A Sensitive Impedimetric Aptasensor Based on Carbon Nanodots Modified Electrode for Detection of 17ß-Estradiol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus and Instrumentation
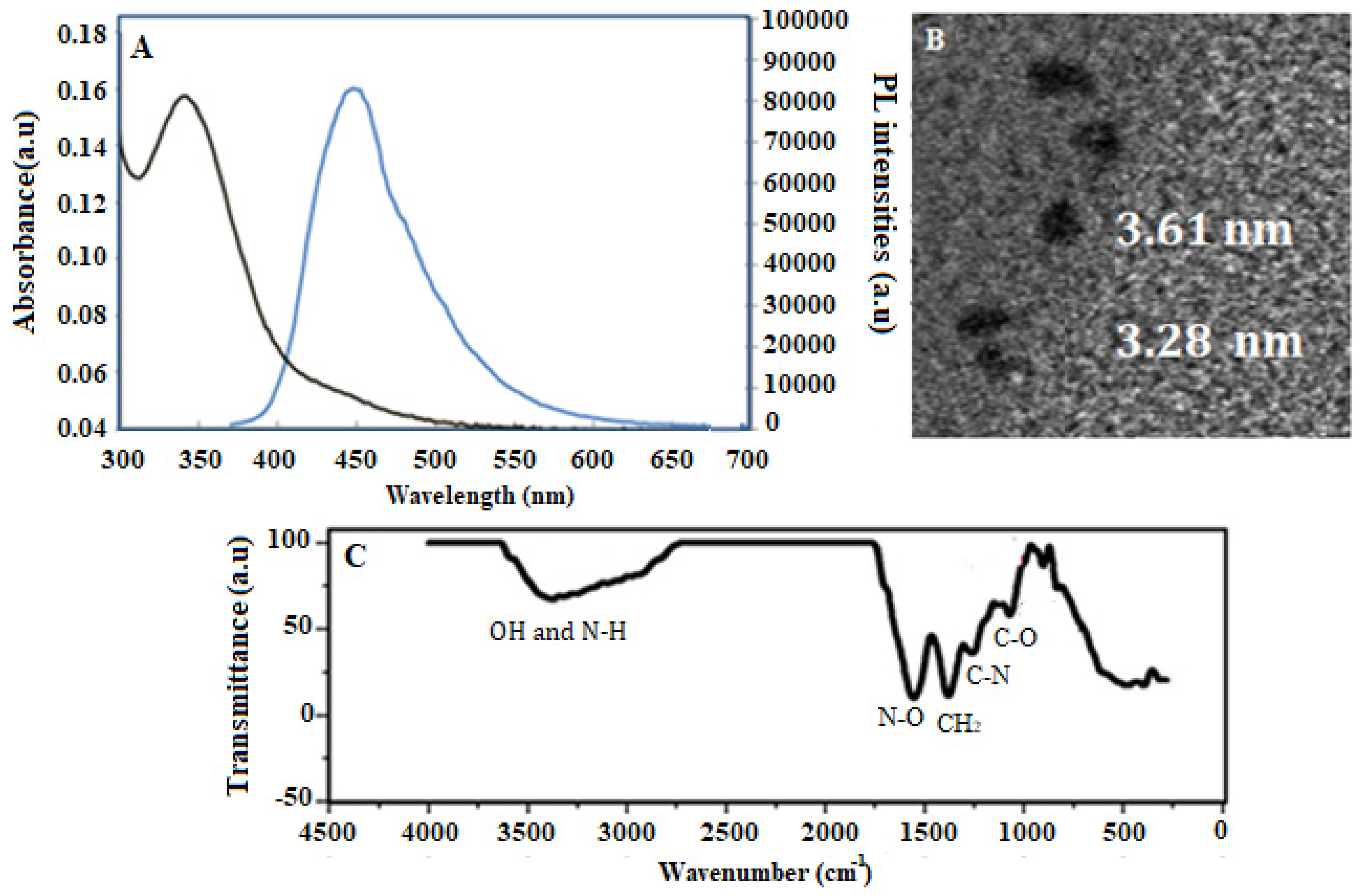
2.3. Synthesis of Carbon Dots
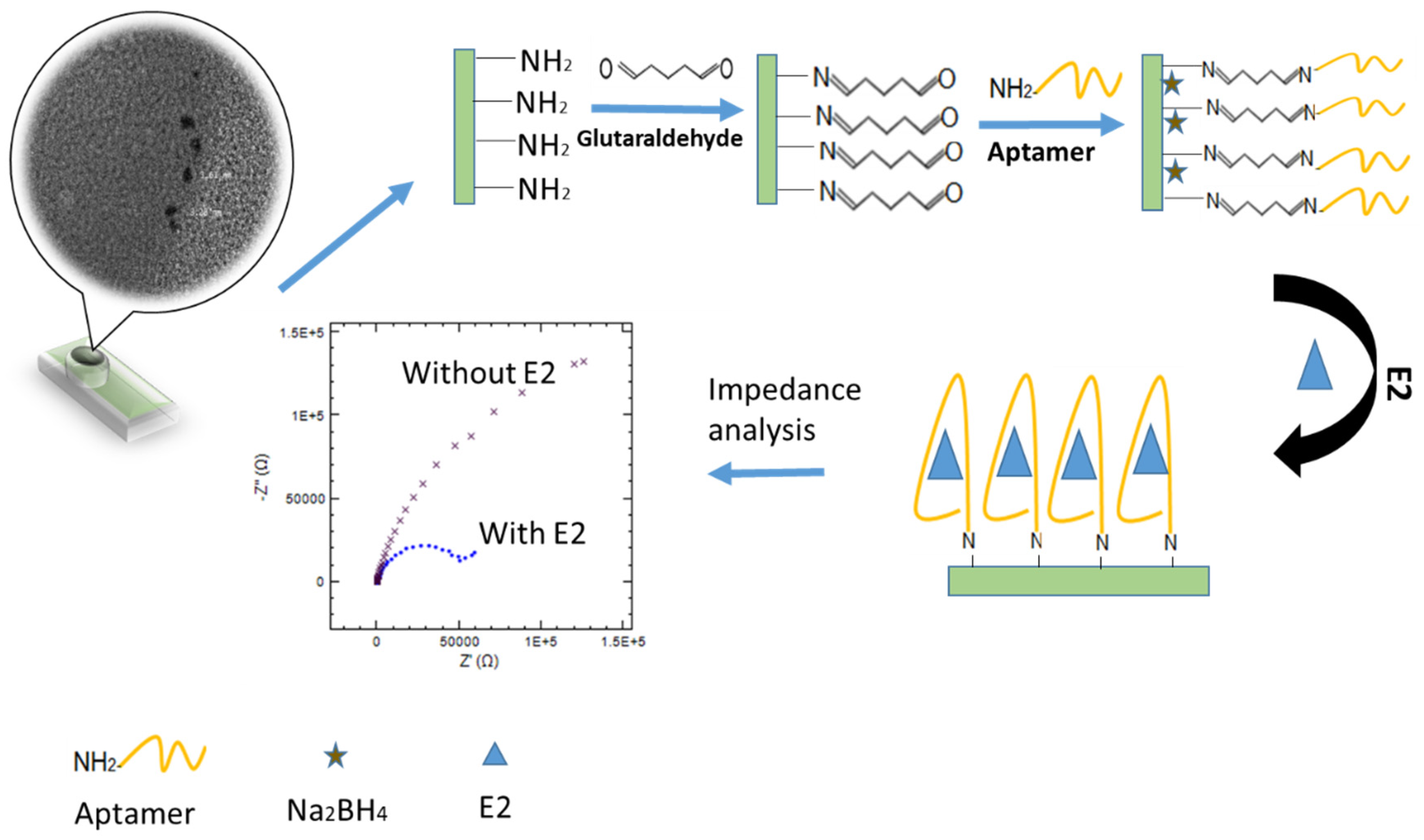
2.4. Electrode Modification
2.5. Aptasensor Preparation
3. Results
3.1. Material Characterization
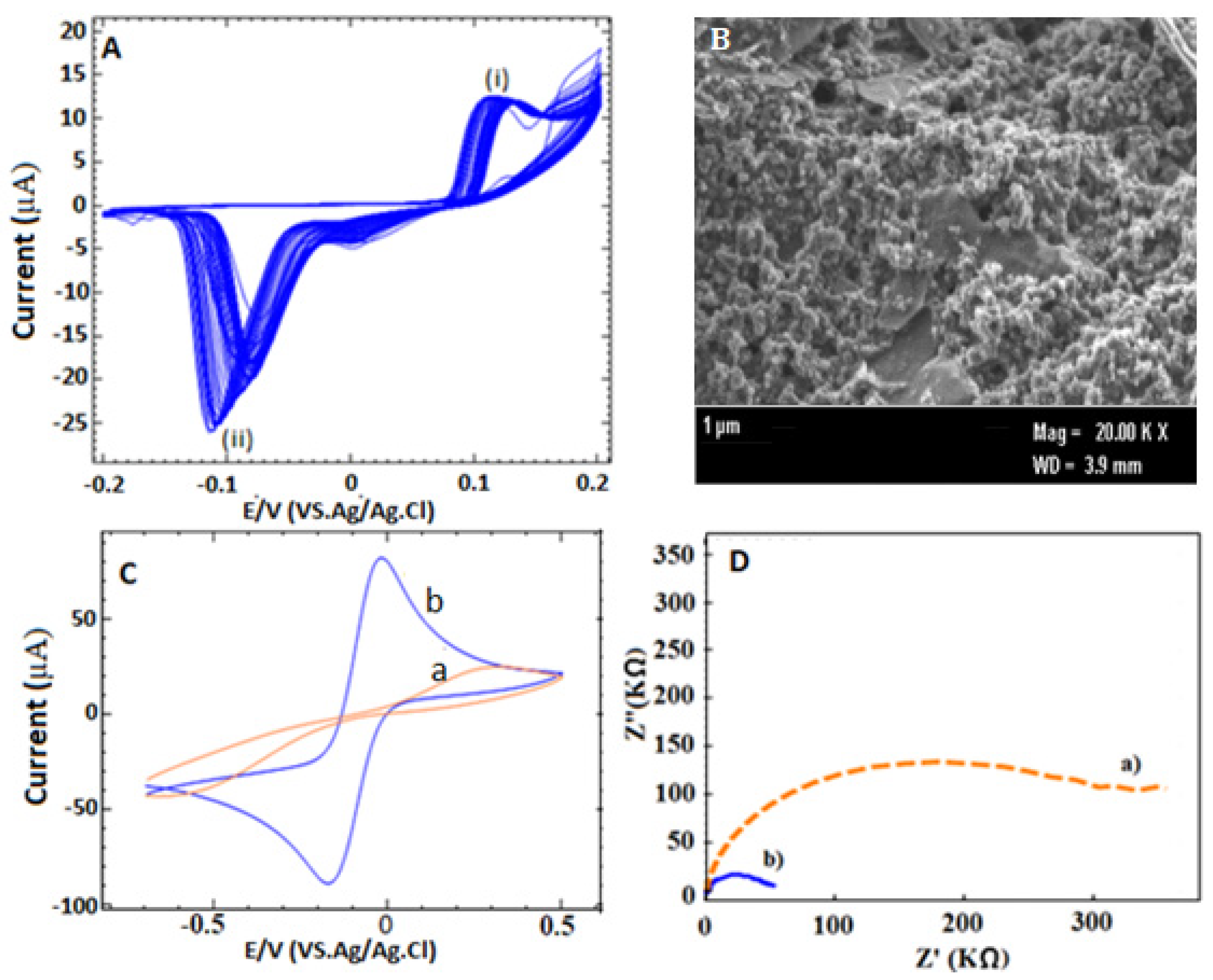
3.2. Electrode Modification and Characterization
3.3. Design and Construction the Developed Aptasensor
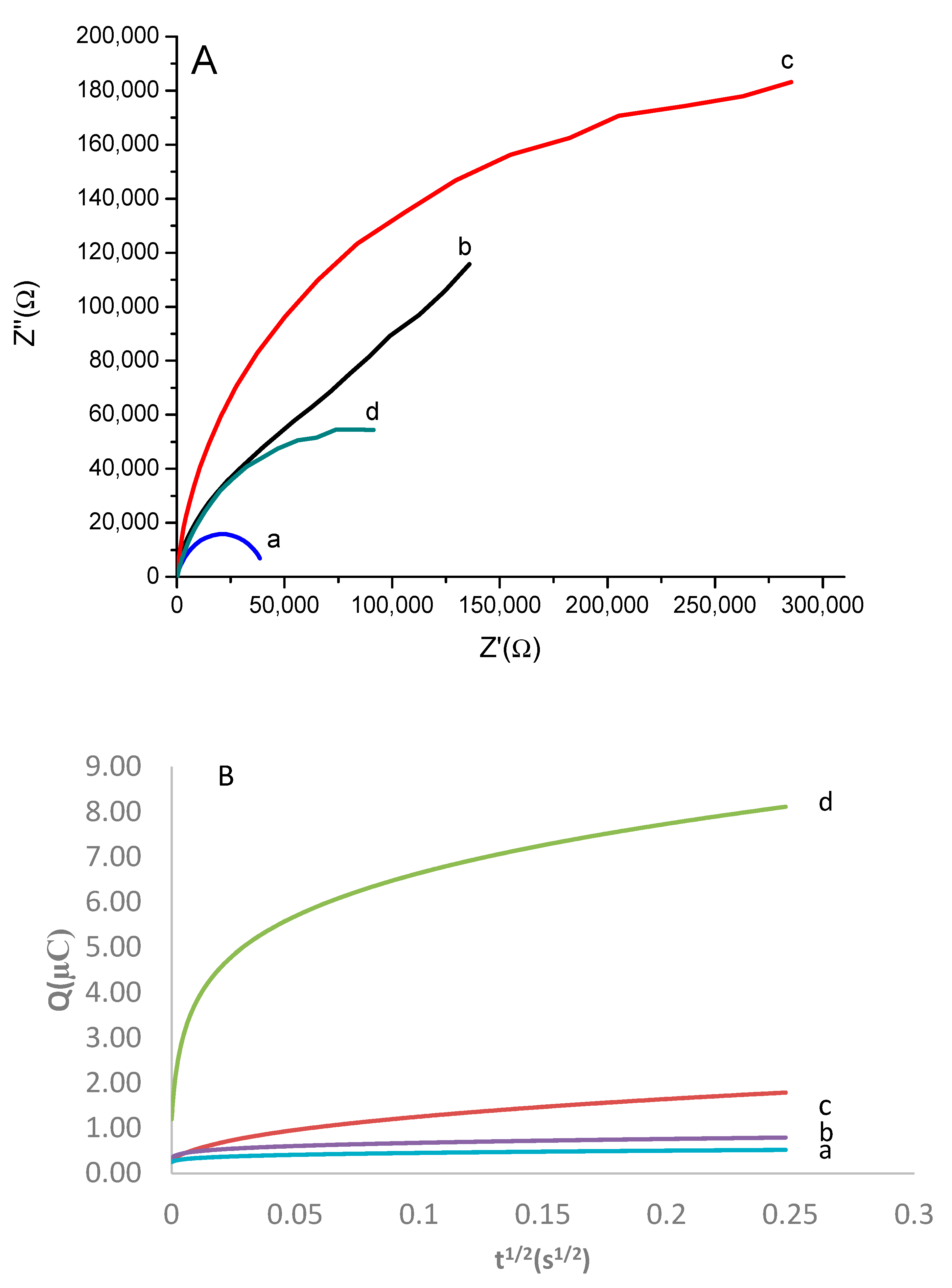
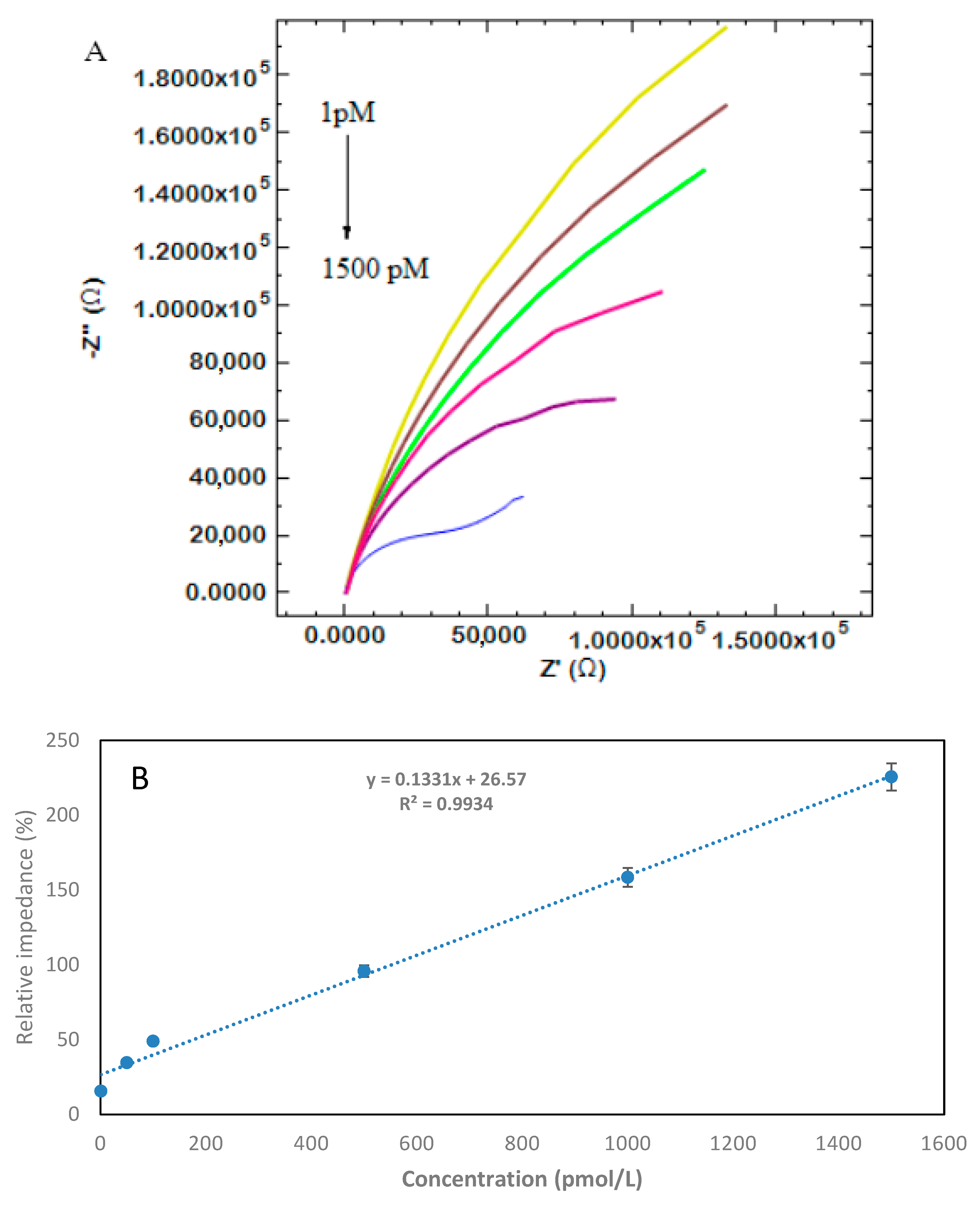
3.4. Analytical Performance of the Developed Aptabiosensor
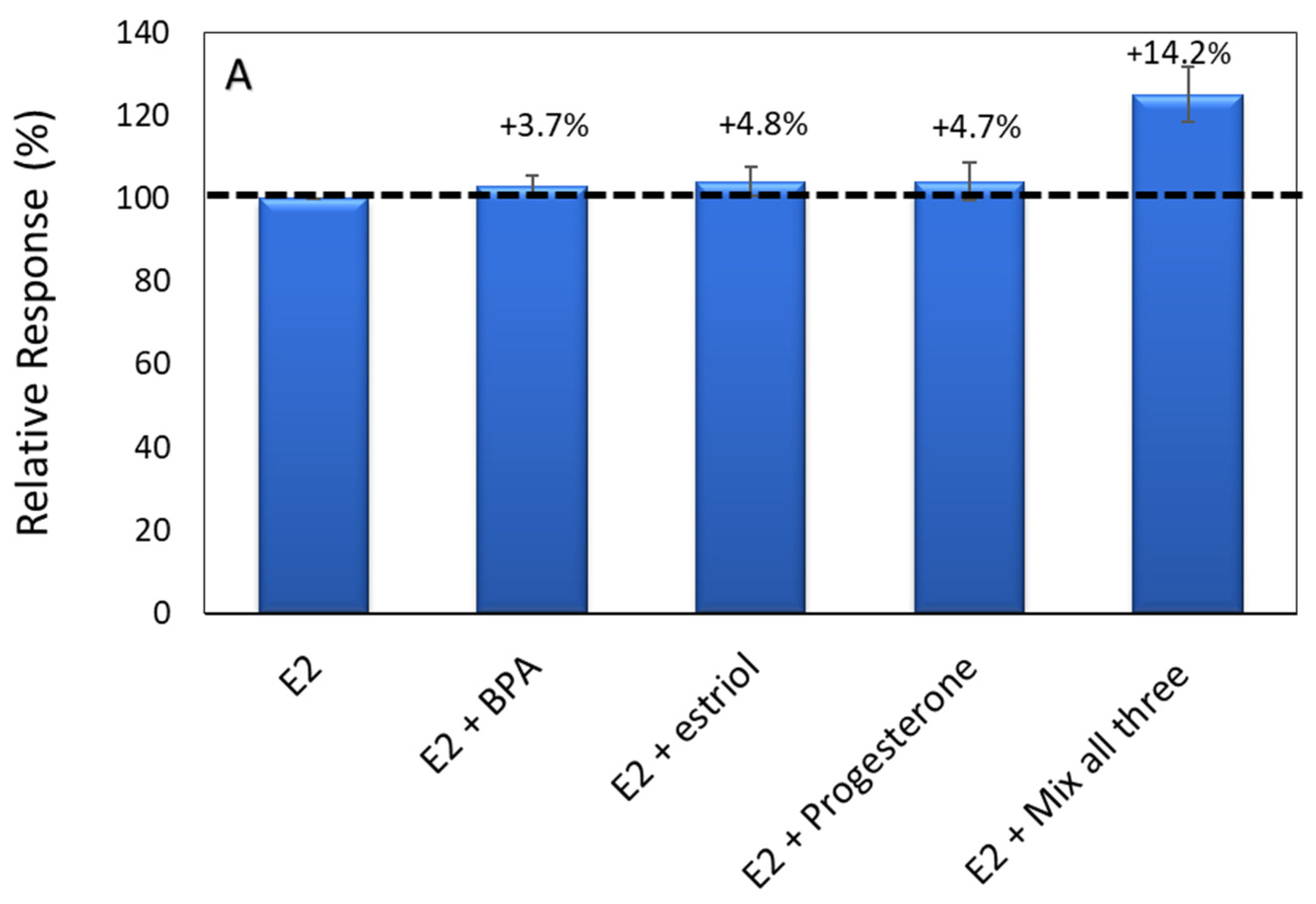
3.5. Specificity and Stability of the Developed Aptasensor
3.6. Analytical Application to Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Rozi, N.; Mat Zaid, M.H.; Tahrim, N.A.; Ikeda, M.; Hanifah, S.A. Polymer-based Biosensor for Estrogenic Endocrine Disrupting Chemicals in Water. Int. J. Environ. Anal. Chem. 2020. [Google Scholar] [CrossRef]
- Sylvain, L.; Denis, H.; Thierry, D.; Farzad, C.P. Emerging Estrogenic Pollutants in the Aquatic Environment and Breast Cancer Genes. Genes 2017, 8, 229. [Google Scholar]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Costa, E.M.; Spritzer, P.M.; Hohl, A.; Bachega, T.A. Effects of endocrine disruptors in the development of the female reproductive tract. Trends Endocrinol. Metab. 2014, 58, 1677–9487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laredo, S.A.; Landeros RRTrainor, B.C. Rapid effects of estrogens on behavior: Environmental modulation and molecular mechanisms. Front. Neuroendocr. 2014, 35, 447–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, J.M.; Nugent, B.M.; McCarthy, M.M. Rapid effects of estrogens on behavior: Environmental modulation and molecular mechanisms. Endocrinology 2010, 151, 4871–4881. [Google Scholar] [CrossRef] [Green Version]
- Russell, J.A.; Malcolm, R.K.; Campbell, K.; Woolfson, A.D.J.; Chromatogr, B. High-performance liquid chromatographic determination of 17beta-estradiol and 17beta-estradiol-3-acetate solubilities and diffusion coefficents in silicone elastromeric intravaginal ring. Chromatogr. B: Biomed. Sci. Appl. 2000, 744, 157–163. [Google Scholar] [CrossRef]
- Choi, M.H.; Kim, K.R.; Chung, B.C. Determination of estrone and 17 beta-estradiol in human hair by gas chromatography-mass spectrometry. Analyst 2000, 125, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Huhtaniemi, I.T.; Tajar, A.; Lee, D.M.; O’Neill, T.W.; Finn, J.D.; Bartfai, G.; Boonen, S.; Casanueva, F.F.; Giwercman, A.; Han, T.S.; et al. Comparison of serum testosterone and estradiol measurements in 3174 European men using platform immunoassay and mass spectrometry; relevance for the diagnostics in aging men. Eur. J. Endocrinol. 2012, 166, 983–991. [Google Scholar] [CrossRef]
- Yanez-Sedeno, P.; Agui, L.; Campuzano, S.; Pingarron, J.M. What Electrochemical Biosensors Can Do for Forensic Science? Unique Features Appl. Biosens. 2019, 9, 127. [Google Scholar]
- Bahadır, E.B.; Sezginturk, M.K. A review on impedimetric biosensors Artificial Cells. Artif. Cell Blood Sub. 2016, 44, 248–262. [Google Scholar]
- Rajeswaran, R.; Ian, I.S.; Candace, S.B.; Bruce, D.H. impedance Biosensors: Applications to Sustainability and Remaining Technical Challenges. ACS Sustain. Chem. Eng. 2014, 2, 1649–1655. [Google Scholar]
- Tran, T.B.; Son, S.J.; Min, J. Nanomaterials in label-free impedimetric biosensor: Current process and future perspectives. BioChip J. 2016, 10, 318–330. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Q.; Cui, D. Recent Advances in Nanotechnology Applied to Biosensors. Sensors 2009, 9, 1033–1053. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A Mini Review on Carbon Quantum Dots: Preparation, Properties, and Electrocatalytic Application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef]
- Peng, Z.; Han, X.; Li, S.; Al-Youbic, A.; OBashammakh, A.S.; SEl-Shahawi MLeblan, R.M. Carbon dots: Biomacromolecule interaction, bioimaging and nanomedicine. Coord. Chem. Rev. 2017, 343, 256–277. [Google Scholar] [CrossRef]
- Wang, T.; Wang, A.; Wang, R.; Liu, Z.; Sun, Y.; Shan, G.; Chen, Y.; Liu, Y. Carbon dots with molecular fluorescence and their application as a “turn-off” fluorescent probe for ferricyanide detection. Sci. Rep. 2019, 9, 10723. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ye, Z.; Ying, Y. New trends in impedimetric biosensors for the detection of foodborne pathogenic bacteria. Sensors 2012, 12, 3449–3471. [Google Scholar] [CrossRef] [Green Version]
- Zheng, F.F.; Zhang, P.; Xi, Y.; Chen, J.J.; Li, L.L.; Zhu, J.J. Aptamer/Graphene Quantum Dots Nanocomposite Capped Fluorescent Mesoporous Silica Nanoparticles for Intracellular Drug Delivery and Real-Time Monitoring of Drug Release. Anal. Chem. 2015, 87, 11739–11745. [Google Scholar] [CrossRef]
- Kaur, H.; Bruno, J.G.; Kumar, A.; Sharma, T.K. Aptamers in the Therapeutics and Diagnostics Pipelines Theranostics. Theranostics 2018, 8, 4016–4032. [Google Scholar] [CrossRef] [PubMed]
- Song, K.M.; Lee, S.; Ban, C. Aptamers and Their Biological Applications. Sensors 2012, 2, 612–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Jung, H.S.; Matsuura, T.; Lee HYKawai, T.; Gu, M.B. Electrochemical detection of 17β-estradiol using DNA aptamer immobilized gold electrode chip. Biosens. Bioelectron. 2007, 22, 2525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Zhang, W.; Ye, J.; Zhan, S.; Xia, B.; Lv, J.; Xu, H.; Du, G.; Wang, A.L. A Label-Free Colorimetric Biosensor for 17β-Estradiol Detection Using Nanoparticles Assembled by Aptamer and Cationic Polymer. Aust. J. Chem. 2016, 69, 12–19. [Google Scholar] [CrossRef]
- Paulo-Mirasol, S.; Martínez-Ferrero, E.; Palomares, E. Direct white light emission from carbon nanodots (C-dots) in solution processed light emitting diodes. Nanoscale 2019, 11, 11315–11321. [Google Scholar] [CrossRef]
- Ziyatdinovaab, G.; Labuda, J. Complex electrochemical and impedimetric evaluation of DNA damage by using DNA biosensor based on a carbon screen-printed electrode. Anal. Methods 2011, 3, 2777–2782. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. Engl. 2013, 52, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Fixe, F.; Dufva, M.; Telleman, P.; Christensen, C.B. Functionalization of poly(methyl methacrylate) (PMMA) as a substrate for DNA microarrays. Nucleic Acids Res. 2004, 32, e9. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Kalytchuk, S.; Zhang, Y.; Shi, H.; Kershaw, S.V.; Rogach, A.L. Thickness-dependent full-color emission tenability in a flexible carbon dot ionogel. J. Phys. Chem. Lett. 2014, 5, 1412–1420. [Google Scholar] [CrossRef]
- Arab, S.; Masoum, S.; Hosseini, E.S. Engineering the Synthesis of Luminescent Carbon Dots with Ultra High-Quantum Yield Using Experimental Design Approach in Order to Develop Sensing Applications. IEEE Sens. J. 2019, 20, 1558–1748. [Google Scholar] [CrossRef]
- Yoo, D.; Park, Y.; Cheon, B.; Park, M.H. Carbon Dots as an Effective Fluorescent Sensing Platform for Metal Ion Detection. Nanoscale Res. Lett. 2019, 14, 272. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Liu, Y.; Li, Y.; He, Z.; Xu, Q.; Chen, Y.; Street, J.; Guof, H. Multicolor carbon nanodots from food waste and their heavy metal ion detection application. RSC Adv. 2018, 8, 23657–23662. [Google Scholar] [CrossRef] [Green Version]
- Futra, D.; Heng, L.Y.; Jaapar, M.Z.; Ulianas, A.; Saeedfara, K.; Ling, T.L. A novel electrochemical sensor for 17β-estradiol from molecularly imprinted polymeric microspheres and multi-walled carbon nanotubes grafted with gold nanoparticles. Anal. Methods 2016, 8, 1381–1389. [Google Scholar] [CrossRef]
- Dickinson, E.J.F.; Limon-Petersen, J.G.; Rees, N.V.; Compton, R.G. How Much Supporting Electrolyte Is Required to Make a Cyclic Voltammetry Experiment Quantitatively “Diffusional”? A Theoretical and Experimental Investigation. J. Phys. Chem. C 2009, 113, 11157–11171. [Google Scholar] [CrossRef]
- Mundinamani, S.P.; Rabinal, M.K. Cyclic Voltammetric Studies on the Role of Electrode, Electrode Surface Modification and Electrolyte Solution of an Electrochemical Cell. IOSR J. Appl. Chem. (IOSR-JAC) 2014, 67, 45–52. [Google Scholar] [CrossRef]
- Pilehvar, S.; Dierckx, T.; Blust, R.; Breugelmans, T.; De Wael, K. An Electrochemical Impedimetric Aptasensing Platform for Sensitive and Selective Detection of Small Molecules Such as Chloramphenicol. Sensors 2014, 14, 12059–12069. [Google Scholar] [CrossRef] [PubMed]
- Rather, J.A.; Khudaish, E.A.; Kannan, P. Graphene-amplified femtosensitive aptasensing of estradiol, an endocrine disruptor. Analyst 2018, 143, 1835–1845. [Google Scholar] [CrossRef]
- Steel, A.M.; Herne, T.M.; Tarlov, M.J. Electrochemical Quantitation of DNA Immobilized on Gold. Anal. Chem. 1998, 70, 4670. [Google Scholar] [CrossRef]
- Balamurugan, S.; Obubuafo, A.; McCarley, R.L.; Soper, S.A.; Spivak, D.A. Effect of Linker Structure on Surface Density of Aptamer Monolayers and their Corresponding Protein Binding Efficiency. Anal. Chem. 2008, 80, 9630–9634. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhang, H.; Liu, L.; Li, C.; Chang, Z.; Zhu, X.; Ye, B.; Xu, M. Fabrication of an antibody-aptamer sandwich assay for electrochemical evaluation of levels of β-amyloid oligomers. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.; Yu, S.; Zhang, J.; Zheng, W.; Niu, L.; Li, G. Poly(3,6-diamino-9-ethylcarbazole) based molecularly imprinted polymer sensor for ultrasensitive and selective detection of 17-β-estradiol in biological fluids. Biosens. Bioelectron. 2018, 104, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Olowu, R.A.; Arotiba, O.; Mailu, S.N.; Waryo, T.T.; Baker, P.; Iwuoh, E. Electrochemical Aptasensor for Endocrine Disrupting 17β-Estradiol Based on a Poly(3,4 ethylenedioxylthiopene)-Gold Nanocomposite Platform. Sensors 2010, 10, 9872–9890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Chen, L.; Zhang, G.; Liu, Q.; Qiu, B.; Cai, Z.; Chen, G. Label-free aptamer-based electrochemical impedance biosensor for 17β-estradiol. Analyst 2012, 137, 819–882. [Google Scholar] [CrossRef]
- Yildirim, N.; Long, F.; Gao, C.; He, M.; Shi, H.C.; Gu, A.Z. Label-free aptamer-based electrochemical impedance biosensor for 17β-estradiol. Environ. Sci. Technol. 2012, 46, 3288–3294. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, G.; Shi, H.; Liu, M. A simple and label-free aptasensor based on nickel hexacyanoferrate nanoparticles as signal probe for highly sensitive detection of 17β-estradiol. Biosen. Bioelectron. 2015, 68, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Liu, M.; Zhuang, L.; Li, Z.; Fan, L.; Zhao, G. A fetomolar level 17β-estradiol electrochemical aptasensor constructed on hierachical dendritic gold modified boron-doped diamond electrode. Electrochim. Acta 2014, 137, 146–153. [Google Scholar] [CrossRef]

| Material | Methods Detection | Linear Range | LOD | References |
|---|---|---|---|---|
| Au/PEDOT/AuNP | SWV | 0.1–100 nM | 0.02 nM | [42] |
| Gold electrode | EIS | 1 × 10−8–1 × 10−11 M | 2.0 pM | [43] |
| β-estradiol6-(O-carboxy-methyl)oxime-BSA | Optical fiber | 5–75 nm | 2.1 nM | [44] |
| (NiHCF NPs)/AuNPs | DPV | 1–1000 pM | 0.8 pM | [45] |
| (PDDA) | Colorimetric | 0–4500 nM | 1.57 nM | [24] |
| CDs | EIS | 1 × 10−7–1 × 10−12 M | 0.5 pM | This work |
| Sample | Spiked (nM) | Determined (nM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| River Water | 0.00 | Non-Detected | - | - |
| 1 | 0.92 | 92.3 | 1.5 | |
| 5 | 4.8 | 96 | 0.9 | |
| 10 | 10.12 | 101.2 | 1.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mat Zaid, M.H.; Abdullah, J.; Rozi, N.; Mohamad Rozlan, A.A.; Abu Hanifah, S. A Sensitive Impedimetric Aptasensor Based on Carbon Nanodots Modified Electrode for Detection of 17ß-Estradiol. Nanomaterials 2020, 10, 1346. https://doi.org/10.3390/nano10071346
Mat Zaid MH, Abdullah J, Rozi N, Mohamad Rozlan AA, Abu Hanifah S. A Sensitive Impedimetric Aptasensor Based on Carbon Nanodots Modified Electrode for Detection of 17ß-Estradiol. Nanomaterials. 2020; 10(7):1346. https://doi.org/10.3390/nano10071346
Chicago/Turabian StyleMat Zaid, Mohd Hazani, Jaafar Abdullah, Normazida Rozi, Aliff Aiman Mohamad Rozlan, and Sharina Abu Hanifah. 2020. "A Sensitive Impedimetric Aptasensor Based on Carbon Nanodots Modified Electrode for Detection of 17ß-Estradiol" Nanomaterials 10, no. 7: 1346. https://doi.org/10.3390/nano10071346
APA StyleMat Zaid, M. H., Abdullah, J., Rozi, N., Mohamad Rozlan, A. A., & Abu Hanifah, S. (2020). A Sensitive Impedimetric Aptasensor Based on Carbon Nanodots Modified Electrode for Detection of 17ß-Estradiol. Nanomaterials, 10(7), 1346. https://doi.org/10.3390/nano10071346





