Synthesis and Studies of Electro-Deposited Yttrium Arsenic Selenide Nanofilms for Opto-Electronic Applications
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
3.1. XRD and Raman Analysis
3.2. Optical Studies
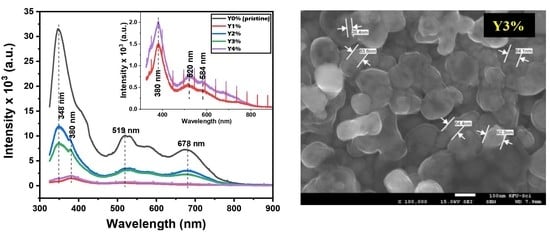
3.2.1. Transmittance (T), Absorption Coefficient (α), Energy Gap (Eg), and PL Plots
3.2.2. Refractive Index (n) and Optical Conductivity (σ)
3.2.3. Complex Dielectric Plots
3.3. Surface Analysis
3.4. Electronic Structures
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iovu, M.S.; Andriesh, A.M.; Buzurniuc, S.A.; Verlan, V.I. Optical properties of As2S3:Pr and As2Se: Dy amorphous composites. Dig. J. Nanomater. Biostruct. 2007, 2, 201–206. [Google Scholar]
- Thylén, L.; Wosinski, L. Integrated photonics in the 21st century. Photonics Res. 2014, 2, 75. [Google Scholar] [CrossRef]
- Frumar, M.; Wagner, T.; Shimakawa, K.; Frumarova, B. Crystalline and Amorphous Chalcogenides, High-Tech Materials with Structural Disorder and Many Important Applications. In Nanomaterials and Nanoarchitectures: A Complex Review of Current Hot Topics and Their Applications; Bardosova, M., Wagner, T., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 151–238. [Google Scholar]
- Milliron, D.J.; Raoux, S.; Shelby, R.M.; Jordan-Sweet, J. Solution-phase deposition and nanopatterning of GeSbSe phase-change materials. Nat. Mater. 2007, 6, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Kohoutek, T.; Wagner, T.; Orava, J.; Krbal, M.; Fejfar, A.; Mates, T.; Kasap, S.O.; Frumar, M. Surface morphology of spin-coated As-S-Se chalcogenide thin films. J. Non. Cryst. Solids 2007, 353, 1437–1440. [Google Scholar] [CrossRef]
- Chung, I.; Kanatzidis, M.G. Metal chalcogenides: A rich source of nonlinear optical materials. Chem. Mater. 2014, 26, 849–869. [Google Scholar] [CrossRef]
- Fick, J.; Knystautas, É.J.; Villeneuve, A.; Schiettekatte, F.; Roorda, S.; Richardson, K.A. High photoluminescence in erbium-doped chalcogenide thin films. J. Non. Cryst. Solids 2000, 272, 200–208. [Google Scholar] [CrossRef]
- Pandey, V.; Tripathi, S.K.; Kumar, A. A study of optical parameters of amorphous Se90inxGe10-x thin films before and after heat treatment. J. Optoelectron. Adv. Mater. 2007, 9, 2010–2015. [Google Scholar]
- Saghaei, H.; Zahedi, A.; Karimzadeh, R.; Parandin, F. Line defects on As2Se3-Chalcogenide photonic crystals for the design of all-optical power splitters and digital logic gates. Superlattices Microstruct. 2017, 110, 133–138. [Google Scholar] [CrossRef]
- Shi, H.; Feng, X.; Tan, F.; Wang, P.; Wang, P. Multi-watt mid-infrared supercontinuum generated from a dehydrated large-core tellurite glass fiber. Opt. Mater. Express 2016, 6, 3967–3976. [Google Scholar] [CrossRef]
- Chenard, F.; Alvarez, O.; Gibson, D.; Shaw, L.B.; Sanghera, J. Mid-infrared imaging fiber bundle. In Advanced Optics for Defense Applications: UV through LWIR II; International Society for Optics and Photonics: Bellingham, WA, USA, 2017; Volume 10181, p. 101810V. [Google Scholar]
- Parani, S.; Bupesh, G.; Manikandan, E.; Pandian, K.; Oluwafemi, O.S. Facile synthesis of mercaptosuccinic acid-capped CdTe/CdS/ZnS core/double shell quantum dots with improved cell viability on different cancer cells and normal cells. J. Nanopart. Res. 2016, 18, 347. [Google Scholar] [CrossRef]
- Slusher, R.E.; Kortan, A.R. Highly nonlinear composite chalcogenide/polymer fibers. In Frontiers in Optics; Optical Society of America: Washington, DC, USA, 2004; p. FWG4. [Google Scholar]
- Ohta, T. Phase-change optical memory promotes the DVD optical disk. J. Optoelectron. Adv. Mater. 2001, 3, 609–626. [Google Scholar]
- Castro, R.A.; Bordovsky, V.A.; Grabko, G.I. Investigation of the Processes of Charge Transfer and Charge Accumulation in As2Se3 Amorphous Layers Prepared by Different Methods. Glass Phys. Chem. 2009, 35, 43–44. [Google Scholar] [CrossRef]
- Orava, J.; Kohoutek, T.; Wagner, T. Deposition techniques for chalcogenide thin films. In Chalcogenide Glasses; Elsevier: Amsterdam, The Netherlands, 2014; pp. 265–309. [Google Scholar]
- Zakery, A. Pulsed laser deposition of chalcogenide films for nonlinear photonic applications. J. Optoelectron. Adv. Mater. 2005, 7, 1143–1155. [Google Scholar]
- Carlie, N.; Anheier, N.C., Jr.; Qiao, H.A.; Bernacki, B.; Phillips, M.C.; Petit, L.; Musgraves, J.D.; Richardson, K. Measurement of the refractive index dispersion of As2Se3 bulk glass and thin films prior to and after laser irradiation and annealing using prism coupling in the near- and mid-infrared spectral range. Rev. Sci. Instrum. 2011, 82, 053103. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Lin, H.; Ogbuu, O.; Li, L.; Danto, S.; Novak, S.; Novak, J.; Musgraves, J.D.; Richardson, K.; Hu, J. Effect of annealing conditions on the physio-chemical properties of spin-coated As_2Se_3 chalcogenide glass films. Opt. Mater. Express 2012, 2, 1723–1732. [Google Scholar] [CrossRef]
- Lai, Y.; Liu, F.; Li, J.; Zhang, Z.; Liu, Y. Nucleation and growth of selenium electro-deposition onto tin oxide electrode. J. Electroanal. Chem. 2010, 639, 187–192. [Google Scholar] [CrossRef]
- Azhniuk, Y.; Dzhagan, V.; Solonenko, D.; Loya, V.; Grytsyshche, I.; Lopushansky, V.; Gomonnai, A.; Zahn, D.R. In-doped As2Se3 thin films studied by Raman and X-ray photoelectron spectroscopies. Appl. Surf. Sci. 2019, 471, 943–949. [Google Scholar] [CrossRef]
- Hristova-Vasileva, T.; Bineva, I.; Dinescu, A.; Danila, M.; Arsova, D. As2Se3 thin films deposited by frequency assisted thermal evaporation—Morphology and structure. J. Phys. Conf. Ser. 2017, 794, 012015. [Google Scholar] [CrossRef]
- Bowen, A.; Li, J.; Lewis, J.; Sivaramakrishnan, K.; Alford, T.L.; Iyer, S. The properties of radio frequency sputtered transparent and conducting ZnO:F films on polyethylene naphthalate substrate. Thin Solid Films 2011, 519, 1809–1816. [Google Scholar] [CrossRef]
- Speakman, S. Estimating Crystallite Size Using XRD. MIT Cent. Mater. Sci. Eng. 2014, 3–8. Available online: http://prism.mit.edu/xray/ (accessed on 29 August 2019).
- Khemiri, N.; Kanzari, M. Determination and analysis of optical constants and dispersion energy parameters of Zn(S,O) thin films. Mater. Chem. Phys. 2018, 214, 185–191. [Google Scholar] [CrossRef]
- Kovanda, V.; Vl, M. Structure of As–Se and As–P–Se glasses studied by Raman spectroscopy. J. Non. Cryst. Solids 2003, 326, 88–92. [Google Scholar] [CrossRef]
- Mikla, V.I. Distinct topological regimes in binary AsxSe1−x glasses. J. Phys. Condens. Matter 1997, 9, 9209–9217. [Google Scholar] [CrossRef]
- Kamitsos, E.I.; Iovu, M.S.; Varsamis, C.; Boolchand, P.; Popescu, M. Raman spectra of AsxSe100-x and As40Se60 glasses doped with metals. Chalcogenide Lett. 2005, 2, 21–25. [Google Scholar]
- Černošek, Z.; Deschamps, M.; Nazabal, V.; Goncalvez, C.; Roiland, C.; Holubová, J.; Černošková, E.; Boussard, C.; Bureau, B. Structure of arsenic selenide glasses by Raman and 77 Se NMR with a multivariate curve resolution approach. J. Non. Cryst. Solids 2016, 447, 322–328. [Google Scholar] [CrossRef]
- Behera, M.; Behera, S.; Naik, R. Optical band gap tuning by laser induced Bi diffusion into As 2 Se 3 fi lm probed by spectroscopic. RSC Adv. 2017, 7, 18428–18437. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Bureau, B.; Rouxel, T.; Gueguen, Y.; Gulbiten, O.; Roiland, C.; Soignard, E.; Yarger, J.L.; Troles, J.; Sangleboeuf, J.C.; et al. Correlation between structure and physical properties of chalcogenide glasses in the As x Se 1−x system. Phys. Rev. B 2010, 82, 1–8. [Google Scholar] [CrossRef]
- Akata, P.N. Study of Composition and Optical Properties of Chemically Deposited Pd-xSb2S3 Thin Films. J. Nanostruct. 2017, 7, 236–245. [Google Scholar]
- Ezema, F.I.; Osuji, R.U. Band gap shift and optical characterization of chemical bath deposited CdSSe thin films on annealing. Chalcogenide Lett. 2007, 4, 69–75. [Google Scholar]
- Ezekoye, B.A.; Ezekoye, V.A.; Offor, P.O.; Utazi, S.C. Synthesis, structural and optical characterizations of cadmium oxide (CdO) thin films by chemical bath deposition (CBD) technique. Int. J. Phys. Sci. 2013, 8, 1597–1601. [Google Scholar] [CrossRef]
- Ekwealor, A.B.C.; Ezema, F.I. Effects of precursor concentration on the optical and structural properties of Fe2O3 thin films synthesized in a polymer matrix by chemical bath deposition. J. Ovonic Res. 2013, 9, 35–43. [Google Scholar]
- Chauhan, R.; Srivastava, A.K.; Tripathi, A.; Srivastava, K.K. Linear and nonlinear optical changes in amorphous As2Se3thin film upon UV exposure. Prog. Nat. Sci. Mater. Int. 2011, 21, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Abdul-ameer, Z.N.; Agool, I.R. Studying structural and optical properties of ZnO-CdO nanocomposites. World Sci. News 2015, 23, 35–45. [Google Scholar]
- Martin, K.; Arguirov, T.; Oehme, M.; Yamamoto, Y.; Tillack, B.; Abrosimov, N.V. Photoluminescence study of Ge containing crystal defects. Phys. Status Solidi A 2011, 208, 754–759. [Google Scholar]
- Depinna, S.P.; Cavenett, B.C.; Lamb, W.E. A new comparison of photoluminescence in crystalline and amorphous arsenic triselenide. Philos. Mag. Part B 1983, 2013, 99–106. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Ayeshamariam, A.; Manikandan, E.; Kennedy, J. Solution processing of CuSe quantum dots: Photocatalytic activity under RhB for UV and visible-light solar irradiation. Mater. Sci. Eng. B 2016, 210, 1–9. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Mohammed, A.D.; Strydom, C.A.; Young, D.A.; Jordaan, A. Colloidal synthesis of monodispersed ZnS and CdS nanocrystals from novel zinc and cadmium complexes. Superlattices Microstruct. 2014, 70, 98–108. [Google Scholar] [CrossRef]
- Singh, N.; Jun, K.; Das, B.K. Synthesis and optical properties of CdS/PVA nanocomposites. Mater. Chem. Phys. 2005, 94, 454–459. [Google Scholar]
- El-gammal, O.A.; Al-hossainy, A.F.; El-brashy, S.A. Spectroscopic, DFT, optical band gap, powder X-ray diffraction and Bleomycindependant DNA studies of Co(II), Ni(II) and Cu(II) complexes derived from macrocyclic Schiff base. J. Mol. Struct. 2018, 1165, 177–195. [Google Scholar] [CrossRef]
- Todorov, R.; Tasseva, J.; Babeva, T. Thin Chalcogenide Films for Photonic Applications. Photonic Cryst. Innov. Syst. Lasers Waveguides Intech Croat. 2012, 143. [Google Scholar] [CrossRef] [Green Version]
- Prince, V.; Singh, R.; Zulfequar, M.; Kumar, A.; Dwivedi, P.K. Electrical and optical properties of solution phase deposited As2S3 and As2Se3 chalcogenide thin films: A comparative study with thermally deposited films. J. Non. Cryst. Solids 2017, 476, 46–51. [Google Scholar] [CrossRef]
- Whyte, G.M.; Awada, C.; Offor, P.O.; Otung, F.U.; Alshoaibi, A.; Aljaafari, A.; Ekwealor, A.B.C.; Maaza, M.; Ezema, F.I. Optical and photoluminescence performance of electrodeposited arsenic selenide thin film doped with erbium ion. Opt. Mater. 2020, 99, 109556. [Google Scholar] [CrossRef]
- Bakr, H.; Hussein, H.F. Optical constants of Zinc sulphide ZnS thin films for different annealing temperature. J. Basrah Res. 2014, 40, 1–11. [Google Scholar]
- Bhargava, A.; Suthar, B. Localized modes in chalcogenide photonic multilayers with As-S-Se defect layer. Chalcogenide Lett. 2009, 6, 529–533. [Google Scholar]
- Perdew, M.; Burke, J.P.; Ernzerhof, K. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Synopsys. QuantumATK (Atomic-Scale Modeling for Semiconductor & Materials Research). 2019. Available online: https://www.synopsys.com/silicon/quantumatk.html (accessed on 29 August 2019).
- Anisimov, V.I.; Kozhevnikov, A.V.; Korotin, M.A.; Lukoyanov, A.V.; Khafizullin, D.A. Orbital density functional as a means to restore the discontinuities in the total-energy derivative and the exchange-correlation potential. J. Phys. Condens. Matter 2007, 19, 106206. [Google Scholar] [CrossRef] [Green Version]
- Ching, W.Y.; Gu, Z.; Xu, Y.-N. Theoretical calculation of the optical properties of Y3Fe5O12. J. Appl. Phys. 2001, 89, 6883. [Google Scholar] [CrossRef]
- Kanoun, M.B.; Reshak, A.H.; Kanoun-Bouayed, N.; Goumri-Said, S. Evidence of Coulomb correction and spin–orbit coupling in rare-earth dioxides CeO2, PrO2 and TbO2: An ab initio study. J. Magn. Magn. Mater. 2012, 324, 1397–1405. [Google Scholar] [CrossRef]
- Alshoaibi, A.; Kanoun, M.B.; Ul, H.B.; AlFaify, S.; Goumri-Said, S. Insights into the Impact of Yttrium Doping at the Ba and Ti Sites of BaTiO3 on the Electronic Structures and Optical Properties: A First-Principles Study. ACS Omega 2020, 5, 15502–15509. [Google Scholar] [CrossRef]
- Antonclli, A.; Tarnow, E.; Joannopoulos, J.D. New insight into the electronic structure of a As2Se3. Phys. Rev. B 1986, 33, 2968. [Google Scholar] [CrossRef]
- Kanoun, M.B.; Shein, I.R.; Goumri-Said, S. Origin of incompressibility and hardness from electronic and mechanical properties of hard material ruthenium diboride. Solid State Commun. 2010, 150, 1095–1098. [Google Scholar] [CrossRef]









| Sample | 2θ (°) | d (Å) | FWHM (β/rad) | (hkl) | Dav (nm) | (line/m2) × 1015 | ε × 10−3 |
|---|---|---|---|---|---|---|---|
| Y0%(pristine) | 26.55 | 3.3534 | 0.0036 | 110 | 40.04 ± 0.009 | 0.62 ± 0.003 | 3.76 ± 0.007 |
| 37.78 | 2.3793 | 0.0039 | 015 | 37.65 ± 0.002 | 0.70 ± 0.005 | 2.84 ± 0.003 | |
| 51.57 | 1.7708 | 0.0050 | 225 | 31.01 ± 0.005 | 1.03 ± 0.009 | 2.56 ± 0.009 | |
| Average values | 36.23± 0.005 | 0.78 ± 0.006 | 3.05 ± 0.006 | ||||
| Y1% | 26.56 | 3.3533 | 0.0041 | 111 | 34.12 ± 0.005 | 0.85 ± 0.008 | 3.42 ± 0.001 |
| 30.70 | 2.9099 | 0.0025 | 220 | 55.51 ± 0.003 | 1.16 ± 0.007 | 3.58 ± 0.008 | |
| 51.58 | 1.7708 | 0.0056 | 211 | 27.32 ± 0.008 | 1.33 ± 0.008 | 2.91 ± 0.005 | |
| Average values | 38.98 ± 0.007 | 1.11 ± 0.007 | 3.30 ± 0.004 | ||||
| Y2% | 26.53 | 3.3570 | 0.0043 | 111 | 33.15 ± 0.007 | 0.90 ± 0.009 | 2.55 ± 0.006 |
| 30.63 | 2.9164 | 0.0029 | 220 | 48.72 ± 0.006 | 0.42 ± 0.001 | 1.69 ± 0.003 | |
| 51.56 | 1.7711 | 0.0057 | 211 | 27.70 ± 0.007 | 1.30 ± 0.001 | 1.98 ± 0.004 | |
| Average values | 36.52 ± 0.006 | 0.87 ± 0.003 | 2.07 ± 0.001 | ||||
| Y3% | 26.56 | 3.3533 | 0.0039 | 111 | 36.34 ± 0.009 | 0.75 ± 0.006 | 2.15 ± 0.001 |
| 30.70 | 2.9099 | 0.0019 | 220 | 74.70 ± 0.009 | 0.17 ± 0.009 | 1.75 ± 0.002 | |
| 51.57 | 1.7708 | 0.0056 | 211 | 27.27 ± 0.006 | 1.34 ± 0.004 | 2.92 ± 0.001 | |
| Average values | 46.10 ± 0.008 | 0.75 ± 0.006 | 2.27 ± 0.001 | ||||
| Y4% | 26.57 | 3.3496 | 0.0021 | 111 | 69.04 ± 0.008 | 0.20 ± 0.009 | 3.18 ± 0.003 |
| 30.71 | 2.9090 | 0.0027 | 220 | 52.78 ± 0.006 | 0.35 ± 0.008 | 3.47 ± 0.009 | |
| 51.58 | 1.7705 | 0.0082 | 211 | 18.78 ± 0.003 | 2.83 ± 0.004 | 4.24 ± 0.001 | |
| Average values | 46.86 ± 0.006 | 1.12 ± 0.007 | 3.93 ± 0.004 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awada, C.; Whyte, G.M.; Offor, P.O.; Whyte, F.U.; Kanoun, M.B.; Goumri-Said, S.; Alshoaibi, A.; Ekwealor, A.B.C.; Maaza, M.; Ezema, F.I. Synthesis and Studies of Electro-Deposited Yttrium Arsenic Selenide Nanofilms for Opto-Electronic Applications. Nanomaterials 2020, 10, 1557. https://doi.org/10.3390/nano10081557
Awada C, Whyte GM, Offor PO, Whyte FU, Kanoun MB, Goumri-Said S, Alshoaibi A, Ekwealor ABC, Maaza M, Ezema FI. Synthesis and Studies of Electro-Deposited Yttrium Arsenic Selenide Nanofilms for Opto-Electronic Applications. Nanomaterials. 2020; 10(8):1557. https://doi.org/10.3390/nano10081557
Chicago/Turabian StyleAwada, Chawki, Goodfriend M. Whyte, Peter O. Offor, Favour U. Whyte, Mohammed Benali Kanoun, Souraya Goumri-Said, Adil Alshoaibi, Azubike B. C. Ekwealor, Malik Maaza, and Fabian I. Ezema. 2020. "Synthesis and Studies of Electro-Deposited Yttrium Arsenic Selenide Nanofilms for Opto-Electronic Applications" Nanomaterials 10, no. 8: 1557. https://doi.org/10.3390/nano10081557
APA StyleAwada, C., Whyte, G. M., Offor, P. O., Whyte, F. U., Kanoun, M. B., Goumri-Said, S., Alshoaibi, A., Ekwealor, A. B. C., Maaza, M., & Ezema, F. I. (2020). Synthesis and Studies of Electro-Deposited Yttrium Arsenic Selenide Nanofilms for Opto-Electronic Applications. Nanomaterials, 10(8), 1557. https://doi.org/10.3390/nano10081557