surface and bulk single-electron-trapped oxygen vacancy of TiO2 in the photocatalytic reduction of CO2. Appl. Catal. B Environ. 2017, 206, 300–307. [Google Scholar] [CrossRef]
- Wang, J.; Liu, P.; Fu, X.; Li, Z.; Han, W.; Wang, X. Relationship between oxygen defects and the photocatalytic property of ZnO nanocrystals in nafion membranes. Langmuir 2009, 25, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, M.K.; Sheppard, L.R.; Bak, T.; Nowotny, J. Defect chemistry of titanium dioxide. Application of defect engineering in processing of TiO2-based photocatalysts. J. Phys. Chem. C 2008, 112, 5275–5300. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, N.; Fu, X.; Xu, Y.J. Selective oxidation of benzyl alcohol over TiO2 nanosheets with exposed {0 0 1} facets: Catalyst deactivation and regeneration. Appl. Catal. A Gen. 2013, 453, 181–187. [Google Scholar] [CrossRef]
- Su, R.; Tiruvalam, R.; He, Q.; Dimitratos, N.; Kesavan, L.; Hammond, C.; Lopez-Sanchez, J.A.; Bechstein, R.; Kiely, C.J.; Hutchings, G.J.; et al. Promotion of phenol photodecomposition over TiO2 using Au, Pd, and Au-Pd nanoparticles. ACS Nano 2012, 6, 6284–6292. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, Z.; Niu, M.; Mao, C.; Cao, D.; Cheng, D.; Feng, P.; Sun, Z. A facile and versatile method for preparation of colored TiO2 with enhanced solar-driven photocatalytic activity. Nanoscale 2014, 6, 10216–10223. [Google Scholar] [CrossRef]
- Katal, R.; Salehi, M.; Davood Abadi Farahani, M.H.; Masudy-Panah, S.; Ong, S.L.; Hu, J. Preparation of a new type of black TiO2 under a vacuum atmosphere for sunlight photocatalysis. ACS Appl. Mater. Interfaces 2018, 10, 35316–35326. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Edit. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Zhou, W.; Du, F.; Zhang, L.; Li, Z.; Zhang, H.; Li, W. Facile synthesis of hierarchical porous tio2 ceramics with enhanced photocatalytic performance for micropolluted pesticide degradation. ACS Appl. Mater. Interfaces 2014, 6, 16653–16660. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.K.H.; Park, D.; Pham, T.N.; An, Y.; Choi, J.S.; Lee, H.-U.; Kwon, O.-H.; Moon, J.-Y.; Kim, K.-T.; Lee, Y.-C. Synthesis of MgAC-Fe3O4/TiO2 hybrid nanocomposites via sol-gel chemistry for water treatment by photo-fenton and photocatalytic reactions. Sci. Rep. 2019, 9, 11855. [Google Scholar] [CrossRef] [Green Version]
- Fabiyi, M.E.; Skelton, R.L. Photocatalytic mineralisation of methylene blue using buoyant TiO2-coated polystyrene beads. J. Photochem. Photobiol. A 2000, 132, 121–128. [Google Scholar] [CrossRef]
- Hosseini, S.N.; Borghei, S.M.; Vossoughi, M.; Taghavinia, N. Immobilization of tio2 on perlite granules for photocatalytic degradation of phenol. Appl. Catal. B Environ. 2007, 74, 53–62. [Google Scholar] [CrossRef]
- Modestov, A.; Glezer, V.; Marjasin, I.; Lev, O. Photocatalytic degradation of chlorinated phenoxyacetic acids by a new buoyant titania-exfoliated graphite composite photocatalyst. J. Phys. Chem. B 1997, 101, 4623–4629. [Google Scholar] [CrossRef]
- Yada, M.; Ohya, M.; Machida, M.; Kijima, T. Mesoporous gallium oxide structurally stabilized by yttrium oxide. Langmuir 2000, 16, 4752–4755. [Google Scholar] [CrossRef]
- Pulido Melián, E.; Nereida Suárez, M.; Jardiel, T.; Calatayud, D.G.; del Campo, A.; Doña-Rodríguez, J.M.; Araña, J.; González Díaz, O.M. Highly photoactive TiO2 microspheres for photocatalytic production of hydrogen. Int. J. Hydrog. Energy 2019, 44, 24653–24666. [Google Scholar] [CrossRef]
- Du, J.; Chen, W.; Zhang, C.; Liu, Y.; Zhao, C.; Dai, Y. Hydrothermal synthesis of porous TiO2 microspheres and their photocatalytic degradation of gaseous benzene. Chem. Eng. J. 2011, 170, 53–58. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, X.; Yin, L.; Huang, W.; Hacohen, Y.R.; Gedanken, A. Sonochemical synthesis of mesoporous titanium oxide with wormhole-like framework structures. Adv. Mater. 2000, 12, 1183–1186. [Google Scholar] [CrossRef]
- Kluson, P.; Kacer, P.; Cajthaml, T.; Kalaji, M. Preparation of titania mesoporous materials using a surfactant-mediated sol-gel method. J. Mater. Chem. 2001, 11, 644–651. [Google Scholar] [CrossRef]
- Yun, H.S.; Miyazawa, K.; Zhou, H.S.; Honma, I.; Kuwabara, M. Synthesis of mesoporous thin TiO2 films with hexagonal pore structures using triblock copolymer templates. Adv. Mater. 2001, 13, 1377–1380. [Google Scholar] [CrossRef]
- Yu, J.C.; Zhang, L.; Yu, J. Direct sonochemical preparation and characterization of highly active mesoporous TiO2 with a bicrystalline framework. Chem. Mater. 2002, 14, 4647–4653. [Google Scholar] [CrossRef]
- Luo, H.; Wang, C.; Yan, Y. Synthesis of mesostructured titania with controlled crystalline framework. Chem. Mater. 2003, 15, 3841–3846. [Google Scholar] [CrossRef]
- Ma, X.; Wang, X.; Yu, C.; Song, Y.; Liang, J.; Min, Q.; Zhang, F. Effects of primary nanobuilding blocks on the photocatalytic performance of TiO2 hierarchical hollow microspheres. J. Alloys Compd. 2019, 773, 352–360. [Google Scholar] [CrossRef]
- Baolong, Z.; Baishun, C.; Keyu, S.; Shangjin, H.; Xiaodong, L.; Zongjie, D.; Kelian, Y. Preparation and characterization of nanocrystal grain TiO2 porous microspheres. Appl. Catal. B Environ. 2003, 40, 253–258. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Wu, Y.; Luo, Y.; Zhang, L. The formation of mesoporous TiO2 spheres via a facile chemical process. J. Phys. Chem. B 2005, 109, 5478–5481. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Li, Y.; Shao, W.; Kang, Y.; Wang, H.; Huang, Z.; Liao, X.; Yin, G. Antibacterial properties of TiO2 ceramic pellets prepared using nano TiO2 powder. J. Wuhan Univ. Technol. 2009, 24, 337. [Google Scholar] [CrossRef]
- Li, R.; Jia, Y.; Bu, N.; Wu, J.; Zhen, Q. Photocatalytic degradation of methyl blue using Fe2O3/TiO2 composite ceramics. J. Alloys Compd. 2015, 643, 88–93. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.; Yoo, K.S.; Lee, T.G. Synthesis of mesoporous TiO2/γ-Al2O3 composite granules with different sol composition and calcination temperature. Powder Technol. 2008, 181, 83–88. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, W.; Zhang, X.; Sun, B.; Wang, L.; Pan, K.; Jiang, B.; Tian, G.; Fu, H. Self-floating amphiphilic black TiO2 foams with 3D macro-mesoporous architectures as efficient solar-driven photocatalysts. Appl. Catal. B Environ. 2017, 206, 336–343. [Google Scholar] [CrossRef]
- Meynen, V.; Cool, P.; Vansant, E.F. Verified syntheses of mesoporous materials. Micropor. Mesopor. Mater. 2009, 125, 170–223. [Google Scholar] [CrossRef]
- Wang, L.; Tomura, S.; Maeda, M.; Ohashi, F.; Inukai, K.; Suzuki, M. Synthesis of mesoporous TiO2 spheres under static condition. Chem. Lett. 2000, 29, 1414–1415. [Google Scholar] [CrossRef]
- Balati, A.; Tek, S.; Nash, K.; Shipley, H. Nanoarchitecture of TiO2 microspheres with expanded lattice interlayers and its heterojunction to the laser modified black TiO2 using pulsed laser ablation in liquid with improved photocatalytic performance under visible light irradiation. J. Colloid Interface Sci. 2019, 541, 234–248. [Google Scholar] [CrossRef]
- Vicent, M.; Sánchez, E.; Santacruz, I.; Moreno, R. Dispersion of TiO2 nanopowders to obtain homogeneous nanostructured granules by spray-drying. J. Eur. Ceram. Soc. 2011, 31, 1413–1419. [Google Scholar] [CrossRef]
- Faure, B.; Sæderup Lindeløv, J.; Wahlberg, M.; Adkins, N.; Jackson, P.; Bergström, L. Spray drying of TiO2 nanoparticles into redispersible granules. Powder Technol. 2010, 203, 384–388. [Google Scholar] [CrossRef]
- Pal, S.; Laera, A.M.; Licciulli, A.; Catalano, M.; Taurino, A. Biphase tio2 microspheres with enhanced photocatalytic activity. Ind. Eng. Chem. Res. 2014, 53, 7931–7938. [Google Scholar] [CrossRef]
- Vicent, M.; Sánchez, E.; Molina, T.; Nieto, M.I.; Moreno, R. Comparison of freeze drying and spray drying to obtain porous nanostructured granules from nanosized suspensions. J. Eur. Ceram. Soc. 2012, 32, 1019–1028. [Google Scholar] [CrossRef] [Green Version]
- Mun, J.Y.; Park, J.Y.; Kwak, M.; Moon, B.K.; Jang, K.; Yang, H.K. Synthesis of TiO2 spheres and their utilization in the enhancement light-extraction efficiency of wleds. Mater. Res. Bull. 2017, 94, 456–462. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Li, H.; Ge, H.; Bian, Z. The enhanced photoreduction of Cr(VI) to Cr(III) using carbon dots coupled TiO2 mesocrystals. Appl. Catal. B Environ. 2018, 226, 213–219. [Google Scholar] [CrossRef]
- Zhang, L.-W.; Fu, H.-B.; Zhu, Y.-F. Efficient TiO2 photocatalysts from surface hybridization of TiO2 particles with graphite-like carbon. Adv. Funct. Mater. 2008, 18, 2180–2189. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Edit. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Singh, S.; Mahalingam, H.; Singh, P.K. Polymer-supported titanium dioxide photocatalysts for environmental remediation: A review. Appl. Catal. A Gen. 2013, 462, 178–195. [Google Scholar] [CrossRef]
- Magalhães, F.; Lago, R.M. Floating photocatalysts based on TiO2 grafted on expanded polystyrene beads for the solar degradation of dyes. Sol. Energy 2009, 83, 1521–1526. [Google Scholar] [CrossRef]
- Baek, M.-H.; Jung, W.-C.; Yoon, J.-W.; Hong, J.-S.; Lee, Y.-S.; Suh, J.-K. Preparation, characterization and photocatalytic activity evaluation of micro- and mesoporous TiO2/spherical activated carbon. J. Ind. Eng. Chem. 2013, 19, 469–477. [Google Scholar] [CrossRef]
- Rosenberg, I.; Brock, J.R.; Heller, A. Collection optics of TiO2 photocatalyst on hollow glass microbeads floating on oil slicks. J. Phys. Chem. A 1992, 96, 3423–3428. [Google Scholar] [CrossRef]
- Berry, R.J.; Mueller, M.R. Photocatalytic decomposition of crude oil slicks using TiO2 on a floating substrate. Microchem. J. 1994, 50, 28–32. [Google Scholar] [CrossRef]
- Syoufian, A.; Nakashima, K. Degradation of methylene blue in aqueous dispersion of hollow titania photocatalyst: Optimization of reaction by peroxydisulfate electron scavenger. J. Colloid Interface Sci. 2007, 313, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Holdich, R.G.; Ipek, I.Y.; Lazrigh, M.; Shama, G. Production and evaluation of floating photocatalytic composite particles formed using pickering emulsions and membrane emulsification. Ind. Eng. Chem. Res. 2012, 51, 12509–12516. [Google Scholar] [CrossRef] [Green Version]
- Goedecke, C.; Sojref, R.; Nguyen, T.Y.; Piechotta, C. Immobilization of photocatalytically active TiO2 nanopowder by high shear granulation. Powder Technol. 2017, 318, 465–470. [Google Scholar] [CrossRef]
- Shelimov, B.N.; Tolkachev, N.N.; Tkachenko, O.P.; Baeva, G.N.; Klementiev, K.V.; Stakheev, A.Y.; Kazansky, V.B. Enhancement effect of TiO2 dispersion over alumina on the photocatalytic removal of NOx admixtures from O2-N2 flow. J. Photochem. Photobiol. A 2008, 195, 81–88. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, X.; Ng, J.; Sun, D.D. Preparation and application of TiO2/Al2O3 microspherical photocatalyst for water treatment. Water Sci. Technol. Water Supply 2009, 9, 39–44. [Google Scholar] [CrossRef]
- Araña, J.; Doña-Rodríguez, J.M.; Cabo, C.G.I.; González-Díaz, O.; Herrera-Melián, J.A.; Pérez-Peña, J. Ftir study of gas-phase alcohols photocatalytic degradation with TiO2 and AC-TiO2. Appl. Catal. B Environ. 2004, 53, 221–232. [Google Scholar] [CrossRef]
- Ouzzine, M.; Romero-Anaya, A.J.; Lillo-Ródenas, M.A.; Linares-Solano, A. Spherical activated carbon as an enhanced support for TiO2/AC photocatalysts. Carbon 2014, 67, 104–118. [Google Scholar] [CrossRef]
- Yang, H.G.; Zeng, H.C. Preparation of hollow anatase TiO2 nanospheres via Ostwald ripening. J. Phys. Chem. B 2004, 108, 3492–3495. [Google Scholar] [CrossRef]
- Alosfur, F.K.M.; Ridha, N.J.; Jumali, M.H.H.; Radiman, S. One-step formation of TiO2 hollow spheres via a facile microwave-assisted process for photocatalytic activity. Nanotechnology 2018, 29, 145707. [Google Scholar] [CrossRef] [PubMed]
- Skrabalak, S.E.; Au, L.; Li, X.; Xia, Y. Facile synthesis of Ag nanocubes and Au nanocages. Nat. Protoc. 2007, 2, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, T.; Ge, J.; Yin, Y. Permeable silica shell through surface-protected etching. Nano Lett. 2008, 8, 2867–2871. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, B.; Liu, Y.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y. An anion exchange approach to Bi2WO6 hollow microspheres with efficient visible light photocatalytic reduction of CO2 to methanol. Chem. Commun. 2012, 48, 9729–9731. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Yin, D.; Li, X.; Li, L.; Mu, J. One-pot template-free preparation of mesoporous TiO2 hollow spheres and their photocatalytic activity. Mater. Res. Bull. 2012, 47, 3065–3069. [Google Scholar] [CrossRef]
- Xie, F.; Wang, J.; Li, Y.; Dou, J.; Wei, M. One-step synthesis of hierarchical SnO2/TiO2 composite hollow microspheres as an efficient scattering layer for dye-sensitized solar cells. Electrochim. Acta 2019, 296, 142–148. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Roy, M.; Kundu, S.; Naskar, M.K. TiO2 hollow microspheres impregnated with biogenic gold nanoparticles for the efficient visible light-induced photodegradation of phenol. J. Phys. Chem. Solids 2019, 129, 329–339. [Google Scholar] [CrossRef]
- Balati, A.; Matta, A.; Nash, K.; Shipley, H.J. Heterojunction of vertically aligned MoS2 layers to hydrogenated black TiO2 and rutile based inorganic hollow microspheres for the highly enhanced visible light arsenic photooxidation. Compos. Part B Eng. 2020, 185, 107785. [Google Scholar] [CrossRef]
- Ren, T.-Z.; Yuan, Z.-Y.; Su, B.-L. Surfactant-assisted preparation of hollow microspheres of mesoporous TiO2. Chem. Phys. Lett. 2003, 374, 170–175. [Google Scholar]
- Zhang, L.; Wan, M.; Wei, Y. Polyaniline/TiO2 microspheres prepared by a template-free method. Synthetic Met. 2005, 151, 1–5. [Google Scholar] [CrossRef]
- Fuhrhop, J.H.; Helfrich, W. Fluid and solid fibers made of lipid molecular bilayers. Chem. Rev. 1993, 93, 1565–1582. [Google Scholar] [CrossRef]
- Kim, B.J.; Oh, S.G.; Han, M.G.; Im, S.S. Preparation of polyaniline nanoparticles in micellar solutions as polymerization medium. Langmuir 2000, 16, 5841–5845. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Z.; Wan, M. Formation mechanism of self-assembled polyaniline micro/nanotubes. Langmuir 2002, 18, 917–921. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, Z.; Wan, M. Nanostructures of polyaniline doped with inorganic acids. Macromolecules 2002, 35, 5937–5942. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, M. Polyaniline/TiO2 composite nanotubes. J. Phys. Chem. B 2003, 107, 6748–6753. [Google Scholar] [CrossRef]
- Zurmühl, C.; Popescu, R.; Gerthsen, D.; Feldmann, C. Microemulsion-based synthesis of nanoscale TiO2 hollow spheres. Solid State Sci. 2011, 13, 1505–1509. [Google Scholar] [CrossRef]
- Hozumi, A.; Yokogawa, Y.; Kameyama, T.; Hiraku, K.; Sugimura, H.; Takai, O.; Okido, M. Photocalcination of mesoporous silica films using vacuum ultraviolet light. Adv. Mater. 2000, 12, 985–987. [Google Scholar] [CrossRef]
- Thurn-Albrecht, T.; Schotter, J.; Kastle, G.A.; Emley, N.; Shibauchi, T.; Krusin-Elbaum, L.; Guarini, K.; Black, C.T.; Tuominen, M.T.; Russell, T.P. Ultrahigh-density nanowire arrays grown in self-assembled diblock copolymer templates. Science 2000, 290, 2126–2129. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Sasaki, T.; Ebina, Y.; Kurashima, K.; Watanabe, M. Fabrication of controllable ultrathin hollow shells by layer-by-layer assembly of exfoliated titania nanosheets on polymer templates. Chem. Mater. 2002, 14, 4827–4832. [Google Scholar] [CrossRef]
- Syoufian, A.; Inoue, Y.; Yada, M.; Nakashima, K. Preparation of submicrometer-sized titania hollow spheres by templating sulfonated polystyrene latex particles. Mater. Lett. 2007, 61, 1572–1575. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, C.-S. Effect of hydrogen peroxide, periodate and persulfate on photocatalysis of 2-chlorobiphenyl in aqueous TiO2 suspensions. Water Res. 1999, 33, 2031–2036. [Google Scholar] [CrossRef]
- Irmak, S.; Kusvuran, E.; Erbatur, O. Degradation of 4-chloro-2-methylphenol in aqueous solution by UV irradiation in the presence of titanium dioxide. Appl. Catal. B Environ. 2004, 54, 85–91. [Google Scholar] [CrossRef]
- Muruganandham, M.; Swaminathan, M. Photocatalytic decolourisation and degradation of reactive orange 4 by TiO2-UV process. Dyes Pig. 2006, 68, 133–142. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.; Hu, Y.; Guo, C.; Qian, H.; Zhang, F.; Lou, X.W. Magnetic-field induced formation of 1D Fe3O4/C/Cds coaxial nanochains as highly efficient and reusable photocatalysts for water treatment. J. Mater. Chem. 2011, 21, 18359–18364. [Google Scholar] [CrossRef]
- Meng, H.L.; Cui, C.; Shen, H.L.; Liang, D.Y.; Xue, Y.Z.; Li, P.G.; Tang, W.H. Synthesis and photocatalytic activity of TiO2@Cds and Cds@TiO2 double-shelled hollow spheres. J. Alloys Compd. 2012, 527, 30–35. [Google Scholar] [CrossRef]
- Xue, C.; Wang, T.; Yang, G.; Yang, B.; Ding, S. A facile strategy for the synthesis of hierarchical TiO2/Cds hollow sphere heterostructures with excellent visible light activity. J. Mater. Chem. A 2014, 2, 7674–7679. [Google Scholar] [CrossRef]
- Iida, M.; Sasaki, T.; Watanabe, M. Titanium dioxide hollow microspheres with an extremely thin shell. Chem. Mater. 1998, 10, 3780–3782. [Google Scholar] [CrossRef]
- McDonald, C.J.; Devon, M.J. Hollow latex particles: Synthesis and applications. Adv. Colloid Interface Sci. 2002, 99, 181–213. [Google Scholar] [CrossRef]
- Ohno, T.; Akiyoshi, M.; Umebayashi, T.; Asai, K.; Mitsui, T.; Matsumura, M. Preparation of S-doped TiO2 photocatalysts and their photocatalytic activities under visible light. Appl. Catal. A Gen. 2004, 265, 115–121. [Google Scholar] [CrossRef]
- Umebayashi, T.; Yamaki, T.; Itoh, H.; Asai, K. Band gap narrowing of titanium dioxide by sulfur doping. Appl. Phys. Lett. 2002, 81, 454–456. [Google Scholar] [CrossRef]
- Martyanov, I.N.; Uma, S.; Rodrigues, S.; Klabunde, K.J. Structural defects cause TiO2-based photocatalysts to be active in visible light. Chem. Commun. 2004, 21, 2476–2477. [Google Scholar] [CrossRef]
- Irie, H.; Washizuka, S.; Hashimoto, K. Hydrophilicity on carbon-doped TiO2 thin films under visible light. Thin Solid Films 2006, 510, 21–25. [Google Scholar] [CrossRef]
- Syoufian, A.; Satriya, O.H.; Nakashima, K. Photocatalytic activity of titania hollow spheres: Photodecomposition of methylene blue as a target molecule. Catal. Commun. 2007, 8, 755–759. [Google Scholar] [CrossRef]
- Dervos, C.T.; Thirios, E.; Novacovich, J.; Vassiliou, P.; Skafidas, P. Permittivity properties of thermally treated TiO2. Mater. Lett. 2004, 58, 1502–1507. [Google Scholar] [CrossRef]
- Destaillats, H.; Hung, H.M.; Hoffmann, M.R. Degradation of alkylphenol ethoxylate surfactants in water with ultrasonic irradiation. Environ. Sci. Technol. 2000, 34, 311–317. [Google Scholar] [CrossRef]
- Nagata, Y.; Nakagawa, M.; Okuno, H.; Mizukoshi, Y.; Yim, B.; Maeda, Y. Sonochemical degradation of chlorophenols in water. Ultrason. Sonochem. 2000, 7, 115–120. [Google Scholar] [CrossRef]
- Stavarache, C.; Yim, B.; Vinatoru, M.; Maeda, Y. Sonolysis of chlorobenzene in Fenton-type aqueous systems. Ultrason. Sonochem. 2002, 9, 291–296. [Google Scholar] [CrossRef]
- Suslick, K.S.; Hammerton, D.A.; Cline, R.E. The sonochemical hot spot. J. Am. Ceram. Soc. 1986, 108, 5641–5642. [Google Scholar] [CrossRef]
- Shimizu, N.; Ogino, C.; Dadjour, M.F.; Murata, T. Sonocatalytic degradation of methylene blue with TiO2 pellets in water. Ultrason. Sonochem. 2007, 14, 184–190. [Google Scholar] [CrossRef]
- Sekiguchi, H.; Saita, Y. Effect of alumina particles on sonolysis degradation of chlorobenzene in aqueous solution. J. Chem. Eng. Jpn. 2001, 34, 1045–1048. [Google Scholar] [CrossRef]
- Marschall, H.B.; Morch, K.A.; Keller, A.P.; Kjeldsen, M. Cavitation inception by almost spherical solid particles in water. Phys. Fluids 2003, 15, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Tuziuti, T.; Yasui, K.; Sivakumar, M.; Iida, Y. Correlation between acoustic cavitation noise and yield enhancement of sonochemical reaction by particle addition. J. Phys. Chem. A 2005, 109, 4869–4872. [Google Scholar] [CrossRef] [PubMed]
- Jasmann, J.R.; Borch, T.; Sale, T.C.; Blotevogel, J. Advanced electrochemical oxidation of 1,4-dioxane via dark catalysis by novel titanium dioxide (TiO2) pellets. Environ. Sci. Technol. 2016, 50, 8817–8826. [Google Scholar] [CrossRef] [PubMed]
- Itatani, K.; Tsujimoto, T.; Kishimoto, A. Thermal and optical properties of transparent magnesium oxide ceramics fabricated by post hot-isostatic pressing. J. Eur. Ceram. Soc. 2006, 26, 639–645. [Google Scholar] [CrossRef]
- Ergun, C. Enhanced phase stability in hydroxylapatite/zirconia composites with hot isostatic pressing. Ceram. Int. 2011, 37, 935–942. [Google Scholar] [CrossRef]
- Ahn, J.P.; Park, J.K.; Kim, G. Effect of compact density on phase transition kinetics from anatase phase to rutile phase during sintering of ultrafine titania powder compacts. Nanostruct. Mater. 1998, 10, 1087–1096. [Google Scholar] [CrossRef]
- Mazaheri, M.; Razavi Hesabi, Z.; Sadrnezhaad, S.K. Two-step sintering of titania nanoceramics assisted by anatase-to-rutile phase transformation. Scripta Mater. 2008, 59, 139–142. [Google Scholar] [CrossRef]
- Kitamura, A.; Kubodera, S.; Yamamoto, H.; Miyamoto, A.; Tsukui, T. Prevention of the color change in hip′ing of zirconia ceramics. In Hot Isostatic Pressing-Theory and Applications; Koizumi, M., Ed.; Springer: Dordrecht, The Netherlands, 1992; pp. 171–174. [Google Scholar]
- Gan, L.; Park, Y.-J.; Park, M.-J.; Kim, H.; Kim, J.-M.; Ko, J.-W.; Lee, J.-W. Facile fabrication of highly transparent yttria ceramics with fine microstructures by a hot-pressing method. J. Am. Ceram. Soc. 2015, 98, 2002–2004. [Google Scholar] [CrossRef]
- Michálek, M.; Michálková, M.; Blugan, G.; Kuebler, J. Effect of carbon contamination on the sintering of alumina ceramics. J. Eur. Ceram. Soc. 2018, 38, 193–199. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Yu, J.G.; Su, Y.R.; Cheng, B. Template-free fabrication and enhanced photocatalytic activity of hierarchical macro-/mesoporous titania. Adv. Funct. Mater. 2007, 17, 1984–1990. [Google Scholar] [CrossRef]
- Wang, G.; Xu, L.; Zhang, J.; Yin, T.; Han, D. Enhanced photocatalytic activity of TiO2 powders (P25) via calcination treatment. Int. J. Photoenergy 2012, 2012, 265760. [Google Scholar] [CrossRef] [Green Version]
- Gamboa, J.A.; Pasquevich, D.M. Effect of chlorine atmosphere on the anatase-rutile transformation. J. Am. Ceram. Soc. 1992, 75, 2934–2938. [Google Scholar] [CrossRef]
- Su, T.; Yang, Y.; Na, Y.; Fan, R.; Li, L.; Wei, L.; Yang, B.; Cao, W. An insight into the role of oxygen vacancy in hydrogenated TiO2 nanocrystals in the performance of dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2015, 7, 3754–3763. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-H.; Lee, E.-J.; Yoon, B.-H.; Song, J.-H.; Kim, H.-E.; Kim, H.-W. Effect of polystyrene addition on freeze casting of ceramic/camphene slurry for ultra-high porosity ceramics with aligned pore channels. J. Am. Ceram. Soc. 2006, 89, 3646–3653. [Google Scholar] [CrossRef]
- Soon, Y.M.; Shin, K.H.; Koh, Y.H.; Lee, J.H.; Kim, H.E. Compressive strength and processing of camphene-based freeze cast calcium phosphate scaffolds with aligned pores. Mater. Lett. 2009, 63, 1548–1550. [Google Scholar] [CrossRef]
- Liu, H.; Du, X.; Xing, X.; Wang, G.; Qiao, S.Z. Highly ordered mesoporous Cr2O3 materials with enhanced performance for gas sensors and lithium ion batteries. Chem. Commun. 2012, 48, 865–867. [Google Scholar] [CrossRef]
- Liu, J.; Yang, T.; Wang, D.W.; Lu, G.Q.; Zhao, D.; Qiao, S.Z. A facile soft-template synthesis of mesoporous polymeric and carbonaceous nanospheres. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Liu, H.; Chen, S.; Wang, G.; Qiao, S.Z. Ordered mesoporous core/shell SnO2/C nanocomposite as high-capacity anode material for lithium-ion batteries. Chem. Eur. J. 2013, 19, 16897–16901. [Google Scholar] [CrossRef]
- Hong, C.; Du, J.; Liang, J.; Zhang, X.; Han, J. Functionally graded porous ceramics with dense surface layer produced by freeze-casting. Ceram. Int. 2011, 37, 3717–3722. [Google Scholar] [CrossRef]
- Araki, K.; Halloran, J.W. New freeze-casting technique for ceramics with sublimable vehicles. J. Am. Ceram. Soc. 2005, 87, 1859–1863. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Sun, F.; Pan, K.; Tian, G.; Jiang, B.; Ren, Z.; Tian, C.; Fu, H. Well-ordered large-pore mesoporous anatase TiO2 with remarkably high thermal stability and improved crystallinity: Preparation, characterization, and photocatalytic performance. Adv. Funct. Mater. 2011, 21, 1922–1930. [Google Scholar] [CrossRef]
- Inagaki, M.; Kojin, F.; Tryba, B.; Toyoda, M. Carbon-coated anatase: The role of the carbon layer for photocatalytic performance. Carbon 2005, 43, 1652–1659. [Google Scholar] [CrossRef]
- Keller, N.; Rebmann, G.; Barraud, E.; Zahraa, O.; Keller, V. Macroscopic carbon nanofibers for use as photocatalyst support. Catal. Today 2005, 101, 323–329. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Bouazza, N.; Berenguer-Murcia, A.; Linares-Salinas, J.J.; Soto, P.; Linares-Solano, A. Photocatalytic oxidation of propene at low concentration. Appl. Catal. B Environ. 2007, 71, 298–309. [Google Scholar] [CrossRef]
- Ibusuki, T.; Takeuchi, K. Removal of low concentration nitrogen oxides through photoassisted heterogeneous catalysis. J. Mol. Catal. 1994, 88, 93–102. [Google Scholar] [CrossRef]
- Takeda, N.; Torimoto, T.; Sampath, S.; Kuwabata, S.; Yoneyama, H. Effects of inert supports for titanium dioxide loading on enhancement of photodecomposition rate of gaseous propionaldehyde. J. Phys. Chem. 1995, 99, 9986–9991. [Google Scholar] [CrossRef]
- Takeda, N.; Ohtani, M.; Torimoto, T.; Kuwabata, S.; Yoneyama, H. Evaluation of diffusibility of adsorbed propionaldehyde on titanium dioxide-loaded adsorbent photocatalyst films from its photodecomposition rate. J. Phys. Chem. B 1997, 101, 2644–2649. [Google Scholar] [CrossRef]
- Tsumura, T.; Kojitani, N.; Umemura, H.; Toyoda, M.; Inagaki, M. Composites between photoactive anatase-type TiO2 and adsorptive carbon. Appl. Surf. Sci. 2002, 196, 429–436. [Google Scholar] [CrossRef]
- Jitianu, A.; Cacciaguerra, T.; Benoit, R.; Delpeux, S.; Béguin, F.; Bonnamy, S. Synthesis and characterization of carbon nanotubes-TiO2 nanocomposites. Carbon 2004, 42, 1147–1151. [Google Scholar] [CrossRef]
- Araña, J.; Doña-Rodríguez, J.M.; Tello Rendón, E.; Garriga, I.; Cabo, C.; González-Díaz, O.; Herrera-Melián, J.A.; Pérez-Peña, J.; Colón, G.; Navío, J.A. TiO2 activation by using activated carbon as a support: Part I. Surface characterisation and decantability study. Appl. Catal. B Environ. 2003, 44, 161–172. [Google Scholar] [CrossRef]
- Bouazza, N.; Lillo-Ródenas, M.A.; Linares-Solano, A. Enhancement of the photocatalytic activity of pelletized TiO2 for the oxidation of propence at low concentration. Appl. Catal. B Environ. 2008, 77, 284–293. [Google Scholar] [CrossRef]
- Pal, B.; Sharon, M.; Nogami, G. Preparation and characterization of TiO2/Fe2O3 binary mixed oxides and its photocatalytic properties. Mater. Chem. Phys. 1999, 59, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.C.; Lee, D.K. Preparation of TiO2-coated hollow glass beads and their application to the control of algal growth in eutrophic water. Microchem. J. 2005, 80, 227–232. [Google Scholar] [CrossRef]
- Feitz, A.J.; Waite, T.D.; Jones, G.J.; Boyden, B.H.; Orr, P.T. Photocatalytic degradation of the blue green algal toxin microcystin-LR in a natural organic-aqueous matrix. Environ. Sci. Technol. 1999, 33, 243–249. [Google Scholar] [CrossRef]
- Hinojosa-Reyes, M.; Arriaga, S.; Diaz-Torres, L.A.; Rodriguez-Gonzalez, V. Gas-phase photocatalytic degradation of ethylbenzene over perlite granules coated with indium doped TiO2. Chem. Eng. J. 2013, 224, 106–113. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.; Han, Y.; Park, J. Preparation of dip-coated TiO2 photocatalyst on ceramic foam pellets. J. Mater. Sci. 2005, 41, 6150–6153. [Google Scholar] [CrossRef]
- Han, H.; Bai, R. Buoyant photocatalyst with greatly enhanced visible-light activity prepared through a low temperature hydrothermal method. Ind. Eng. Chem. Res. 2009, 48, 2891–2898. [Google Scholar] [CrossRef]
- Velásquez, J.; Valencia, S.; Rios, L.; Restrepo, G.; Marín, J. Characterization and photocatalytic evaluation of polypropylene and polyethylene pellets coated with P25 TiO2 using the controlled-temperature embedding method. Chem. Eng. J. 2012, 203, 398–405. [Google Scholar] [CrossRef]
- Rajaraman, T.S.; Parikh, S.P.; Gandhi, V.G. Black TiO2: A review of its properties and conflicting trends. Chem. Eng. J. 2019, 389, 123918. [Google Scholar] [CrossRef]









| Advantages | Disadvantages | Future Perspectives | |
|---|---|---|---|
| TiO2 microscale structures |
|
|
|
| TiO2 macroscale structures |
|
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, V.K.H.; Tran, V.V.; Moon, J.-Y.; Park, D.; Lee, Y.-C. Titanium Dioxide Microscale and Macroscale Structures: A Mini-Review. Nanomaterials 2020, 10, 1190. https://doi.org/10.3390/nano10061190
Bui VKH, Tran VV, Moon J-Y, Park D, Lee Y-C. Titanium Dioxide Microscale and Macroscale Structures: A Mini-Review. Nanomaterials. 2020; 10(6):1190. https://doi.org/10.3390/nano10061190
Chicago/Turabian StyleBui, Vu Khac Hoang, Vinh Van Tran, Ju-Young Moon, Duckshin Park, and Young-Chul Lee. 2020. "Titanium Dioxide Microscale and Macroscale Structures: A Mini-Review" Nanomaterials 10, no. 6: 1190. https://doi.org/10.3390/nano10061190
APA StyleBui, V. K. H., Tran, V. V., Moon, J.-Y., Park, D., & Lee, Y.-C. (2020). Titanium Dioxide Microscale and Macroscale Structures: A Mini-Review. Nanomaterials, 10(6), 1190. https://doi.org/10.3390/nano10061190










