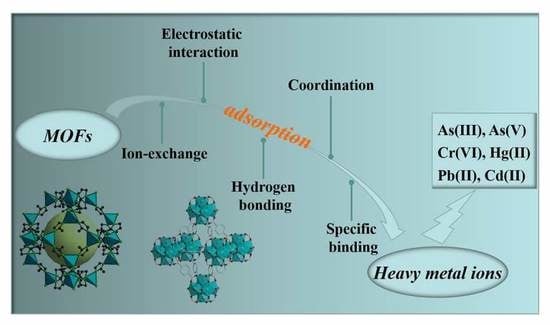
Recent Progress in Heavy Metal Ion Decontamination Based on Metal–Organic Frameworks
Abstract
:1. Introduction
2. Recent Studies on MOFs for the Removal of Heavy Metal Ions
2.1. Adsorptive Removal of Arsenic
2.1.1. ZIF Family MOFs for Arsenic Adsorption
2.1.2. Materials of Institute Lavoisier (MIL) Family MOFs for Arsenic Adsorption
2.1.3. Zr-Based MOFs for Adsorption of Arsenic
2.2. Adsorptive Removal of Chromium
2.3. Adsorptive Removal of Mercury
2.3.1. Binding with Sulfur Atoms
2.3.2. Binding with Other Functional Groups
2.4. Adsorptive Removal of Lead
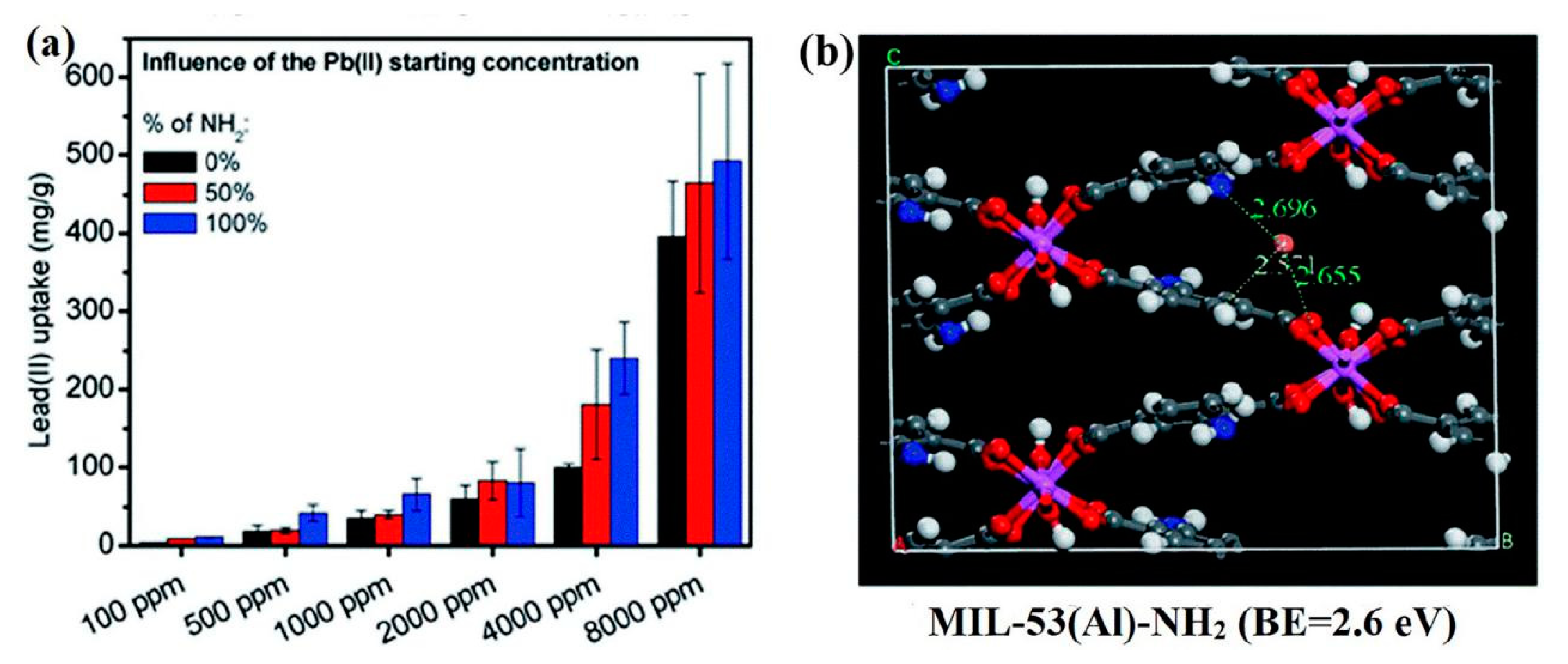
2.4.1. Binding with Amino Groups
2.4.2. Binding with Other Functional Groups
2.5. Adsorptive Removal of Cadmium
3. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Xu, S.; Lv, Y.; Zeng, X.; Cao, D. ZIF-derived nitrogen-doped porous carbons as highly efficient adsorbents for removal of organic compounds from wastewater. Chem. Eng. J. 2017, 323, 502–511. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Norton-Brandão, D.; Scherrenberg, S.M.; van Lier, J.B. Reclamation of used urban waters for irrigation purposes—A review of treatment technologies. J. Environ. Manag. 2013, 122, 85–98. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, X.; Guo, L.; Lan, J.; Zhang, L.; Cao, D. Heavy metal ion removal of wastewater by zeolite-imidazolate frameworks. Sep. Purif. Technol. 2018, 194, 462–469. [Google Scholar] [CrossRef]
- Ali, I. New generation adsorbents for water treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef]
- Li, X.; Wang, B.; Cao, Y.; Zhao, S.; Wang, H.; Feng, X.; Zhou, J.; Ma, X. Water Contaminant Elimination Based on Metal-Organic Frameworks and Perspective on Their Industrial Applications. ACS Sustain. Chem. Eng. 2019, 7, 4548–4563. [Google Scholar] [CrossRef]
- Xiang, Z.; Hu, Z.; Cao, D.; Yang, W.; Lu, J.; Han, B.; Wang, W. Metal-organic frameworks with incorporated carbon nanotubes: Improving carbon dioxide and methane storage capacities by lithium doping. Angew. Chemie Int. Ed. 2011, 50, 491–494. [Google Scholar] [CrossRef]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.W. Hydrogen storage in metal-organic frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, C.; Zhang, Q.; Chen, L. Metal-Organic Frameworks for Carbon Dioxide Capture and Methane Storage. Adv. Energy Mater. 2017, 7, 1601296. [Google Scholar] [CrossRef]
- Manos, G.; Dunne, L.J. Predicting the features of methane adsorption in large pore metal-organic frameworks for energy storage. Nanomaterials 2018, 8, 818. [Google Scholar] [CrossRef] [Green Version]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Van de Voorde, B.; Bueken, B.; Denayer, J.; De Vos, D. Adsorptive separation on metal-organic frameworks in the liquid phase. Chem. Soc. Rev. 2014, 43, 5766–5788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adil, K.; Belmabkhout, Y.; Pillai, R.S.; Cadiau, A.; Bhatt, P.M.; Assen, A.H.; Maurin, G.; Eddaoudi, M. Gas/vapour separation using ultra-microporous metal-organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Y.; Li, D.S.; Bu, X.; Feng, P. Metal–Organic Frameworks for Separation. Adv. Mater. 2018, 30, 1705189–1705223. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liang, H.; Peng, F.; Liu, Z.; Liu, J.; Qiao, Z. Computational screening of metal-organic framework membranes for the separation of 15 gas mixtures. Nanomaterials 2019, 9, 467. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Lin, W. Metal-organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef]
- Gascon, J.; Corma, A.; Kapteijn, F.; Llabrés, I.; Xamena, F.X. Metal organic framework catalysis: Quo vadis? ACS Catal. 2014, 4, 361–378. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.Y. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.C.; Li, J.R.; Lv, X.L.; Zhang, Y.Q.; Guo, G. Photocatalytic organic pollutants degradation in metal-organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Xiao, J.D.; Jiang, H.L. Metal-Organic Frameworks for Photocatalysis and Photothermal Catalysis. Acc. Chem. Res. 2018, 52, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, S.; Luo, J.; Huang, R.; Cai, H.; Yan, W.; Yang, H. A novel Tb@Sr-MOF as self-calibrating luminescent sensor for nutritional antioxidant. Nanomaterials 2018, 8, 796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, L.; Guo, S.; Xu, J.; Chen, X.; Zhu, T.; Zhao, T. A ratiometric fluorescent nano-probe for rapid and specific detection of tetracycline residues based on a dye-doped functionalized nanoscaled metal–organic framework. Nanomaterials 2019, 9, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal-organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Kuo, C.H.; Chou, L.Y.; Liu, D.Y.; Weerapana, E.; Tsung, C.K. Optimized metal-organic-framework nanospheres for drug delivery: Evaluation of small-molecule encapsulation. ACS Nano 2014, 8, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, M.; Xie, Z. Nanoscale metal-organic frameworks for drug delivery: A conventional platform with new promise. J. Mater. Chem. B 2018, 6, 707–717. [Google Scholar] [CrossRef]
- Sava Gallis, D.F.; Rohwer, L.E.S.; Rodriguez, M.A.; Barnhart-Dailey, M.C.; Butler, K.S.; Luk, T.S.; Timlin, J.A.; Chapman, K.W. Multifunctional, Tunable Metal-Organic Framework Materials Platform for Bioimaging Applications. ACS Appl. Mater. Interfaces 2017, 9, 22268–22277. [Google Scholar] [CrossRef]
- Wang, H.S. Metal–organic frameworks for biosensing and bioimaging applications. Coord. Chem. Rev. 2017, 349, 139–155. [Google Scholar] [CrossRef]
- Escorihuela, J.; Sahuquillo, Ó.; García-Bernabé, A.; Giménez, E.; Compañ, V. Phosphoric Acid Doped Polybenzimidazole (PBI)/Zeolitic Imidazolate Framework Composite Membranes with Significantly Enhanced Proton Conductivity under Low Humidity Conditions. Nanomaterials 2018, 8, 775. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Gil, D.; Figueiredo, F.M.L. High surface proton conduction in nanostructured ZIF-8. Nanomaterials 2019, 9, 1369. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, T.; Zhang, L.; Zhang, J.; Aggarwal, S. Removal of arsenic(III) from aqueous solution using metal organic framework-graphene oxide nanocomposite. Nanomaterials 2018, 8, 1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Huang, Y.; Xiao, A.; Qiu, H.; Liu, L. Preparation of magnetic Fe3O4/MIL-88A nanocomposite and its adsorption properties for bromophenol blue dye in aqueous solution. Nanomaterials 2019, 9, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Shi, X.; Li, J.; Kumar, P.; Liu, B. Selective dye adsorption by zeolitic imidazolate framework-8 loaded UiO-66-NH2. Nanomaterials 2019, 9, 1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehghan, A.; Mohammadi, A.A.; Yousefi, M.; Najafpoor, A.A.; Shams, M.; Rezania, S. Enhanced kinetic removal of ciprofloxacin onto metal-organic frameworks by sonication, process optimization and metal leaching study. Nanomaterials 2019, 9, 1422. [Google Scholar] [CrossRef] [Green Version]
- Kobielska, P.A.; Howarth, A.J.; Farha, O.K.; Nayak, S. Metal–organic frameworks for heavy metal removal from water. Coord. Chem. Rev. 2018, 358, 92–107. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal–organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef]
- Wen, J.; Fang, Y.; Zeng, G. Progress and prospect of adsorptive removal of heavy metal ions from aqueous solution using metal-organic frameworks: A review of studies from the last decade. Chemosphere 2018, 201, 627–643. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, P.; Zhou, H.; Sharma, V.K. Water-stable metal-organic frameworks for aqueous removal of heavy metals and radionuclides: A review. Chemosphere 2018, 209, 783–800. [Google Scholar] [CrossRef]
- Wang, C.; Luan, J.; Wu, C. Metal-organic frameworks for aquatic arsenic removal. Water Res. 2019, 158, 370–382. [Google Scholar]
- Mon, M.; Bruno, R.; Ferrando-soria, J.; Armentano, D.; Pardo, E. Metal-organic framework technologies for water remediation: Towards a sustainable ecosystem. J. Mater. Chem. A 2018, 6, 4912–4947. [Google Scholar] [CrossRef]
- Rasheed, T.; Ahmad, A.; Bilal, M.; Hussain, T.; Rizwan, K. Metal-organic frameworks based adsorbents: A review from removal perspective of various environmental contaminants from wastewater. Chemosphere 2020, 259, 127369. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Xu, J.; Bu, X.H. Recent advances about metal–organic frameworks in the removal of pollutants from wastewater. Coord. Chem. Rev. 2019, 378, 17–31. [Google Scholar] [CrossRef]
- He, X.; Deng, F.; Shen, T.; Yang, L.; Chen, D.; Luo, J.; Luo, X.; Min, X.; Wang, F. Exceptional adsorption of arsenic by zirconium metal-organic frameworks: Engineering exploration and mechanism insight. J. Colloid Interface Sci. 2019, 539, 223–234. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.N.; Li, Z.; Zhang, B.; Zhu, M.; Hu, X.; Zhang, Y.; Li, F. Zeolitic imidazolate framework-8 with high efficiency in trace arsenate adsorption and removal from water. J. Phys. Chem. C 2014, 118, 27382–27387. [Google Scholar] [CrossRef]
- Wu, Y.N.; Zhou, M.; Zhang, B.; Wu, B.; Li, J.; Qiao, J.; Guan, X.; Li, F. Amino acid assisted templating synthesis of hierarchical zeolitic imidazolate framework-8 for efficient arsenate removal. Nanoscale 2014, 6, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jian, M.; Liu, R.; Yao, J.; Zhang, X. Highly efficient removal of arsenic(III) from aqueous solution by zeolitic imidazolate frameworks with different morphology. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 358–366. [Google Scholar] [CrossRef]
- Jian, M.; Wang, H.; Liu, R.; Qu, J.; Wang, H.; Zhang, X. Self-assembled one-dimensional MnO2@zeolitic imidazolate framework-8 nanostructures for highly efficient arsenite removal. Environ. Sci. Nano 2016, 3, 1186–1194. [Google Scholar] [CrossRef]
- Jian, M.; Liu, B.; Zhang, G.; Liu, R.; Zhang, X. Adsorptive removal of arsenic from aqueous solution by zeolitic imidazolate framework-8 (ZIF-8) nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 67–76. [Google Scholar] [CrossRef]
- Vu, T.A.; Le, G.H.; Dao, C.D.; Dang, L.Q.; Nguyen, K.T.; Nguyen, Q.K.; Dang, P.T.; Tran, H.T.K.; Duong, Q.T.; Nguyen, T.V.; et al. Arsenic removal from aqueous solutions by adsorption using novel MIL-53(Fe) as a highly efficient adsorbent. RSC Adv. 2015, 5, 5261–5268. [Google Scholar] [CrossRef]
- Zhu, B.J.; Yu, X.Y.; Jia, Y.; Peng, F.M.; Sun, B.; Zhang, M.Y.; Luo, T.; Liu, J.H.; Huang, X.J. Iron and 1,3,5-benzenetricarboxylic metal-organic coordination polymers prepared by solvothermal method and their application in efficient As(V) removal from aqueous solutions. J. Phys. Chem. C 2012, 116, 8601–8607. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.N.; Li, Z.; Zhu, M.; Li, F. Characteristics of arsenate removal from water by metal-organic frameworks (MOFs). Water Sci. Technol. 2014, 70, 1391–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, J.; Wang, X.; Zhou, Y.; Jiang, L.; Wang, C. Selective adsorption of arsenate and the reversible structure transformation of the mesoporous metal-organic framework MIL-100(Fe). Phys. Chem. Chem. Phys. 2016, 18, 10864–10867. [Google Scholar] [CrossRef] [PubMed]
- Folens, K.; Leus, K.; Nicomel, N.R.; Meledina, M.; Turner, S.; Van Tendeloo, G.; Laing, G.D.; Van Der Voort, P. Fe3O4@MIL-101 – A Selective and Regenerable Adsorbent for the Removal of As Species from Water. Eur. J. Inorg. Chem. 2016, 2016, 4395–4401. [Google Scholar] [CrossRef]
- Yang, J.C.; Yin, X.B. CoFe2O4@MIL-100(Fe) hybrid magnetic nanoparticles exhibit fast and selective adsorption of arsenic with high adsorption capacity. Sci. Rep. 2017, 7, 40955. [Google Scholar] [CrossRef]
- Li, Z.Q.; Yang, J.C.; Sui, K.W.; Yin, N. Facile synthesis of metal-organic framework MOF-808 for arsenic removal. Mater. Lett. 2015, 160, 412–414. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Chen, J.P.; Li, K. Superior removal of arsenic from water with zirconium metal-organic framework UiO-66. Sci. Rep. 2015, 5, 16613. [Google Scholar] [CrossRef] [Green Version]
- Li, L.L.; Feng, X.Q.; Han, R.P.; Zang, S.Q.; Yang, G. Cr(VI) removal via anion exchange on a silver-triazolate MOF. J. Hazard. Mater. 2017, 321, 622–628. [Google Scholar] [CrossRef]
- Fu, H.R.; Xu, Z.X.; Zhang, J. Water-stable metal-organic frameworks for fast and high dichromate trapping via single-crystal-to-single-crystal ion exchange. Chem. Mater. 2015, 27, 205–210. [Google Scholar] [CrossRef]
- Niu, H.; Zheng, Y.; Wang, S.; He, S.; Cai, Y. Stable hierarchical microspheres of 1D Fe-gallic acid MOFs for fast and efficient Cr(VI) elimination by a combination of reduction, metal substitution and coprecipitation. J. Mater. Chem. A 2017, 5, 16600–16604. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, C.; Xu, H.; Gao, Y.; Tan, X.; Alsaedi, A.; Hayat, T. Cr(VI) Reduction and Immobilization by Core-Double-Shell Structured Magnetic Polydopamine@Zeolitic Idazolate Frameworks-8 Microspheres. ACS Sustain. Chem. Eng. 2017, 5, 6795–6802. [Google Scholar] [CrossRef]
- Rapti, S.; Pournara, A.; Sarma, D.; Papadas, I.T.; Armatas, G.S.; Hassan, Y.S.; Alkordi, M.H.; Kanatzidis, M.G.; Manos, M.J. Rapid, green and inexpensive synthesis of high quality UiO-66 amino-functionalized materials with exceptional capability for removal of hexavalent chromium from industrial waste. Inorg. Chem. Front. 2016, 3, 635–644. [Google Scholar] [CrossRef]
- Rapti, S.; Pournara, A.; Sarma, D.; Papadas, I.T.; Armatas, G.S.; Tsipis, A.C.; Lazarides, T.; Kanatzidis, M.G.; Manos, M.J. Selective capture of hexavalent chromium from an anion-exchange column of metal organic resin-alginic acid composite. Chem. Sci. 2016, 7, 2427–2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapti, S.; Sarma, D.; Diamantis, S.A.; Skliri, E.; Armatas, G.S.; Tsipis, A.C.; Hassan, Y.S.; Alkordi, M.; Malliakas, C.D.; Kanatzidis, M.G.; et al. All in one porous material: Exceptional sorption and selective sensing of hexavalent chromium by using a Zr4+ MOF. J. Mater. Chem. A 2017, 5, 14707–14719. [Google Scholar] [CrossRef]
- Mon, M.; Lloret, F.; Ferrando-Soria, J.; Martí-Gastaldo, C.; Armentano, D.; Pardo, E. Selective and Efficient Removal of Mercury from Aqueous Media with the Highly Flexible Arms of a BioMOF. Angew. Chemie Int. Ed. 2016, 55, 11167–11172. [Google Scholar] [CrossRef]
- Mon, M.; Qu, X.; Ferrando-Soria, J.; Pellicer-Carreño, I.; Sepúlveda-Escribano, A.; Ramos-Fernandez, E.V.; Jansen, J.C.; Armentano, D.; Pardo, E. Fine-tuning of the confined space in microporous metal-organic frameworks for efficient mercury removal. J. Mater. Chem. A 2017, 5, 20120–20125. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Chen, Q.; Jiang, F.; Yuan, D.; Qian, J.; Lv, G.; Xue, H.; Liu, L.; Jiang, H.L.; Hong, M. In situ large-scale construction of sulfur-functionalized metal-organic framework and its efficient removal of Hg(II) from water. J. Mater. Chem. A 2016, 4, 15370–15374. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Luo, X.; Shao, P.; Yang, J.; Sun, D. Thiol-Functionalized Zr-Based Metal-Organic Framework for Capture of Hg(II) through a Proton Exchange Reaction. ACS Sustain. Chem. Eng. 2018, 6, 8494–8502. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Ai, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Combined experimental and theoretical investigation on selective removal of mercury ions by metal organic frameworks modified with thiol groups. Chem. Eng. J. 2018, 354, 790–801. [Google Scholar] [CrossRef]
- Luo, F.; Chen, J.L.; Dang, L.L.; Zhou, W.N.; Lin, H.L.; Li, J.Q.; Liu, S.J.; Luo, M.B. High-performance Hg2+ removal from ultra-low-concentration aqueous solution using both acylamide- and hydroxyl-functionalized metal-organic framework. J. Mater. Chem. A 2015, 3, 9616–9620. [Google Scholar] [CrossRef]
- Luo, X.; Shen, T.; Ding, L.; Zhong, W.; Luo, J.; Luo, S. Novel thymine-functionalized MIL-101 prepared by post-synthesis and enhanced removal of Hg2+ from water. J. Hazard. Mater. 2016, 306, 313–322. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Du, T.; Zhang, W.; Zhu, W.; Yang, C.; Yue, T.; Sun, J.; Li, T.; Wang, J. NH2-MIL-53(Al) Metal-Organic Framework as the Smart Platform for Simultaneous High-Performance Detection and Removal of Hg2+. Inorg. Chem. 2019, 58, 12573–12581. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, L.; Gharib, M.; Morsali, A. The targeted design of dual-functional metal-organic frameworks (DF-MOFs) as highly efficient adsorbents for Hg2+ ions: Synthesis for purpose. Dalt. Trans. 2019, 48, 17831–17839. [Google Scholar] [CrossRef] [PubMed]
- Del Toral, M.A.; Porter, A.; Schock, M.R. Detection and Evaluation of Elevated Lead Release from Service Lines: A Field Study. Environ. Sci. Technol. 2013, 47, 9300–9307. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Li, J.; Zhang, Z.; Yang, F.; Dong, R.; Chen, L. Novel Pb2+ Ion Imprinted Polymers Based on Ionic Interaction via Synergy of Dual Functional Monomers for Selective Solid-Phase Extraction of Pb2+ in Water Samples. ACS Appl. Mater. Interfaces 2014, 6, 305–313. [Google Scholar] [CrossRef]
- Liu, B.; Khan, A.; Kim, K.; Kukkar, D.; Zhang, M. The adsorptive removal of lead ions in aquatic media: Performance comparison between advanced functional materials and conventional materials. Crit. Rev. Environ. Sci. Technol. 2019, 1–43. [Google Scholar] [CrossRef]
- Luo, X.; Ding, L.; Luo, J. Adsorptive removal of Pb(II) ions from aqueous samples with amino-functionalization of metal-organic frameworks MIL-101(Cr). J. Chem. Eng. Data 2015, 60, 1732–1743. [Google Scholar] [CrossRef]
- Ricco, R.; Konstas, K.; Styles, M.J.; Richardson, J.J.; Babarao, R.; Suzuki, K.; Scopece, P.; Falcaro, P. Lead(II) uptake by aluminium based magnetic framework composites (MFCs) in water. J. Mater. Chem. A 2015, 3, 19822–19831. [Google Scholar] [CrossRef]
- Yin, N.; Wang, K.; Xia, Y.; Li, Z. Novel melamine modified metal-organic frameworks for remarkably high removal of heavy metal Pb (II). Desalination 2018, 430, 120–127. [Google Scholar] [CrossRef]
- Ke, F.; Jiang, J.; Li, Y.; Liang, J.; Wan, X.; Ko, S. Highly selective removal of Hg2+ and Pb2+ by thiol-functionalized Fe3O4@metal-organic framework core-shell magnetic microspheres. Appl. Surf. Sci. 2017, 413, 266–274. [Google Scholar] [CrossRef]
- Wang, N.; Ouyang, X.K.; Yang, L.Y.; Omer, A.M. Fabrication of a Magnetic Cellulose Nanocrystal/Metal-Organic Framework Composite for Removal of Pb(II) from Water. ACS Sustain. Chem. Eng. 2017, 5, 10447–10458. [Google Scholar] [CrossRef]
- Yu, C.; Han, X.; Shao, Z.; Liu, L.; Hou, H. High Efficiency and Fast Removal of Trace Pb(II) from Aqueous Solution by Carbomethoxy-Functionalized Metal-Organic Framework. Cryst. Growth Des. 2018, 18, 1474–1482. [Google Scholar] [CrossRef]
- Liang, X.X.; Wang, N.; Qu, Y.L.; Yang, L.Y.; Wang, Y.G.; Ouyang, X.K. Facile preparation of metal-organic framework (MIL-125)/chitosan beads for adsorption of Pb(II) from aqueous solutions. Molecules 2018, 23, 1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Li, L.; Shen, S.; Chen, D.; Han, W. Highly efficient removal of Pb2+ by a sandwich structure of metal-organic framework/GO composite with enhanced stability. New J. Chem. 2019, 43, 1032–1037. [Google Scholar] [CrossRef]
- Fu, L.; Wang, S.; Lin, G.; Zhang, L.; Liu, Q.; Zhou, H.; Kang, C.; Wan, S.; Li, H.; Wen, S. Post-modification of UiO-66-NH2 by resorcyl aldehyde for selective removal of Pb(II) in aqueous media. J. Clean. Prod. 2019, 229, 470–479. [Google Scholar] [CrossRef]
- Liu, Q.; Li, S.; Yu, H.; Zeng, F.; Li, X.; Su, Z. Covalently crosslinked zirconium-based metal-organic framework aerogel monolith with ultralow-density and highly efficient Pb(II) removal. J. Colloid Interface Sci. 2020, 561, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, G.; Chen, H.; Hu, X.; Niu, Z.; Ma, S. Functionalized metal-organic framework as a new platform for efficient and selective removal of cadmium(II) from aqueous solution. J. Mater. Chem. A 2015, 3, 15292–15298. [Google Scholar] [CrossRef]
- Xue, H.; Chen, Q.; Jiang, F.; Yuan, D.; Lv, G.; Liang, L.; Liu, L.; Hong, M. A regenerative metal-organic framework for reversible uptake of Cd(II): From effective adsorption to: In situ detection. Chem. Sci. 2016, 7, 5983–5988. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Gu, J.; Yin, N. Efficient Removal of Pb(II) and Cd(II) Using NH2-Functionalized Zr-MOFs via Rapid Microwave-Promoted Synthesis. Ind. Eng. Chem. Res. 2017, 56, 1880–1887. [Google Scholar] [CrossRef]
- Roushani, M.; Saedi, Z.; Baghelani, Y.M. Removal of cadmium ions from aqueous solutions using TMU-16-NH2 metal organic framework. Environ. Nanotechnol. Monit. Manag. 2017, 7, 89–96. [Google Scholar]
- Liu, C.; Wang, P.; Liu, X.; Yi, X.; Liu, D.; Zhou, Z. Ultrafast Removal of Cadmium(II) by Green Cyclodextrin Metal–Organic-Framework-Based Nanoporous Carbon: Adsorption Mechanism and Application. Chem. Asian J. 2018, 261–268. [Google Scholar] [CrossRef]
- Jamshidifard, S.; Koushkbaghi, S.; Hosseini, S.; Rezaei, S.; Karamipour, A.; Jafari rad, A.; Irani, M. Incorporation of UiO-66-NH2 MOF into the PAN/chitosan nanofibers for adsorption and membrane filtration of Pb(II), Cd(II) and Cr(VI) ions from aqueous solutions. J. Hazard. Mater. 2019, 368, 10–20. [Google Scholar] [CrossRef] [PubMed]




| Adsorbates | MOFs/MOF-Based Materials | Adsorption Capacity (mg g−1) | Equilibrium Time | pH | Selectivity | Reusability | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|---|
| As(V) | ZIF-8 | 76.5 | 7 h | 4 | vs. Cl−, F−, NO3−, SO42−, PO43− | Reusable | Inner-sphere complex | [44] |
| ZIF-8 | 90.92 | 10 h | — | — | Reusable | Ion exchange | [45] | |
| MIL-53(Fe) | 21.27 | 90 min | 5 | — | — | Lewis acid–base and electrostatic interactions | [49] | |
| Fe-BTC | 12.3 | 10 min | 4 | — | — | Ion exchange | [50] | |
| MIL-53(Al) | 105.6 | 11 h | 8 | vs. CO32−, Cl−, NO3−, SO42− | — | Electrostatic attraction, hydrogen bond | [51] | |
| MIL-100(Fe) | 110 | 1 h | — | vs. Cl−, SO42−, NO3−, CO32− | Reusable | Coordination | [52] | |
| MOF-808 | 24.83 | 30 min | 4 | — | Reusable | Ion exchange | [55] | |
| UiO-66 | 303.34 | 48 h | 2 | vs. CO32−, Cl−, NO3−, SO42− | — | Ion exchange | [56] | |
| As(III) | Cubic ZIF-8 | 122.6 | 10 h | 8.5 | — | — | Ion exchange | [46] |
| Leaf-shaped ZIF-8 | 108.1 | 10 h | 8.5 | — | — | Ion exchange | [46] | |
| Dodecahedral ZIF-8 | 117.5 | 10 h | 8.5 | vs. CO32−, PO42−, SO42−, Cl− | Reusable | Ion exchange | [46] | |
| β-MnO2@ZIF-8 | 140.27 | 24 h | 7 | — | — | Oxidation | [47] | |
| As(V)/As(III) | ZIF-8 | 60.03/49.49 | 7 h/13 h | 7 | vs. SO42−, PO42−, CO32−, NO3− | — | Ion exchange | [48] |
| Fe3O4@MIL-101(Cr) | 80.0/121.5 | 24 h/24 h | 7 | vs. various oxyanions, Ca2+, Mg2+ | — | Ion exchange | [53] | |
| CoFe2O4@MIL-100(Fe) | 114.8/143.6 | 2 min/2 min | 4–10 | vs. various oxyanions, Congo red, humic acid | — | Ion exchange/hydrogen bonding | [54] |
| Adsorbates | MOFs/MOF-Based Materials | Adsorption Capacity (mg g−1) | Equilibrium Time | pH | Selectivity | Reusability | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|---|
| CrO42– | {[Ag8(tz)6](NO3)2·6H2O}n | 37 | NA | 6 | vs. NO3−, CO32− | — | Ion exchange | [57] |
| Cr2O72− | FIR-53 | 74 | 10 min | — | vs. Cl−, Br−, NO3− | Reusable | Ion exchange | [58] |
| FIR-54 | 103 | 30 min | — | — | Reusable | Ion exchange | [58] | |
| Fe-gallic acid MOF | 1709.2 | 72 h | 4 | vs. various ions | Limited | Multiple | [59] | |
| MP@ZIF-8 | 136.56 | 16 h | 5 | — | — | Adsorption reduction | [60] | |
| MOR-1-HA | 242–280 | 3 min | 3 | vs. Cl−, Br−, CO32−, SO42− | Reusable | Ion exchange | [61,62] | |
| MOR-1 (protonated) | 247–321 | NA | 3 | — | — | Ion exchange | [61,62] | |
| MOR-1 (non-protonated) | 267 | >1 h | 3 | — | — | Ion exchange | [61,62] | |
| MOR-2-HA | 162.8 | 1 min | 3 | — | Reusable | Ion exchange | [63] |
| MOFs/MOF-Based Materials | Adsorption Capacity (mg g−1) | Equilibrium Time | pH | Selectivity | Reusability | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| {CaIICuII6[(S,S)-methox]3 (OH)2(H2O)}·16H2O | 900 | <200 min | 7 | vs. CH3Hg+ | — | Binding with S | [64] |
| {Cu4II[(S,S)-methox]2}·5H2O | 1 HgCl2 per formula | 15 min | 7 | vs. various cations | Reusable | Binding with thiol | [65] |
| FJI-H12 | 439.8 | <50 min | 7 | vs. Mn2+, Ba2+, Ni2+, Cd2+ | Reusable | Binding with SCN− | [66] |
| Zr-DMBD | 171.5 | 10 min | 6 | vs. various cations | Reusable | Coordination with S2− | [67] |
| UiO-66–SH | 785 | 20 min | 4 | vs. various cations | Reusable | Interact with thiol | [68] |
| [Zn(hip)(L)]·(DMF)(H2O) | 250 | 1 h | 5 | — | — | Contact with C=O | [69] |
| MIL-101-thymine | 59.28 | 300 min | 6 | vs. various cations | Limited | Coordination with N | [70] |
| NH2–ML–53(Al) | 153.85 | 60 min | 6 | — | Reusable | Coordination with –NH2 | [71] |
| TMU-32S–65% | 1428 | 17 min | 4.4 | vs. various cations | Reusable | Electrostatic interaction, chelation | [72] |
| MOFs/MOF-Based Materials | Adsorption Capacity (mg g−1) | Equilibrium Time | pH | Selectivity | Reusability | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| ED-MIL-101(Cr) | 81.09 | 30 min | 6 | vs. various cations | Limited | Specific binding | [76] |
| MIL-53(Al) MFC | 492.4 | 120 min | — | — | — | Ion exchange | [77] |
| Melamine-MOFs | 205 | 120 min | 6 | — | — | Coordination interaction | [78] |
| Thiol-functionalized Fe3O4@Cu3(btc)2 | 215.05 | 120 min | 5.92 | vs. various cations | Reusable | Binding with thiol | [79] |
| MCNC@Zn-BTC | 558.66 | 30 min | 5.45 | vs. Cu2+, Zn2+, Cd2+ | Reusable | — | [80] |
| {[Cd(ADB)L2]·1.5 DMF·2H2O}n | 63.052 | 5 min | 7 | vs. various cations | Reusable | Pb/O=C−O interaction | [81] |
| MIL-125-CS | 407.50 | 180 min | 6 | — | Reusable | — | [82] |
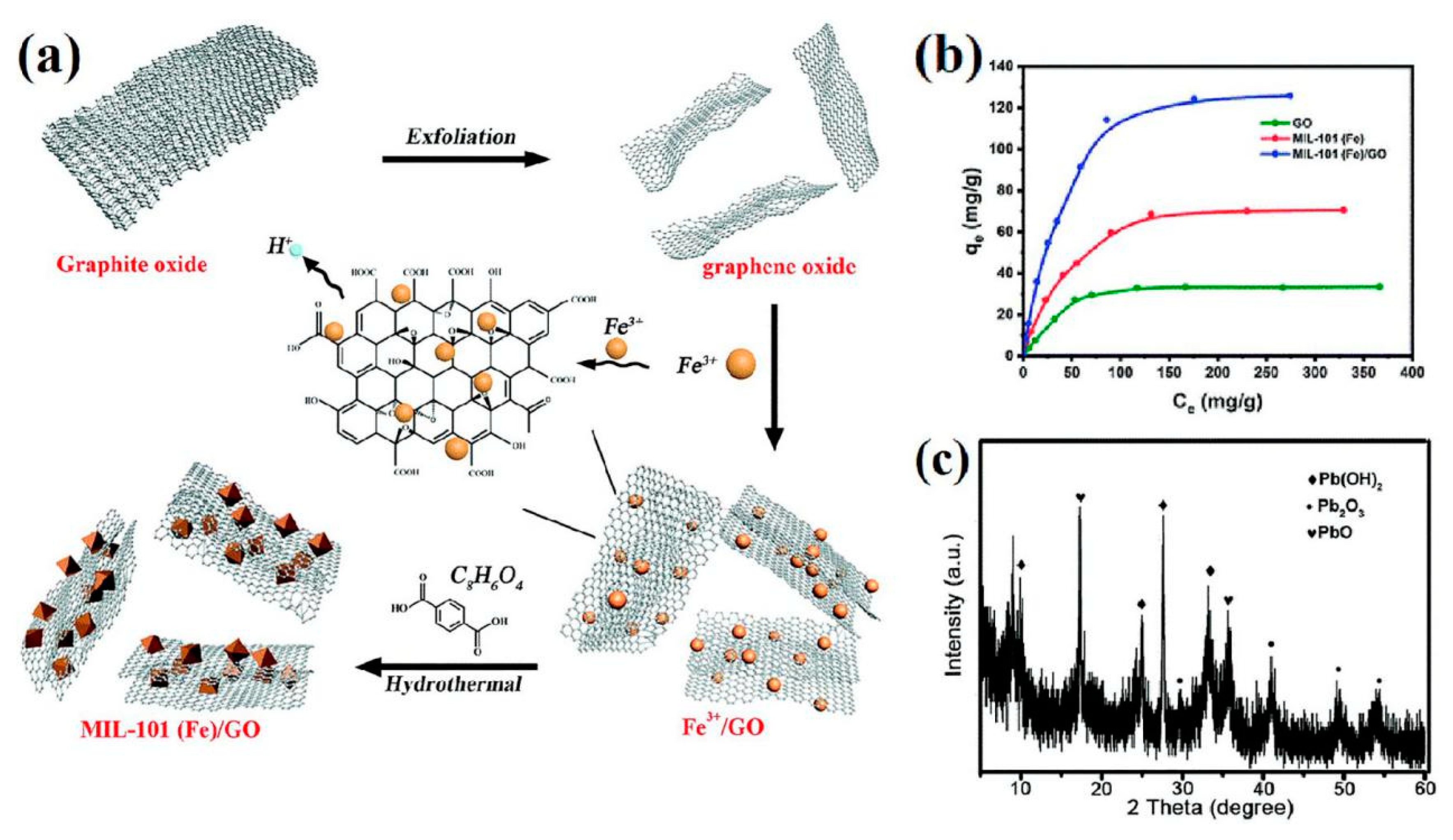
| MIL-101(Fe)/GO | 128.6 | 15 min | 6 | — | Reusable | Ion exchange | [83] |
| UiO-66–RSA | 189.8 | 150 min | 4 | vs. various cations | Reusable | Complexation reaction | [84] |
| UNCAM | 102.03 | 300 min | 5 | — | Reusable | Coordination interaction | [85] |
| MOFs/MOF-Based Materials | Adsorption Capacity (mg g−1) | Equilibrium Time | pH | Selectivity | Reusability | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| Cu3(BTC)2–SO3H | 88 | 10 min | 6 | vs. various cations | Reusable | Ion exchange | [86] |
| FJI-H9 | 225 | NA | — | vs. various cations | Reusable | Cd–O interactions | [87] |
| NH2-functionalized Zr-MOFs | 177.35 | 120 min | 6 | — | — | Coordination interaction | [88] |
| TMU-16–NH2 | 126.6 | 90 min | 6 | — | — | Ion exchange | [89] |
| γ-CD MOF-NPC | 140.85 | 60 min | 7 | vs. Na+, Ca2+, Mg2+, NO3−, SO42−, Cl− | — | Ion exchange | [90] |
| PAN/chitosan/UiO-66–NH2 | 415.6 | 60 min | 6 | — | Reusable | — | [91] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Bai, X.; Ye, Z. Recent Progress in Heavy Metal Ion Decontamination Based on Metal–Organic Frameworks. Nanomaterials 2020, 10, 1481. https://doi.org/10.3390/nano10081481
Chen Y, Bai X, Ye Z. Recent Progress in Heavy Metal Ion Decontamination Based on Metal–Organic Frameworks. Nanomaterials. 2020; 10(8):1481. https://doi.org/10.3390/nano10081481
Chicago/Turabian StyleChen, Yajie, Xue Bai, and Zhengfang Ye. 2020. "Recent Progress in Heavy Metal Ion Decontamination Based on Metal–Organic Frameworks" Nanomaterials 10, no. 8: 1481. https://doi.org/10.3390/nano10081481
APA StyleChen, Y., Bai, X., & Ye, Z. (2020). Recent Progress in Heavy Metal Ion Decontamination Based on Metal–Organic Frameworks. Nanomaterials, 10(8), 1481. https://doi.org/10.3390/nano10081481