Effects of Functionalized Fullerenes on ROS Homeostasis Determine Their Cytoprotective or Cytotoxic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Functionalized Fullerenes
2.2. Cell Culture
2.3. MTT Assay
2.4. Antibodies
2.5. Flow Cytometry Analysis (FCA)
2.6. Fluorescence Microscopy
2.7. Immunocytochemistry
2.8. Reactive Oxygen Species Assays
2.9. NADH-Stimulated Lucigenin-Enhanced Chemiluminescence
2.10. Quantification of mRNA Levels
2.11. Statistical Analysis
3. Results
3.1. Fluorescence Spectra
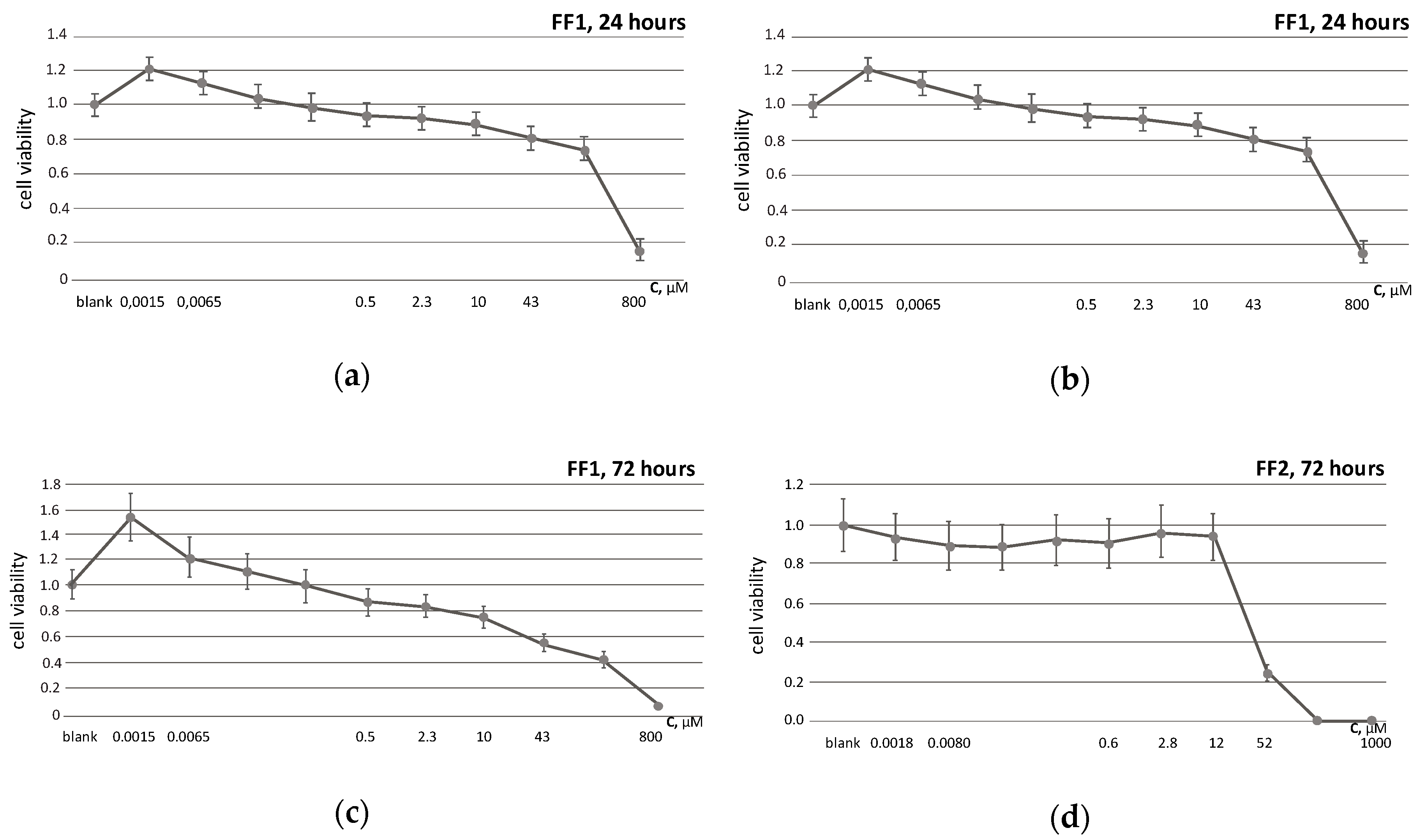
3.2. Cell Viability
3.3. Uptake and Localization of FFs inside Human Fetal Lung Fibroblasts
3.4. Intracellular ROS Visualization
3.5. Superoxide Scavenging Potential of FFs
3.6. NOX4 Expression
3.7. FFs Effects on NRF2 Pathway
3.8. FFs Effects on Oxidative DNA Modifications, Double-Strand Breaks and Damage Response Genes Expression
3.9. FFs Effects on Cell Proliferation and Cell Cycle
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Fu, Y.F.; Liu, X.; Feng, G.; Xiong, D.; Mu, G.F.; Chen, F.P. ROS promote Ox-LDL-induced platelet activation by up-regulating autophagy through the inhibition of the PI3K/AKT/mTOR pathway. Cell. Physiol. Biochem. 2018, 50, 1779–1793. [Google Scholar] [CrossRef] [PubMed]
- Jalmi, S.K.; Sinha, A.K. ROS mediated MAPK signaling in abiotic and biotic stress- striking similarities and differences. Front. Plant Sci. 2015, 6, 769. [Google Scholar] [CrossRef] [Green Version]
- Zepeda, A.B.; Pessoa, A., Jr.; Castillo, R.L.; Figueroa, C.A.; Pulgar, V.M.; Farias, J.G. Cellular and molecular mechanisms in the hypoxic tissue: Role of HIF-1 and ROS. Cell. Biochem. Funct. 2013, 31, 451–459. [Google Scholar] [CrossRef]
- Liu, J.; Du, L. PERK pathway is involved in oxygen-glucose-serum deprivation-induced NF-kB activation via ROS generation in spinal cord astrocytes. Biochem. Biophys. Res. Commun. 2015, 467, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Dansen, T.B. ROS induced p53 activation: DNA damage, redox signaling or both? Antioxid. Redox Signal. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Zhang, Q.; Fu, J.; Woods, C.G.; Hou, Y.; Corkey, B.E.; Collins, S.; Andersen, M.E. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol. Appl. Pharmacol. 2010, 244, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Seifermann, S.M.; Pierrat, P.; Brase, S. Synthesis of highly functionalized C60 fullerene derivatives and their applications in material and life sciences. Org. Biomol. Chem. 2015, 13, 25–54. [Google Scholar] [CrossRef] [Green Version]
- Bosi, S.; Da Ros, T.; Spalluto, G.; Prato, M. Fullerene derivatives: An attractive tool for biological applications. Eur. J. Med. Chem. 2003, 38, 913–923. [Google Scholar] [CrossRef]
- Partha, R.; Conyers, J.L. Biomedical applications of functionalized fullerene-based nanomaterials. Int. J. Nanomed. 2009, 4, 261–275. [Google Scholar]
- Markovic, Z.; Trajkovic, V. Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials 2008, 29, 3561–3573. [Google Scholar] [CrossRef] [PubMed]
- Sheka, E. Fullerenes: Nanochemistry, Nanomagnetism, Nanomedicine, Nanophotonics; CRC Press: Boca Raton, FL, USA, 2011; p. 293. [Google Scholar]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alshamsan, A. Mechanism of ROS scavenging and antioxidant signalling by redox metallic and fullerene nanomaterials: Potential implications in ROS associated degenerative disorders. Biochim. Biophys. Acta 2017, 1861, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, V.; Kraevaya, O.; Ershova, E.; Kameneva, L.; Malinovskaya, E.; Dolgikh, O.; Konkova, M.; Voronov, I.; Zhilenkov, A.; Veiko, N.; et al. Antioxidant properties of fullerene derivatives depend on their chemical structure: A study of two fullerene derivatives on HELFs. Oxid. Med. Cell. Longev. 2019, 2019, 4398695. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, X.; Zhao, Y. Mechanisms of antioxidant activities of fullerenols from first-principles calculation. J. Phys. Chem. A 2018, 122, 8183–8190. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, V.A.; Smirnova, Y.O.; Prazdnova, E.V.; Soldatov, A.V. Possible mechanisms of fullerene C(6)(0) antioxidant action. Biomed. Res. Int. 2013, 2013, 821498. [Google Scholar] [CrossRef] [Green Version]
- Andrievsky, G.V.; Bruskov, V.I.; Tykhomyrov, A.A.; Gudkov, S.V. Peculiarities of the antioxidant and radioprotective effects of hydrated C60 fullerene nanostuctures in vitro and in vivo. Free Radic. Biol. Med. 2009, 47, 786–793. [Google Scholar] [CrossRef]
- Yang, J.; Alemany, L.B.; Driver, J.; Hartgerink, J.D.; Barron, A.R. Fullerene-derivatized amino acids: Synthesis, characterization, antioxidant properties, and solid-phase peptide synthesis. Chemistry 2007, 13, 2530–2545. [Google Scholar] [CrossRef]
- Dugan, L.L.; Lovett, E.G.; Quick, K.L.; Lotharius, J.; Lin, T.T.; O’Malley, K.L. Fullerene-based antioxidants and neurodegenerative disorders. Parkinsonism Relat. Disord. 2001, 7, 243–246. [Google Scholar] [CrossRef]
- Nielsen, G.D.; Roursgaard, M.; Jensen, K.A.; Poulsen, S.S.; Larsen, S.T. In vivo biology and toxicology of fullerenes and their derivatives. Basic Clin. Pharmacol. Toxicol. 2008, 103, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Aschberger, K.; Johnston, H.J.; Stone, V.; Aitken, R.J.; Tran, C.L.; Hankin, S.M.; Peters, S.A.; Christensen, F.M. Review of fullerene toxicity and exposure--appraisal of a human health risk assessment, based on open literature. Regul. Toxicol. Pharmacol. 2010, 58, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, N.; Pressac, M.; Hadchouel, M.; Szwarc, H.; Wilson, S.R.; Moussa, F. Fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005, 5, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.J.; Lao, F.; Fu, P.P.; Wamer, W.G.; Zhao, Y.; Wang, P.C.; Qiu, Y.; Sun, B.; Xing, G.; Dong, J.; et al. The scavenging of reactive oxygen species and the potential for cell protection by functionalized fullerene materials. Biomaterials 2009, 30, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.H.; Zhao, X. Chemically modified fullerene derivatives as photosensitizers in photodynamic therapy: A first-principles study. J. Comput. Chem. 2012, 33, 861–867. [Google Scholar] [CrossRef]
- Ito, O. Photosensitizing electron transfer processes of fullerenes, carbon nanotubes, and carbon nanohorns. Chem. Rec. 2017, 17, 326–362. [Google Scholar] [CrossRef]
- Markovic, Z.; Todorovic-Markovic, B.; Kleut, D.; Nikolic, N.; Vranjes-Djuric, S.; Misirkic, M.; Vucicevic, L.; Janjetovic, K.; Isakovic, A.; Harhaji, L.; et al. The mechanism of cell-damaging reactive oxygen generation by colloidal fullerenes. Biomaterials 2007, 28, 5437–5448. [Google Scholar] [CrossRef]
- Ikeda, A.; Doi, Y.; Nishiguchi, K.; Kitamura, K.; Hashizume, M.; Kikuchi, J.; Yogo, K.; Ogawa, T.; Takeya, T. Induction of cell death by photodynamic therapy with water-soluble lipid-membrane-incorporated [60]fullerene. Org. Biomol. Chem. 2007, 5, 1158–1160. [Google Scholar] [CrossRef]
- Nishizawa, C.; Hashimoto, N.; Yokoo, S.; Funakoshi-Tago, M.; Kasahara, T.; Takahashi, K.; Nakamura, S.; Mashino, T. Pyrrolidinium-type fullerene derivative-induced apoptosis by the generation of reactive oxygen species in HL-60 cells. Free Radic. Res. 2009, 43, 1240–1247. [Google Scholar] [CrossRef]
- Hao, T.; Zhou, J.; Lu, S.; Yang, B.; Wang, Y.; Fang, W.; Jiang, X.; Lin, Q.; Li, J.; Wang, C. Fullerene mediates proliferation and cardiomyogenic differentiation of adipose-derived stem cells via modulation of MAPK pathway and cardiac protein expression. Int. J. Nanomed. 2016, 11, 269–283. [Google Scholar] [CrossRef] [Green Version]
- Nie, X.; Tang, J.; Liu, Y.; Cai, R.; Miao, Q.; Zhao, Y.; Chen, C. Fullerenol inhibits the cross-talk between bone marrow-derived mesenchymal stem cells and tumor cells by regulating MAPK signaling. Nanomedicine 2017, 13, 1879–1890. [Google Scholar] [CrossRef]
- Kornev, A.; Peregudov, A.; Martynenko, V.; Balzarini, J.; Hoorelbeke, B.; Troshin, P. Synthesis and antiviral activity of highly water-soluble polycarboxylic derivatives of [70]fullerene. Chem. Commun. 2011, 47, 8298–8300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraevaya, O.A.; Peregudov, A.S.; Godovikov, I.A.; Shchurik, E.V.; Martynenko, V.M.; Shestakov, A.F.; Balzarini, J.; Schols, D.; Troshin, P.A. Direct arylation of C60Cl6 and C70Cl8 with carboxylic acids: A synthetic avenue to water-soluble fullerene derivatives with promising antiviral activity. Chem. Commun. 2020, 56, 1179–1182. [Google Scholar] [CrossRef]
- Kobzar, O.L.; Trush, V.V.; Tanchuk, V.Y.; Zhilenkov, A.V.; Troshin, P.A.; Vovk, A.I. Fullerene derivatives as a new class of inhibitors of protein tyrosine phosphatases. Bioorg Med. Chem. Lett. 2014, 24, 3175–3179. [Google Scholar] [CrossRef] [PubMed]
- Ershova, E.S.; Sergeeva, V.A.; Tabakov, V.J.; Kameneva, L.A.; Porokhovnik, L.N.; Voronov, I.I.; Khakina, E.A.; Troshin, P.A.; Kutsev, S.I.; Veiko, N.N.; et al. Functionalized fullerene increases NF-kappaB activity and blocks genotoxic effect of oxidative stress in serum-starving human embryo lung diploid fibroblasts. Oxid. Med. Cell. Longev. 2016, 2016, 9895245. [Google Scholar] [CrossRef] [Green Version]
- Ershova, E.S.; Sergeeva, V.; Chausheva, A.I.; Zheglo, D.G.; Nikitina, V.; Smirnova, T.D.; Kameneva, L.V.; Porokhovnik, L.N.; Kutsev, S.I.; Troshin, P.A.; et al. Toxic and DNA damaging effects of a functionalized fullerene in human embryonic lung fibroblasts. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2016, 805, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.A.; Krutskikh, A.; Curry, B.J.; McLaughlin, E.A.; Aitken, R.J. Identification of cytochrome P450-reductase as the enzyme responsible for NADPH-dependent lucigenin and tetrazolium salt reduction in rat epididymal sperm preparations. Biol. Reprod. 2004, 71, 307–318. [Google Scholar] [CrossRef]
- Sun, P.; Nie, X.; Chen, X.; Yin, L.; Luo, J.; Sun, L.; Wan, C.; Jiang, S. Nrf2 signaling elicits a neuroprotective role against PFOS-mediated oxidative damage and apoptosis. Neurochem. Res. 2018, 43, 2446–2459. [Google Scholar] [CrossRef]
- Kooy, N.W.; Royall, J.A.; Ischiropoulos, H. Oxidation of 2′,7′-dichlorofluorescin by peroxynitrite. Free Radic. Res. 1997, 27, 245–254. [Google Scholar] [CrossRef]
- Melino, G.; Savini, I.; Guerrieri, P.; Finazzi-Agro, A. Redox buffering ability of lymphoid cells evaluated by the oxidation of 2′,7′-dichlorofluorescin. Free Radic. Res. Commun. 1990, 11, 213–221. [Google Scholar] [CrossRef]
- Royall, J.A.; Ischiropoulos, H. Evaluation of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch. Biochem. Biophys. 1993, 302, 348–355. [Google Scholar] [CrossRef]
- Rota, C.; Chignell, C.F.; Mason, R.P. Evidence for free radical formation during the oxidation of 2’-7’-dichlorofluorescin to the fluorescent dye 2’-7’-dichlorofluorescein by horseradish peroxidase: Possible implications for oxidative stress measurements. Free Radic. Biol. Med. 1999, 27, 873–881. [Google Scholar] [CrossRef]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilski, P.; Belanger, A.G.; Chignell, C.F. Photosensitized oxidation of 2′,7′-dichlorofluorescin: Singlet oxygen does not contribute to the formation of fluorescent oxidation product 2′,7′-dichlorofluorescein. Free Radic. Biol. Med. 2002, 33, 938–946. [Google Scholar] [CrossRef]
- Bonini, M.G.; Rota, C.; Tomasi, A.; Mason, R.P. The oxidation of 2′,7′-dichlorofluorescin to reactive oxygen species: A self-fulfilling prophesy? Free Radic. Biol. Med. 2006, 40, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Maiorino, M.; Ursini, F. Signaling functions of reactive oxygen species. Biochemistry 2010, 49, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Rampon, C.; Volovitch, M.; Joliot, A.; Vriz, S. Hydrogen Peroxide and Redox Regulation of Developments. Antioxidants 2018, 7, 159. [Google Scholar] [CrossRef] [Green Version]
- Takac, I.; Schroder, K.; Zhang, L.; Lardy, B.; Anilkumar, N.; Lambeth, J.D.; Shah, A.M.; Morel, F.; Brandes, R.P. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011, 286, 13304–13313. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Chen, X. The human Nox4: Gene, structure, physiological function and pathological significance. J. Drug Target. 2015, 23, 888–896. [Google Scholar] [CrossRef]
- Radermacher, K.A.; Wingler, K.; Langhauser, F.; Altenhofer, S.; Kleikers, P.; Hermans, J.J.; Hrabe de Angelis, M.; Kleinschnitz, C.; Schmidt, H.H. Neuroprotection after stroke by targeting NOX4 as a source of oxidative stress. Antioxid. Redox Signal. 2013, 18, 1418–1427. [Google Scholar] [CrossRef] [Green Version]
- Gorin, Y.; Block, K. Nox4 and diabetic nephropathy: With a friend like this, who needs enemies? Free Radic. Biol. Med. 2013, 61, 130–142. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Haigh, S.; Barman, S.; Fulton, D.J. From form to function: The role of Nox4 in the cardiovascular system. Front. Physiol. 2012, 3, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Block, K.; Gorin, Y.; Abboud, H.E. Subcellular localization of Nox4 and regulation in diabetes. Proc. Natl. Acad. Sci. USA 2009, 106, 14385–14390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diebold, I.; Petry, A.; Hess, J.; Gorlach, A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol. Biol. Cell 2010, 21, 2087–2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Zhang, Y.; Wang, Z.; Wang, L.; Wei, X.; Zhang, B.; Wen, Z.; Fang, H.; Pang, Q.; Yi, F. Regulation of NADPH oxidase activity is associated with miRNA-25-mediated NOX4 expression in experimental diabetic nephropathy. Am. J. Nephrol. 2010, 32, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Hubackova, S.; Kucerova, A.; Michlits, G.; Kyjacova, L.; Reinis, M.; Korolov, O.; Bartek, J.; Hodny, Z. IFNgamma induces oxidative stress, DNA damage and tumor cell senescence via TGFbeta/SMAD signaling-dependent induction of Nox4 and suppression of ANT2. Oncogene 2016, 35, 1236–1249. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-Kinase/Akt Signaling and Redox Metabolism in Cancer. Front. Oncol. 2018, 8, 160. [Google Scholar] [CrossRef]
- Nguyen, M.V.; Lardy, B.; Rousset, F.; Hazane-Puch, F.; Zhang, L.; Trocme, C.; Serrander, L.; Krause, K.H.; Morel, F. Quinone compounds regulate the level of ROS production by the NADPH oxidase Nox4. Biochem. Pharmacol. 2013, 85, 1644–1654. [Google Scholar] [CrossRef]
- Brewer, A.C.; Murray, T.V.; Arno, M.; Zhang, M.; Anilkumar, N.P.; Mann, G.E.; Shah, A.M. Nox4 regulates Nrf2 and glutathione redox in cardiomyocytes in vivo. Free Radic. Biol. Med. 2011, 51, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Yao, B.; Li, N.; Ma, L.; Deng, Y.; Yang, Y.; Zeng, C.; Yang, Z.; Liu, B. Nrf2 mediates redox adaptation in NOX4-overexpressed non-small cell lung cancer cells. Exp. Cell Res. 2017, 352, 245–254. [Google Scholar] [CrossRef]
- Weyemi, U.; Dupuy, C. The emerging role of ROS-generating NADPH oxidase NOX4 in DNA-damage responses. Mutat. Res. 2012, 751, 77–81. [Google Scholar] [CrossRef]
- Tang, C.T.; Gao, Y.J.; Ge, Z.Z. NOX4, a new genetic target for anti-cancer therapy in digestive system cancer. J. Dig. Dis. 2018, 19, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Hakami, N.Y.; Wong, H.; Shah, M.H.; Dusting, G.J.; Jiang, F.; Peshavariya, H.M. Smad-independent pathway involved in transforming growth factor beta1-induced Nox4 expression and proliferation of endothelial cells. Naunyn Schmiedebergs Arch. Pharmacolacol. 2015, 388, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, N.; Weber, R.; Zhang, M.; Brewer, A.; Shah, A.M. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.T.; Lin, X.L.; Wu, S.; Liang, Q.; Yang, L.; Gao, Y.J.; Ge, Z.Z. NOX4-driven ROS formation regulates proliferation and apoptosis of gastric cancer cells through the GLI1 pathway. Cell. Signal. 2018, 46, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.A.; Fulton, D. Adventitial fibroblast Nox4 expression and ROS signaling in pulmonary arterial hypertension. Adv. Exp. Med. Biol. 2017, 967, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Salmeen, A.; Park, B.O.; Meyer, T. The NADPH oxidases NOX4 and DUOX2 regulate cell cycle entry via a p53-dependent pathway. Oncogene 2010, 29, 4473–4484. [Google Scholar] [CrossRef] [Green Version]
- Chaabane, F.; Boubaker, J.; Loussaif, A.; Neffati, A.; Kilani-Jaziri, S.; Ghedira, K.; Chekir-Ghedira, L. Antioxidant, genotoxic and antigenotoxic activities of daphne gnidium leaf extracts. BMC Complement. Altern. Med. 2012, 12, 153. [Google Scholar] [CrossRef] [Green Version]
- Hricoviniova, J.; Sevcovicova, A.; Hricoviniova, Z. Evaluation of the genotoxic, DNA-protective and antioxidant profile of synthetic alkyl gallates and gallotannins using in vitro assays. Toxicol. Vitr. 2020, 65, 104789. [Google Scholar] [CrossRef]
- Rubio, L.; Annangi, B.; Vila, L.; Hernandez, A.; Marcos, R. Antioxidant and anti-genotoxic properties of cerium oxide nanoparticles in a pulmonary-like cell system. Arch. Toxicol. 2016, 90, 269–278. [Google Scholar] [CrossRef]


















© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostyuk, S.V.; Proskurnina, E.V.; Savinova, E.A.; Ershova, E.S.; Kraevaya, O.A.; Kameneva, L.V.; Umryukhin, P.E.; Dolgikh, O.A.; Kutsev, S.I.; Troshin, P.A.; et al. Effects of Functionalized Fullerenes on ROS Homeostasis Determine Their Cytoprotective or Cytotoxic Properties. Nanomaterials 2020, 10, 1405. https://doi.org/10.3390/nano10071405
Kostyuk SV, Proskurnina EV, Savinova EA, Ershova ES, Kraevaya OA, Kameneva LV, Umryukhin PE, Dolgikh OA, Kutsev SI, Troshin PA, et al. Effects of Functionalized Fullerenes on ROS Homeostasis Determine Their Cytoprotective or Cytotoxic Properties. Nanomaterials. 2020; 10(7):1405. https://doi.org/10.3390/nano10071405
Chicago/Turabian StyleKostyuk, Svetlana V., Elena V. Proskurnina, Ekaterina A. Savinova, Elizaveta S. Ershova, Olga A. Kraevaya, Larisa V. Kameneva, Pavel E. Umryukhin, Olga A. Dolgikh, Sergey I. Kutsev, Pavel A. Troshin, and et al. 2020. "Effects of Functionalized Fullerenes on ROS Homeostasis Determine Their Cytoprotective or Cytotoxic Properties" Nanomaterials 10, no. 7: 1405. https://doi.org/10.3390/nano10071405
APA StyleKostyuk, S. V., Proskurnina, E. V., Savinova, E. A., Ershova, E. S., Kraevaya, O. A., Kameneva, L. V., Umryukhin, P. E., Dolgikh, O. A., Kutsev, S. I., Troshin, P. A., & Veiko, N. N. (2020). Effects of Functionalized Fullerenes on ROS Homeostasis Determine Their Cytoprotective or Cytotoxic Properties. Nanomaterials, 10(7), 1405. https://doi.org/10.3390/nano10071405






