Surface Modification of Iron Oxide-Based Magnetic Nanoparticles for Cerebral Theranostics: Application and Prospection
Abstract
:1. Introduction
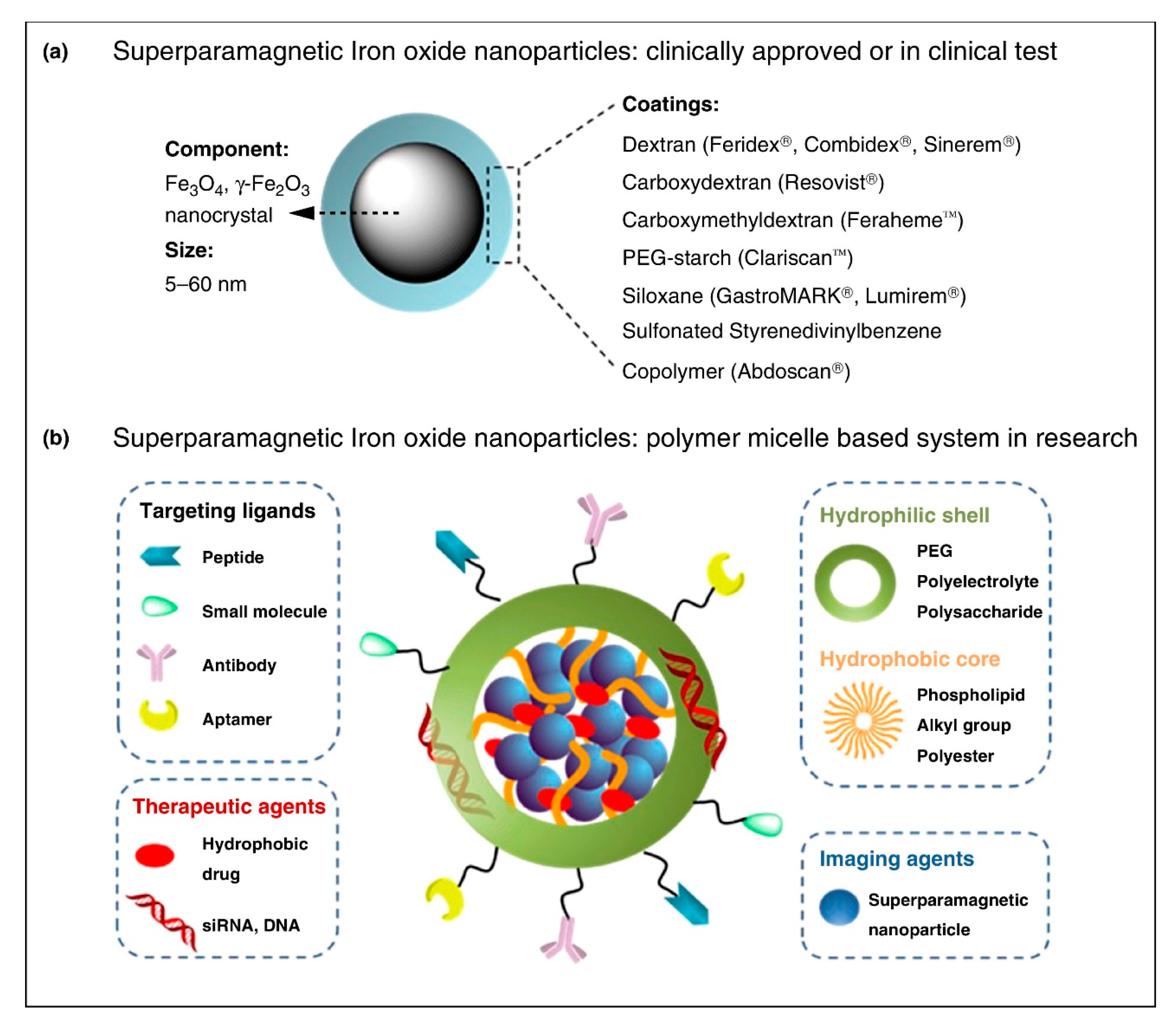
1.1. Characteristics of Iron-Based Nanoparticles
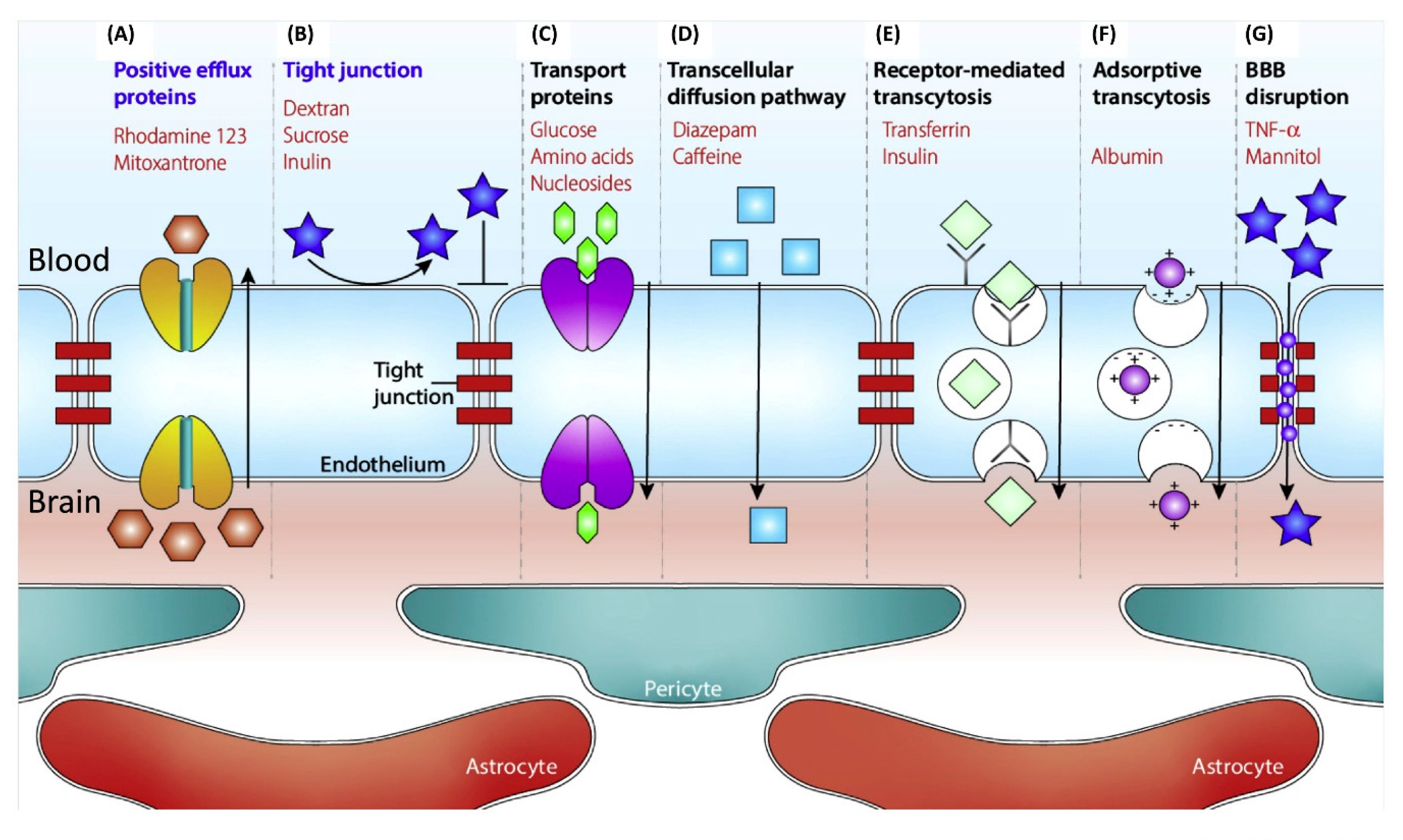
1.2. Treatment Difficulties and Current Solutions of Brain Diseases
2. Application of Different Coating of INOPs in Cerebral Theranostics
2.1. Small Molecules Coating
2.1.1. Silicon
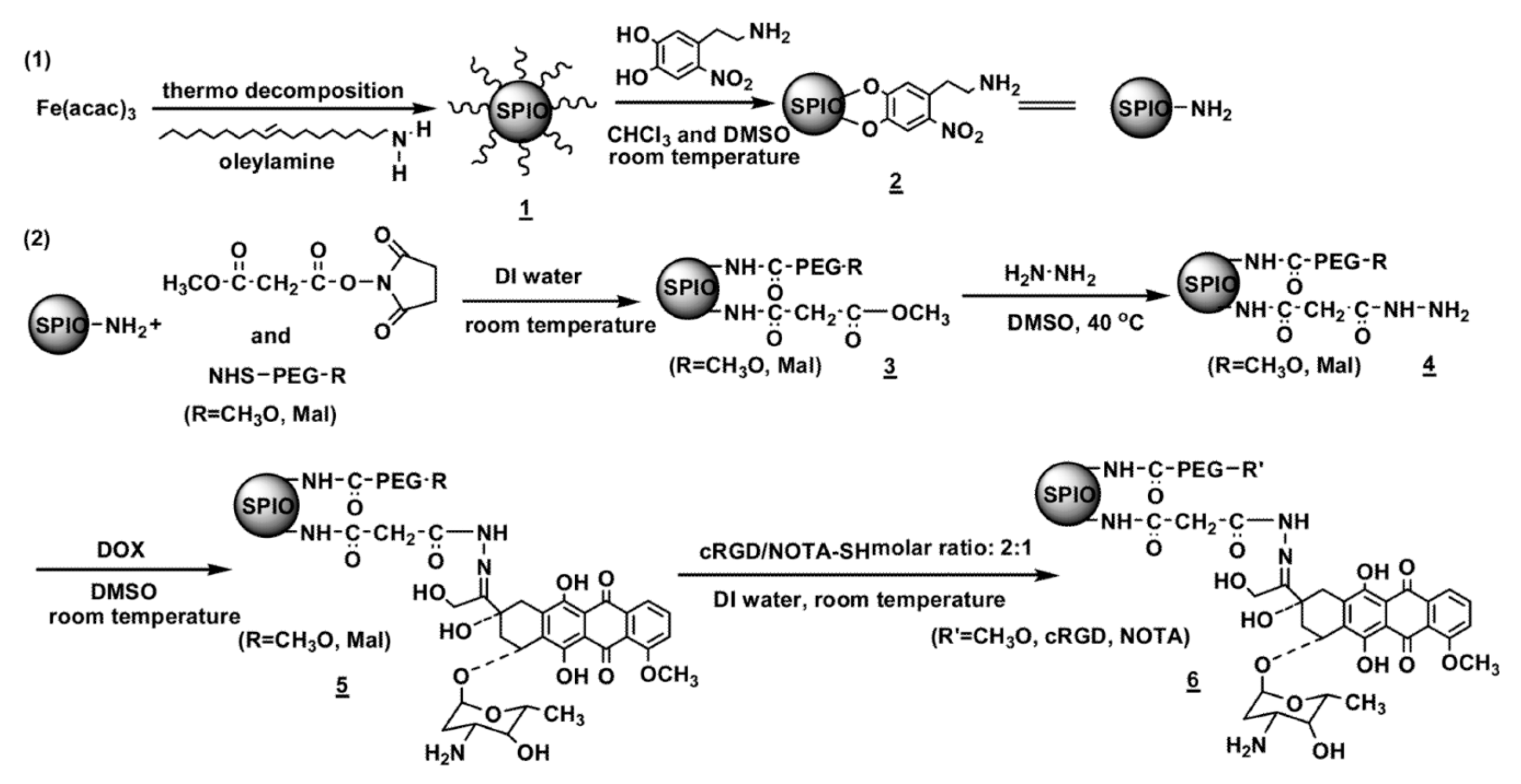
2.1.2. Catechol
2.2. Polymers Coating
2.2.1. Dextran
2.2.2. Chitosan
2.2.3. Poly (Ethylene Glycol) PEG
2.2.4. Polyethyleneimine (PEI)
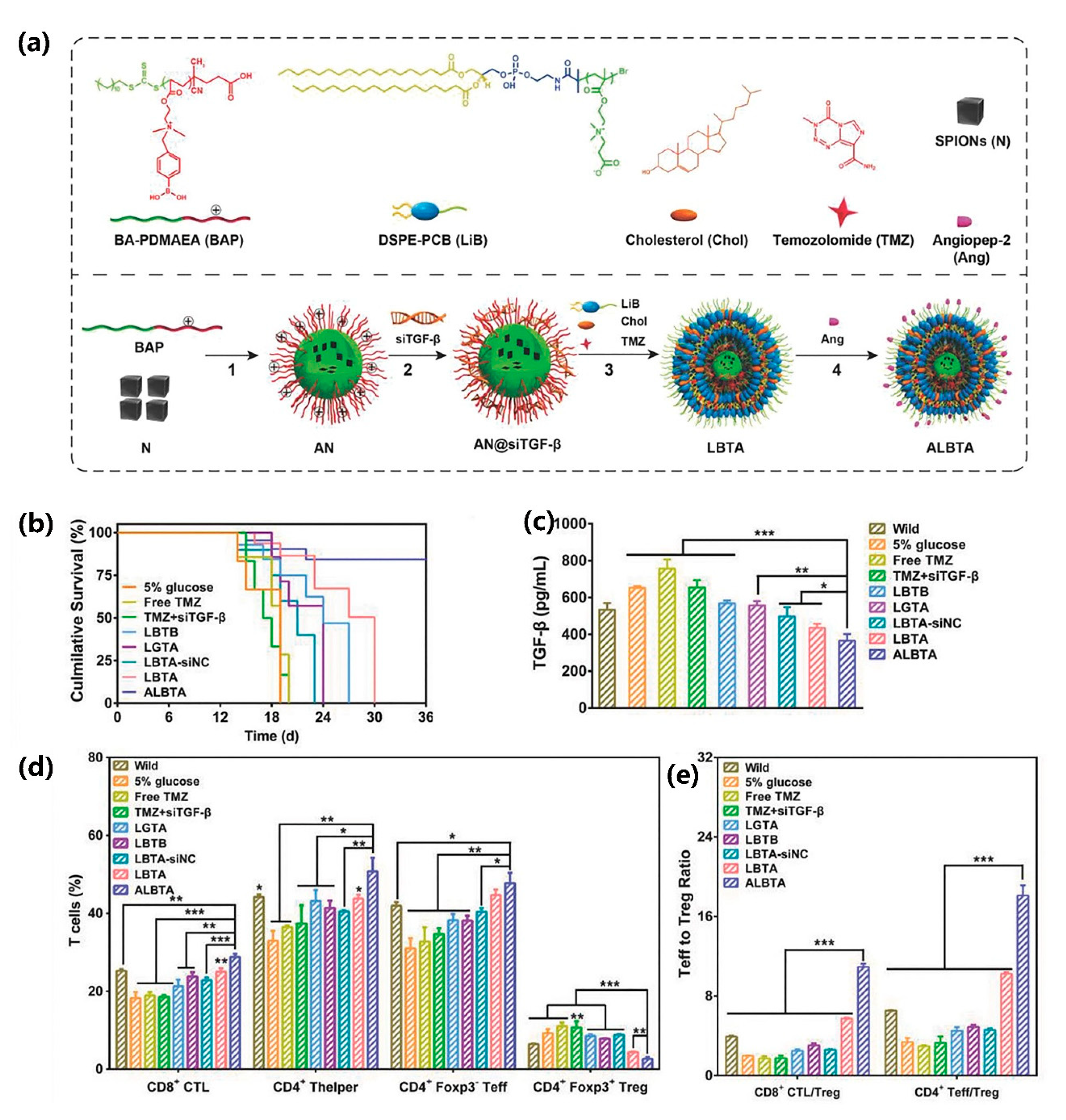
2.2.5. Poly (Carboxybetaine) PCB
2.2.6. PLGA
2.3. Lipid Molecule Coating
2.4. Lipid and Polymers Coating
2.5. Other Coatings
3. Conclusions and Prospection
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, A.H.; Salabas, E.L.; Schuth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. Engl. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gu, H.; Xu, B. Multifunctional magnetic nanoparticles: Design, synthesis, and biomedical applications. Acc. Chem. Res. 2009, 42, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Xing, R.; Xu, Z.; Hou, Y.; Gao, S.; Sun, S. Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. Adv. Mater. 2010, 22, 2729–2742. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Hola, K.; Subr, V.; Bakandritsos, A.; Tucek, J.; Zboril, R. Targeted drug delivery with polymers and magnetic nanoparticles: Covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Ho, D.; Sun, X.; Sun, S. Monodisperse magnetic nanoparticles for theranostic applications. Acc. Chem. Res. 2011, 44, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Amiri, H.; Saeidi, K.; Borhani, P.; Manafirad, A.; Ghavami, M.; Zerbi, V. Alzheimer’s disease: Pathophysiology and applications of magnetic nanoparticles as MRI theranostic agents. ACS Chem. Neurosci. 2013, 4, 1417–1429. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Shin, T.H.; Cheon, J.; Weissleder, R. Recent developments in magnetic diagnostic systems. Chem. Rev. 2015, 115, 10690–10724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Li, Y.; Liu, R.; Shen, Q.; Li, Y.; Shi, Z.; Shen, J.; Ji, W.; Zhang, X. Switchable nanoparticle for programmed gene-chem delivery with enhanced neuronal recovery and CT imaging for neurodegenerative disease treatment. Mater. Horiz. 2019, 6, 1923–1929. [Google Scholar] [CrossRef]
- Yousaf, M.Z.; Yu, J.; Hou, Y.L.; Gao, S. Magnetic nanoparticle-based cancer nanodiagnostics. Chin. Phys. B 2013, 22, 58702. [Google Scholar] [CrossRef]
- Barrow, M.; Taylor, A.; Murray, P.; Rosseinsky, M.J.; Adams, D.J. Design considerations for the synthesis of polymer coated iron oxide nanoparticles for stem cell labelling and tracking using MRI. Chem. Soc. Rev. 2015, 44, 6733–6748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, S.; Ma, C.; Zhu, M.-Q.; Ju, W.-N.; Yang, Y.; Wang, X. Application of Iron Oxide Nanoparticles in the diagnosis and treatment of neurodegenerative diseases with emphasis on alzheimer’s disease. Front. Cell. Neurosci. 2020, 14, 21. [Google Scholar] [CrossRef] [Green Version]
- Farka, Z.; Jurik, T.; Kovar, D.; Trnkova, L.; Skladal, P. Nanoparticle-based immunochemical biosensors and assays: Recent advances and challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Dai, F.; Ma, G.; Zhang, X. Theranostic gold nanomicelles made from biocompatible comb-like polymers for thermochemotherapy and multifunctional imaging with rapid clearance. Adv. Mater. 2015, 27, 3645–3653. [Google Scholar] [CrossRef]
- Kievit, F.M.; Zhang, M. Surface engineering of iron oxide nanoparticles for targeted cancer therapy. Acc. Chem. Res. 2011, 44, 853–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knuchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Nejadnik, H.; Daldrup-Link, H.E. Next-generation superparamagnetic iron oxide nanoparticles for cancer theranostics. Drug Discov. Today 2017, 22, 1421–1429. [Google Scholar] [CrossRef]
- Subramani, K.; Hosseinkhani, H.; Khraisat, A.; Hosseinkhani, M.; Pathak, Y. Targeting nanoparticles as drug delivery systems for cancer treatment. Curr. Nanosci. 2009, 5, 135–140. [Google Scholar] [CrossRef]
- Meng, X.X.; Wan, J.Q.; Jing, M.; Zhao, S.G.; Cai, W.; Liu, E.Z. Specific targeting of gliomas with multifunctional superparamagnetic iron oxide nanoparticle optical and magnetic resonance imaging contrast agents1. Acta Pharmacol. Sin. 2007, 28, 2019–2026. [Google Scholar] [CrossRef] [Green Version]
- Mizrahy, S.; Gutkin, A.; Decuzzi, P.; Peer, D. Targeting central nervous system pathologies with nanomedicines. J. Drug Target. 2019, 27, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Furtado, D.; Bjornmalm, M.; Ayton, S.; Bush, A.I.; Kempe, K.; Caruso, F. Overcoming the blood-brain barrier: The role of nanomaterials in treating neurological diseases. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Liu, R.; Ji, W.; Li, Y.; Liu, L.; Zhang, X. Delivery systems for theranostics in neurodegenerative diseases. Nano Res. 2018, 11, 5535–5555. [Google Scholar] [CrossRef]
- Meng, J.; Agrahari, V.; Youm, I. Advances in targeted drug delivery approaches for the central nervous system tumors: The inspiration of nanobiotechnology. J. Neuroimmune Pharmacol. 2017, 12, 84–98. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Deng, Y.; Zou, Y.; Li, C.; Guo, X.; Xiong, L.; Gao, Y.; Li, F.; Zhao, D. Highly water-dispersible biocompatible magnetite particles with low cytotoxicity stabilized by citrate groups. Angew. Chem. Int. Ed. 2009, 48, 5875–5879. [Google Scholar] [CrossRef]
- Rockenberger, J.; Scher, E.C.; Alivisatos, A.P. A new nonhydrolytic single-precursor approach to surfactant-capped nanocrystals of transition metal oxides. J. Am. Chem. Soc. 1999, 121, 11595–11596. [Google Scholar] [CrossRef]
- Mohagheghpour, E.; Moztarzadeh, F.; Rabiee, M.; Tahriri, M.; Ashuri, M.; Sameie, H.; Salimi, R.; Moghadas, S. Micro-Emulsion synthesis, surface modification, and photophysical properties of Zn1-x MnXS nanocrystals for biomolecular recognition. IEEE Trans. Nanobioscience 2012, 11, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Lemine, O.M.; Omri, K.; Zhang, B.; El Mir, L.; Sajieddine, M.; Alyamani, A.; Bououdina, M. Sol-gel synthesis of 8 nm magnetite (Fe3O4) nanoparticles and their magnetic properties. Superlattices Microstruct. 2012, 52, 793–799. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, D.; Bu, W.; Ehlerding, E.B.; Cai, W.; Shi, J. Engineering of inorganic nanoparticles as magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2017, 46, 7438–7468. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.W.; Huh, Y.M.; Choi, J.S.; Lee, J.H.; Song, H.T.; Kim, S.; Yoon, S.; Kim, K.S.; Shin, J.S.; Suh, J.S.; et al. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef]
- Mou, Y.B.; Xing, Y.; Ren, H.Y.; Cui, Z.H.; Zhang, Y.; Yu, G.J.; Urba, W.J.; Hu, Q.G.; Hu, H.M. The effect of superparamagnetic Iron Oxide nanoparticle surface charge on antigen cross-presentation. Nanoscale Res. Lett. 2017, 12, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roca, A.G.; Gutierrez, L.; Gavilan, H.; Fortes Brollo, M.E.; Veintemillas-Verdaguer, S.; del Puerto Morales, M. Design strategies for shape-controlled magnetic iron oxide nanoparticles. Adv. Drug Deliv. Rev. 2019, 138, 68–104. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.G.D.; Veloso, S.R.S.; Castanheira, E.M.S. Shape Anisotropic Iron Oxide-based magnetic nanoparticles: Synthesis and biomedical applications. Int. J. Mol. Sci. 2020, 21, 2455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohapatra, J.; Mitra, A.; Tyagi, H.; Bahadur, D.; Aslam, M. Iron oxide nanorods as high-performance magnetic resonance imaging contrast agents. Nanoscale 2015, 7, 9174–9184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.; Choi, Y.; Lee, Y.; Park, M.; Moon, W.K.; Choi, S.H.; Hyeon, T. Water-dispersible ferrimagnetic Iron Oxide nanocubes with extremely high r(2) relaxivity for highly sensitive in vivo MRI of tumors. Nano Lett. 2012, 12, 3127–3131. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, Z.; Bao, J.; Wang, Z.; Hu, J.; Chi, X.; Ni, K.; Wang, R.; Chen, X.; Chen, Z.; et al. Octapod iron oxide nanoparticles as high-performance T-2 contrast agents for magnetic resonance imaging. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef]
- Saito, S.; Tsugeno, M.; Koto, D.; Mori, Y.; Yoshioka, Y.; Nohara, S.; Murase, K. Impact of surface coating and particle size on the uptake of small and ultrasmall superparamagnetic iron oxide nanoparticles by macrophages. Int. J. Nanomed. 2012, 7, 5415–5421. [Google Scholar]
- Marino, A.; Camponovo, A.; Degl’Innocenti, A.; Bartolucci, M.; Tapeinos, C.; Martinelli, C.; De Pasquale, D.; Santoro, F.; Mollo, V.; Arai, S.; et al. Multifunctional temozolomide-loaded lipid superparamagnetic nanovectors: Dual targeting and disintegration of glioblastoma spheroids by synergic chemotherapy and hyperthermia treatment. Nanoscale 2019, 11, 21227–21248. [Google Scholar] [CrossRef]
- Fang, J.-H.; Chiu, T.-L.; Huang, W.-C.; Lai, Y.-H.; Hu, S.-H.; Chen, Y.-Y.; Chen, S.-Y. Dual-targeting lactoferrin-conjugated polymerized magnetic polydiacetylene-assembled nanocarriers with self-responsive fluorescence/magnetic resonance imaging for in vivo brain tumor therapy. Adv. Healthc. Mater. 2016, 5, 688–695. [Google Scholar] [CrossRef]
- Du, C.; Liu, X.; Hu, H.; Li, H.; Yu, L.; Geng, D.; Chen, Y.; Zhang, J. Dual-targeting and excretable ultrasmall SPIONs for T-1-weighted positive MR imaging of intracranial glioblastoma cells by targeting the lipoprotein receptor-related protein. J. Mater. Chem. B 2020, 8, 2296–2306. [Google Scholar] [CrossRef]
- Zhou, Q.; Shao, S.; Wang, J.; Xu, C.; Xiang, J.; Piao, Y.; Zhou, Z.; Yu, Q.; Tang, J.; Liu, X.; et al. Enzyme-activatable polymer-drug conjugate augments tumour penetration and treatment efficacy. Nat. Nanotechnol. 2019, 14, 799–809. [Google Scholar] [CrossRef]
- Zhao, X.; Shang, T.; Zhang, X.; Ye, T.; Wang, D.; Rei, L. Passage of magnetic tat-conjugated Fe3O4@SiO2 nanoparticles across in vitro blood-brain barrier. Nanoscale Res. Lett. 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Canul-Tec, J.C.; Assal, R.; Cirri, E.; Legrand, P.; Brier, S.; Chamot-Rooke, J.; Reyes, N. Structure and allosteric inhibition of excitatory amino acid transporter 1. Nature 2017, 544, 446–451. [Google Scholar] [CrossRef]
- Patching, S.G. Glucose transporters at the blood-brain barrier: Function, regulation and gateways for drug delivery. Mol. Neurobiol. 2017, 54, 1046–1077. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Xu, C.; Sun, P.; Wu, J.; Yan, C.; Hu, M.; Yan, N. Crystal structure of the human glucose transporter GLUT1. Nature 2014, 510, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Yang, X.; Fu, M.; Zhai, G. Recent progress of drug nanoformulations targeting to brain. J. Control. Release 2018, 291, 37–64. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.B.; Thomsen, M.S.; Moos, T. Targeted drug delivery to the brain using magnetic nanoparticles. Ther. Deliv. 2015, 6, 1145–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazur, J.; Roy, K.; Kanwar, J.R. Recent advances in nanomedicine and survivin targeting in brain cancers. Nanomedicine 2018, 13, 105–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, C.; He, Q. Pharmaceutical application of magnetic Iron Oxide nanoparticles. Sci. Adv. Mater. 2015, 7, 672–685. [Google Scholar] [CrossRef]
- Glaser, T.; Han, I.; Wu, L.; Zeng, X. Targeted Nanotechnology in Glioblastoma Multiforme. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, M.P.; Arce, M.; Yameen, B.; Vilos, C. Targeted brain delivery nanoparticles for malignant gliomas. Nanomedicine 2017, 12, 59–72. [Google Scholar] [CrossRef]
- Bredlau, A.L.; Dixit, S.; Chen, C.; Broome, A.-M. Nanotechnology applications for diffuse intrinsic pontine glioma. Curr. Neuropharmacol. 2017, 15, 104–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Xu, C.-L.; Liu, C.-M. Drug delivery strategies to enhance the permeability of the blood-brain barrier for treatment of glioma. Drug Des. Dev. Ther. 2015, 9, 2089–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranowska-Wojcik, E.; Szwajgier, D. Alzheimer’s disease: Review of current nanotechnological therapeutic strategies. Expert Rev. Neurother. 2020, 20, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z.; Lu, Z.; Yang, Q.; Liu, L.; Jiang, Z.; Zhang, L.; Zhang, X.; Qing, H. “Cell-addictive” dual-target traceable nanodrug for Parkinson’s disease treatment via flotillins pathway. Theranostics 2018, 8, 5469–5481. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Cutler, E.G.; Cho, H. Therapeutic nanoplatforms and delivery strategies for neurological disorders. Nano Converg. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Haeberlein, S.L.B.; Harris, T.J.R. Promising Targets for the treatment of neurodegenerative diseases. Clin. Pharmacol. Ther. 2015, 98, 492–501. [Google Scholar] [CrossRef]
- Lo, D.; Grossberg, G.T. Use of memantine for the treatment of dementia. Expert Rev. Neurother. 2011, 11, 1359–1370. [Google Scholar] [CrossRef]
- Shega, J.W.; Ellner, L.; Lau, D.T.; Maxwell, T.L. Cholinesterase inhibitor and N-Methyl-D-Aspartic Acid receptor antagonist use in older adults with end-stage dementia: A survey of hospice medical directors. J. Palliat. Med. 2009, 12, 779–783. [Google Scholar] [CrossRef]
- Vassar, R. BACE1 inhibitor drugs in clinical trials for Alzheimer’s disease. Alzheimers Res. Ther. 2014, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, R.; Vassar, R. Targeting the beta secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol. 2014, 13, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Ly, P.T.T.; Wu, Y.; Zou, H.; Wang, R.; Zhou, W.; Kinoshita, A.; Zhang, M.; Yang, Y.; Cai, F.; Woodgett, J.; et al. Inhibition of GSK3 beta-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J. Clin. Investig. 2013, 123, 224–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, R.; Wang, X.-L.; Wu, J.-M.; Tang, Y.; Qiu, W.-Q.; Shen, X.; Teng, J.-F.; Pan, R.; Zhao, Y.; Yu, L.; et al. Polyphenols isolated from lychee seed inhibit Alzheimer’s disease-associated Tau through improving insulin resistance via the IRS-1/PI3K/Akt/GSK-3 beta pathway. J. Ethnopharmacol. 2020, 251. [Google Scholar] [CrossRef]
- Zhou, H.; Gong, Y.; Liu, Y.; Huang, A.; Zhu, X.; Liu, J.; Yuan, G.; Zhang, L.; Wei, J.-A.; Liu, J. Intelligently thermoresponsive flower-like hollow nano-ruthenium system for sustained release of nerve growth factor to inhibit hyperphosphorylation of tau and neuronal damage for the treatment of Alzheimer’s disease. Biomaterials 2020, 237. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shal, B.; Ding, W.; Ali, H.; Kim, Y.S.; Khan, S. Anti-neuroinflammatory Potential of Natural Products in Attenuation of Alzheimer’s Disease. Front. Pharmacol. 2018, 9, 17. [Google Scholar] [CrossRef]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuca, K.; Musilek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Reich, S.G.; Savitt, J.M. Parkinson’s Disease. Med. Clin. N. Am. 2019, 103, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jiang, C.Z.; Roy, V.A.L. Designed synthesis and surface engineering strategies of magnetic iron oxide nanoparticles for biomedical applications. Nanoscale 2016, 8, 19421–19474. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Hu, B.; Chen, J.; Zhang, Z.; Zhang, X.; Fan, Z. Superparamagnetic Iron Oxide Nanoparticles as magnetic resonance imaging contrast agent for myocardial infarction. J. Biomater. Tissue Eng. 2016, 6, 713–718. [Google Scholar] [CrossRef]
- Lee, H.; Yu, M.K.; Park, S.; Moon, S.; Min, J.J.; Jeong, Y.Y.; Kang, H.-W.; Jon, S. Thermally cross-linked superparamagnetic iron oxide nanoparticles: Synthesis and application as a dual Imaging probe for cancer in vivo. J. Am. Chem. Soc. 2007, 129, 12739–12745. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhang, L.L.; Huang, F.R.; Du, L. Investigation on poly (methylsilylene ethynylene phenylene ethynylene) co (tetramethyldisiloxane ethynylene phenylene ethynylene). Chin. Chem. Lett. 2010, 21, 738–742. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Hirt, A.M.; Lozach, P.-Y.; Teleki, A.; Krumeich, F.; Pratsinis, S.E. Hybrid, silica-coated, janus-like plasmonic-magnetic nanoparticles. Chem. Mater. 2011, 23, 1985–1992. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.L.; Li, Y.Y.; Sun, K.; Fan, J.B.; Zhang, P.C.; Meng, J.X.; Wang, S.T.; Jiang, L. Dual-responsive surfaces modified with phenylboronic acid-containing polymer brush to reversibly capture and release cancer cells. J. Am. Chem. Soc. 2013, 135, 7603–7609. [Google Scholar] [CrossRef]
- He, Q.G.; Zeng, L.; Wu, W.; Hu, R.; Huang, J.K. Preparation and magnetic comparison of silane-functionalized magnetite nanoparticles. Sens. Mater. 2010, 22, 285–295. [Google Scholar]
- Wu, W.; Wu, Z.H.; Yu, T.; Jiang, C.Z.; Kim, W.S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 43. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Maier-Hauff, K.; van Landeghem, F.K.H.; Waldoefner, N.; Teichgraeber, U.; Pinkernelle, J.; Bruhn, H.; Neumann, F.; Thiesen, B.; et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J. Neuro Oncol. 2006, 78, 7–14. [Google Scholar] [CrossRef]
- Veiseh, O.; Gunn, J.W.; Kievit, F.M.; Sun, C.; Fang, C.; Lee, J.S.H.; Zhang, M. Inhibition of tumor-cell invasion with chlorotoxin-bound superparamagnetic nanoparticles. Small 2009, 5, 256–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.; Sun, W.; Lu, L.; Xiao, Z.; Wei, H.; Shi, W.; Wang, Y.; Han, S.; Shuai, X. I6P7 peptide modified superparamagnetic iron oxide nanoparticles for magnetic resonance imaging detection of low-grade brain gliomas. J. Mater. Chem. B 2019, 7, 6139–6147. [Google Scholar] [CrossRef]
- Wang, L.; Jang, G.; Ban, D.K.; Sant, V.; Seth, J.; Kazmi, S.; Patel, N.; Yang, Q.; Lee, J.; Janetanakit, W.; et al. Multifunctional stimuli responsive polymer-gated iron and gold-embedded silica nano golf balls: Nanoshuttles for targeted on-demand theranostics. Bone Res. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef]
- Yu, B.; Liu, J.; Liu, S.; Zhou, F. Pdop layer exhibiting zwitterionicity: A simple electrochemical interface for governing ion permeability. Chem. Commun. 2010, 46, 5900–5902. [Google Scholar] [CrossRef]
- Richard, S.; Saric, A.; Boucher, M.; Slomianny, C.; Geffroy, F.; Mériaux, S.; Lalatonne, Y.; Motte, L. Anti-oxidative theranostic iron oxide nanoparticles towards brain tumors imaging and ROS production. ACS Chem. Biol. 2018. [Google Scholar] [CrossRef]
- Yang, X.; Hong, H.; Grailer, J.J.; Rowland, I.J.; Javadi, A.; Hurley, S.A.; Xiao, Y.; Yang, Y.; Zhang, Y.; Nickles, R.; et al. cRGD-functionalized, DOX-conjugated, and Cu-64-labeled superparamagnetic iron oxide nanoparticles for targeted anticancer drug delivery and PET/MR imaging. Biomaterials 2011, 32, 4151–4160. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Kievit, F.M.; Gunn, J.W.; Ratner, B.D.; Zhang, M. A ligand-mediated nanovector for targeted gene delivery and transfection in cancer cells. Biomaterials 2009, 30, 649–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Z.; Wei, J.; Yu, C.; Han, X.; Qin, X.; Zhang, C.; Liao, W.; Li, L.; Huang, W. Non-viral nanocarriers for intracellular delivery of microRNA therapeutics. J. Mater. Chem. B 2019, 7, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
- Molday, R.S.; Mackenzie, D. Immunospecific ferromagnetic iron-dextran reagents for the labeling and magnetic separation of cells. J. Immunol. Methods 1982, 52, 353–367. [Google Scholar] [CrossRef]
- Wan, X.Y.; Song, Y.Q.; Song, N.J.; Li, J.H.; Yang, L.; Li, Y.; Tan, H. The preliminary study of immune superparamagnetic Iron Oxide Nanoparticles for the detection of lung cancer in magnetic resonance imaging. Carbohydr. Res. 2016, 419, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.M.; Li, S.H.; Kim, H.H.; Kim, H.; Lee, H.B.; Muhammed, M.; Kim, D.K. Complete separation of magnetic nanoparticles via chemical cleavage of dextran by ethylenediamine for intracellular uptake. J. Mater. Chem. 2010, 20, 444–447. [Google Scholar] [CrossRef]
- Tassa, C.; Shaw, S.Y.; Weissleder, R. Dextran-Coated Iron Oxide Nanoparticles: A Versatile Platform for Targeted Molecular Imaging, Molecular Diagnostics, and Therapy. Acc. Chem. Res. 2011, 44, 842–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, T.-H.; Hsu, S.-C.; Wu, S.-H.; Hsiao, J.-K.; Lin, C.-P.; Yao, M.; Huang, D.-M. Dextran-coated iron oxide nanoparticle-improved therapeutic effects of human mesenchymal stem cells in a mouse model of Parkinson’s disease. Nanoscale 2018, 10, 2998–3007. [Google Scholar] [CrossRef]
- Pisanic, T.R.; Blackwell, J.D.; Shubayev, V.I.; Finones, R.R.; Jin, S. Nanotoxicity of Iron Oxide Nanoparticle internalization in growing neurons. Biomaterials 2007, 28, 2572–2581. [Google Scholar] [CrossRef]
- Katebi, S.; Esmaeili, A.; Ghaedi, K.; Zarrabi, A. Superparamagnetic iron oxide nanoparticles combined with NGF and quercetin promote neuronal branching morphogenesis of PC12 cells. Int. J. Nanomed. 2019, 14, 2157–2169. [Google Scholar] [CrossRef] [Green Version]
- Artursson, P.; Lindmark, T.; Davis, S.S.; Illum, L. Effect of chitosan on the permeability of monolayers of intestinal epithelial-cells (caco-2). Pharm. Res. 1994, 11, 1358–1361. [Google Scholar] [CrossRef]
- Guo, L.L.; Chen, H.; He, N.Y.; Deng, Y. Effects of surface modifications on the physicochemical properties of iron oxide nanoparticles and their performance as anticancer drug carriers. Chin. Chem. Lett. 2018, 29, 1829–1833. [Google Scholar] [CrossRef]
- Kim, E.H.; Lee, H.S.; Kwak, B.K.; Kim, B.K. Synthesis of ferrofluid with magnetic nanoparticles by sonochemical method for MRI contrast agent. J. Magn. Magn. Mater. 2005, 289, 328–330. [Google Scholar] [CrossRef]
- Bhattarai, S.R.; Kim, S.Y.; Jang, K.Y.; Lee, K.C.; Yi, H.K.; Lee, D.Y.; Kim, H.Y.; Hwang, P.H. Laboratory formulated magnetic nanoparticles for enhancement of viral gene expression in suspension cell line. J. Virol. Methods 2008, 147, 213–218. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H.; Beygzadeh, M. Novel high flux antifouling nanofiltration membranes for dye removal containing carboxymethyl chitosan coated Fe3O4 nanoparticles. Desalination 2014, 349, 145–154. [Google Scholar] [CrossRef]
- Kamalzare, S.; Noormohammadi, Z.; Rahimi, P.; Atyabi, F.; Irani, S.; Tekie, F.S.M.; Mottaghitalab, F. Carboxymethyl dextran-trimethyl chitosan coated superparamagnetic iron oxide nanoparticles: An effective siRNA delivery system for HIV-1 Nef. J. Cell. Physiol. 2019, 234, 20554–20565. [Google Scholar] [CrossRef] [PubMed]
- Barrow, M.; Taylor, A.; Nieves, D.J.; Bogart, L.K.; Mandal, P.; Collins, C.M.; Moore, L.R.; Chalmers, J.J.; Levy, R.; Williams, S.R.; et al. Tailoring the surface charge of dextran-based polymer coated SPIONs for modulated stem cell uptake and MRI contrast. Biomater. Sci. 2015, 3, 608–616. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Q.; Chang, Y.Y.; Zhang, D.X.; Tian, B.M.; Yang, Y.; Wei, F. Transferrin-conjugated drug/dye-co-encapsulated magnetic nanocarriers for active-targeting fluorescent/magnetic resonance imaging and anti-tumor effects in human brain tumor cells. RSC Adv. 2016, 6, 105661–105675. [Google Scholar] [CrossRef]
- Walia, S.; Sharma, S.; Kulurkar, P.M.; Patial, V.; Acharya, A. A bimodal molecular imaging probe based on chitosan encapsulated magneto-fluorescent nanocomposite offers biocompatibility, visualization of specific cancer cells in vitro and lung tissues in vivo. Int. J. Pharm. 2016, 498, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.J.; Bentley, M.D.; Harris, J.M. Chemistry for peptide and protein PEGylation. Adv. Drug Deliv. Rev. 2012, 64, 116–127. [Google Scholar] [CrossRef]
- Zhang, Y.; Kohler, N.; Zhang, M.Q. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials 2002, 23, 1553–1561. [Google Scholar] [CrossRef]
- Meng, Q.; Hu, H.; Zhou, L.; Zhang, Y.; Yu, B.; Shen, Y.; Cong, H. Logical design and application of prodrug platforms. Polym. Chem. 2019, 10, 306–324. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Stiller, S.; Hoth, A.; Kaufner, L.; Pison, U.; Cartier, R. One-pot synthesis of PEGylated ultrasmall iron-oxide nanoparticles and their in vivo evaluation as magnetic resonance imaging contrast agents. Biomacromolecules 2006, 7, 3132–3138. [Google Scholar] [CrossRef] [PubMed]
- Kohler, N.; Fryxell, G.E.; Zhang, M.Q. A bifunctional poly(ethylene glycol) silane immobilized on metallic oxide-based nanoparticles for conjugation with cell targeting agents. J. Am. Chem. Soc. 2004, 126, 7206–7211. [Google Scholar] [CrossRef] [PubMed]
- Stephen, Z.R.; Gebhart, R.N.; Jeon, M.; Blair, A.A.; Ellenbogen, R.G.; Silber, J.R.; Zhang, M. pH-Sensitive O6-Benzylguanosine polymer modified magnetic nanoparticles for treatment of glioblastomas. Bioconjugate Chem. 2017, 28, 194–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohler, N.; Sun, C.; Fichtenholtz, A.; Gunn, J.; Fang, C.; Zhang, M.Q. Methotrexate-immobilized poly(ethylene glycol) magnetic nanoparticles for MR imaging and drug delivery. Small 2006, 2, 785–792. [Google Scholar] [CrossRef]
- Kievit, F.M.; Wang, F.Y.; Fang, C.; Mok, H.; Wang, K.; Silber, J.R.; Ellenbogen, R.G.; Zhang, M. Doxorubicin loaded iron oxide nanoparticles overcome multidrug resistance in cancer in vitro. J. Control. Release 2011, 152, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Miller, D.W. Salinomycin-loaded Iron Oxide Nanoparticles for glioblastoma therapy. Nanomaterials 2020, 10, 447. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Lv, Z.; Sun, Y.; Chi, Z.; Qing, G. Recent advancements in polyethyleneimine-based materials and their biomedical, biotechnology, and biomaterial applications. J. Mater. Chem. B 2020, 8, 2951–2973. [Google Scholar] [CrossRef]
- Yan, H.; Zhu, D.; Zhou, Z.; Liu, X.; Piao, Y.; Zhang, Z.; Liu, X.; Tang, J.; Shen, Y. Facile synthesis of semi-library of low charge density cationic polyesters from poly(alkylene maleate)s for efficient local gene delivery. Biomaterials 2018, 178, 559–569. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.; Zhang, X. Design, preparation and application of nucleic acid delivery carriers. Biotechnol. Adv. 2014, 32, 804–817. [Google Scholar] [CrossRef]
- Steitz, B.; Hofmann, H.; Kamau, S.W.; Hassa, P.O.; Hottiger, M.O.; von Rechenberg, B.; Hofmann-Amtenbrink, M.; Petri-Fink, A. Characterization of PEI-coated superparamagnetic iron oxide nanoparticles for transfection: Size distribution, colloidal properties and DNA interaction. J. Magn. Magn. Mater. 2007, 311, 300–305. [Google Scholar] [CrossRef]
- Chertok, B.; David, A.E.; Yang, V.C. Polyethyleneimine-modified iron oxide nanoparticles for brain tumor drug delivery using magnetic targeting and intra-carotid administration. Biomaterials 2010, 31, 6317–6324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, F.; Du, M.; Liu, Y.; Liu, G.; Liu, Q.; Zhang, X. Folic acid-conjugated glucose and dextran coated iron oxide nanoparticles as MRI contrast agents for diagnosis and treatment response of rheumatoid arthritis. J. Mater. Chem. B 2014, 2, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Mok, H.; Veiseh, O.; Fang, C.; Kievit, F.M.; Wang, F.Y.; Park, J.O.; Zhang, M. pH-Sensitive siRNA nanovector for targeted gene silencing and cytotoxic effect in cancer cells. Mol. Pharm. 2010, 7, 1930–1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veiseh, O.; Kievit, F.M.; Fang, C.; Mu, N.; Jana, S.; Leung, M.C.; Mok, H.; Ellenbogen, R.G.; Park, J.O.; Zhang, M. Chlorotoxin bound magnetic nanovector tailored for cancer cell targeting, imaging, and siRNA delivery. Biomaterials 2010, 31, 8032–8042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Wang, C.; Zhan, H.; Yao, L.; Zhang, J.; Gao, R.; Tang, X.; Chong, T.; Liu, W.; Tang, Y. A high-loading drug delivery system based on magnetic nanomaterials modified by hyperbranched phenylboronic acid for tumor-targeting treatment with pH response. Colloids Surf. B-Biointerfaces 2019, 182. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-Y.; Chiang, P.-H.; Hsiao, W.-C.; Chuang, C.-C.; Chang, C.-W. Redox-Sensitive Polymer/SPIO Nanocomplexes for Efficient Magnetofection and MR Imaging of Human Cancer Cells. Langmuir 2015, 31, 6523–6531. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Q.; Jiang, Q.; Huang, Y.; Liu, H.; Zhao, Y.; Cao, W.; Ma, G.; Dai, F.; Liang, X.; et al. Enhanced endosomal/lysosomal escape by distearoyl phosphoethanolamine-polycarboxybetaine lipid for systemic delivery of siRNA. J. Control. Release 2014, 176, 104–114. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Ji, W.; Lu, Z.; Liu, L.; Shi, Y.; Ma, G.; Zhang, X. Positively charged polyprodrug amphiphiles with enhanced drug loading and reactive oxygen species-responsive release ability for traceable synergistic therapy. J. Am. Chem. Soc. 2018, 140, 4164–4171. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Y.; Hu, B.; Lu, Z.; Zhang, J.; Zhang, X. Traceable nanoparticle delivery of small interfering rna and retinoic acid with temporally release ability to control neural stem cell differentiation for alzheimer’s disease therapy. Adv. Mater. 2016, 28, 6345–6352. [Google Scholar] [CrossRef]
- Martins, C.; Sousa, F.; Araujo, F.; Sarmento, B. Functionalizing PLGA and PLGA derivatives for drug delivery and tissue regeneration applications. Adv. Healthc. Mater. 2018, 7, 24. [Google Scholar] [CrossRef]
- Ganipineni, L.P.; Ucakar, B.; Joudiou, N.; Bianco, J.; Danhier, P.; Zhao, M.; Bastiancich, C.; Gallez, B.; Danhier, F.; Preat, V. Magnetic targeting of paclitaxel-loaded poly(lactic-co-glycolic acid)-based nanoparticles for the treatment of glioblastoma. Int. J. Nanomed. 2018, 13, 4509–4521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soenen, S.J.; Vande Velde, G.; Ketkar-Atre, A.; Himmelreich, U.; De Cuyper, M. Magnetoliposomes as magnetic resonance imaging contrast agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2011, 3, 197–211. [Google Scholar] [CrossRef]
- Sawant, R.R.; Torchilin, V.P. Liposomes as ‘smart’ pharmaceutical nanocarriers. Soft Matter 2010, 6, 4026–4044. [Google Scholar] [CrossRef]
- Ji, W.H.; Xiao, Z.B.; Liu, G.Y.; Zhang, X. Development and application of nano-flavor-drug carriers in neurodegenerative diseases. Chin. Chem. Lett. 2017, 28, 1829–1834. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Li, Y.; Liu, R.; Yang, J.; Shi, Y.; Ma, G.; Zhang, Z.; Zhang, X. Enhanced retention and anti-tumor efficacy of liposomes by changing their cellular uptake and pharmacokinetics behavior. Biomaterials 2015, 41, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mullis, A.S.; Schlichtmann, B.W.; Narasimhan, B.; Cademartiri, R.; Mallapragada, S.K. Ligand-cascading nano-delivery devices to enable multiscale targeting of anti-neurodegenerative therapeutics. Biomed. Mater. 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Dai, F.; Fan, Z.; Ma, G.; Tang, Q.; Zhang, X. Nanotheranostics: Congo Red/Rutin-MNPs with Enhanced Magnetic Resonance Imaging and H2O2-Responsive Therapy of Alzheimer’s Disease in APPswe/PS1dE9 Transgenic Mice. Adv. Mater. 2015, 27, 5499–5505. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-L.; Yang, J.-J.; Zhuge, D.-L.; Lin, M.-T.; Zhu, Q.-Y.; Jin, B.-H.; Tong, M.-Q.; Shen, B.-X.; Xiao, J.; Zhao, Y.-Z. Glioma-targeted delivery of a theranostic liposome integrated with quantum dots, superparamagnetic Iron Oxide, and cilengitide for dual-imaging guiding cancer surgery. Adv. Healthc. Mater. 2018, 7. [Google Scholar] [CrossRef]
- Luchini, A.; Vitiello, G. Understanding the nano-bio interfaces: Lipid-coatings for inorganic nanoparticles as promising strategy for biomedical applications. Front. Chem. 2019, 7, 16. [Google Scholar] [CrossRef]
- Gill, K.K.; Kaddoumi, A.; Nazzal, S. PEG-lipid micelles as drug carriers: Physiochemical attributes, formulation principles and biological implication. J. Drug Target. 2015, 23, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Hu, S.Q.; Mao, W.W.; Xiang, J.J.; Zhou, Z.X.; Liu, X.R.; Tang, J.B.; Shen, Y.Q. Assemblies of peptide-cytotoxin conjugates for tumor-homing chemotherapy. Adv. Funct. Mater. 2019, 29, 10. [Google Scholar]
- Lukyanov, A.N.; Gao, Z.G.; Mazzola, L.; Torchilin, V.P. Polyethylene glycol-diacyllipid micelles demonstrate increased acculumation in subcutaneous tumors in mice. Pharm. Res. 2002, 19, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, Y.; Shi, Y.; Li, Y.; Xiao, Z.; Zhang, X. Traceable nanoparticles with spatiotemporally controlled release ability for synergistic glioblastoma multiforme treatment. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Qiao, C.; Yang, J.; Shen, Q.; Liu, R.; Li, Y.; Shi, Y.; Chen, J.; Shen, Y.; Xiao, Z.; Weng, J.; et al. Traceable nanoparticles with dual targeting and ROS response for RNAi-Based immunochemotherapy of intracranial glioblastoma treatment. Adv. Mater. 2018, 30, e1705054. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Monga, Y.; Zboril, R.; Sharma, R.K. Silica-decorated magnetic nanocomposites for catalytic applications. Coord. Chem. Rev. 2015, 288, 118–143. [Google Scholar] [CrossRef]
- Chen, Y.J.; Tao, J.A.; Xiong, F.; Zhu, J.B.; Gu, N.; Zhang, Y.H.; Ding, Y.; Ge, L.A. Synthesis, self-assembly, and characterization of PEG-coated iron oxide nanoparticles as potential MRI contrast agent. Drug Dev. Ind. Pharm. 2010, 36, 1235–1244. [Google Scholar]
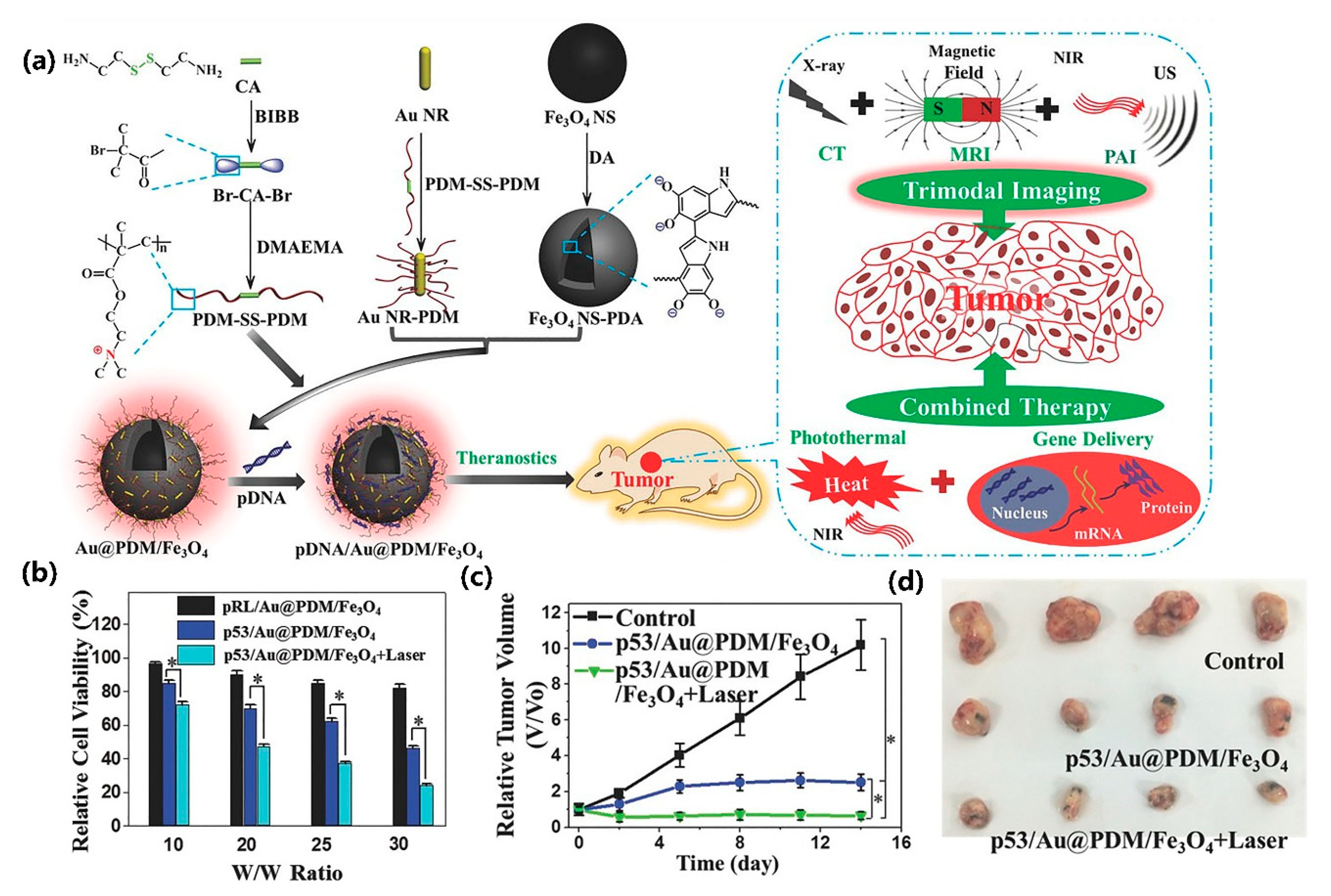
- Hu, Y.; Zhou, Y.; Zhao, N.; Liu, F.; Xu, F.-J. Multifunctional pDNA-Conjugated polycationic Au Nanorod-Coated Fe3O4 hierarchical nanocomposites for trimodal imaging and combined photothermal/gene therapy. Small 2016, 12, 2459–2468. [Google Scholar] [CrossRef]
- Yong, C.; Chen, X.; Xiang, Q.; Li, Q.; Xing, X. Recyclable magnetite-silver heterodimer nanocomposites with durable antibacterial performance. Bioact. Mater. 2018, 3, 80–86. [Google Scholar] [CrossRef]
- Zhao, S.; Yu, X.; Qian, Y.; Chen, W.; Shen, J. Multifunctional magnetic iron oxide nanoparticles: An advanced platform for cancer theranostics. Theranostics 2020, 10, 6278–6309. [Google Scholar] [CrossRef]
- Zhou, J.; Atsina, K.-B.; Himes, B.T.; Strohbehn, G.W.; Saltzman, W.M. Novel delivery strategies for glioblastoma. Cancer J. 2012, 18, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, M.; Simchi, A.; Imani, M.; Haefeli, U.O. Superparamagnetic Iron Oxide Nanoparticles with rigid cross-linked polyethylene glycol fumarate coating for application in imaging and drug delivery. J. Phys. Chem. C 2009, 113, 8124–8131. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Coating Classification | Coating (Examples) | Characteristics |
|---|---|---|
| Small molecule | Catechol, carboxylic, phosphate, sulfate, citrate, silane | reducing loss of original magnetic properties [72], antioxidant activity [86] |
| Polymer | Dextran, Chitosan, PEG, PEI, PCB, PLGA | magnetic reduction [105], providing positive charge [117], reduce reticuloendothelial (RES) clearance [108], proton sponge effect [127] |
| Lipid molecule | DSPE-PEG, DSPE-PCB | biocompatible protective barrier [132] |
| Polymer and lipid molecule | BAP + DSPE-PCB PAE + DSPE-PEG | combination of lipid and polymers [141,142,143] |
| Other coatings | Au, MgO | function modification [140,147] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Lu, Z.; Li, Y.; Yang, J.; Zhang, X. Surface Modification of Iron Oxide-Based Magnetic Nanoparticles for Cerebral Theranostics: Application and Prospection. Nanomaterials 2020, 10, 1441. https://doi.org/10.3390/nano10081441
Wu Y, Lu Z, Li Y, Yang J, Zhang X. Surface Modification of Iron Oxide-Based Magnetic Nanoparticles for Cerebral Theranostics: Application and Prospection. Nanomaterials. 2020; 10(8):1441. https://doi.org/10.3390/nano10081441
Chicago/Turabian StyleWu, Yanyue, Zhiguo Lu, Yan Li, Jun Yang, and Xin Zhang. 2020. "Surface Modification of Iron Oxide-Based Magnetic Nanoparticles for Cerebral Theranostics: Application and Prospection" Nanomaterials 10, no. 8: 1441. https://doi.org/10.3390/nano10081441
APA StyleWu, Y., Lu, Z., Li, Y., Yang, J., & Zhang, X. (2020). Surface Modification of Iron Oxide-Based Magnetic Nanoparticles for Cerebral Theranostics: Application and Prospection. Nanomaterials, 10(8), 1441. https://doi.org/10.3390/nano10081441







