Photo-Induced Black Phase Stabilization of CsPbI3 QDs Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CsPbI3 QDs
2.2. Quantum Dot Solution Preparation
2.3. Solar Cell Fabrication
2.4. Photoelectric Characterization
2.5. Structural Characterization
2.6. Optical Characterization
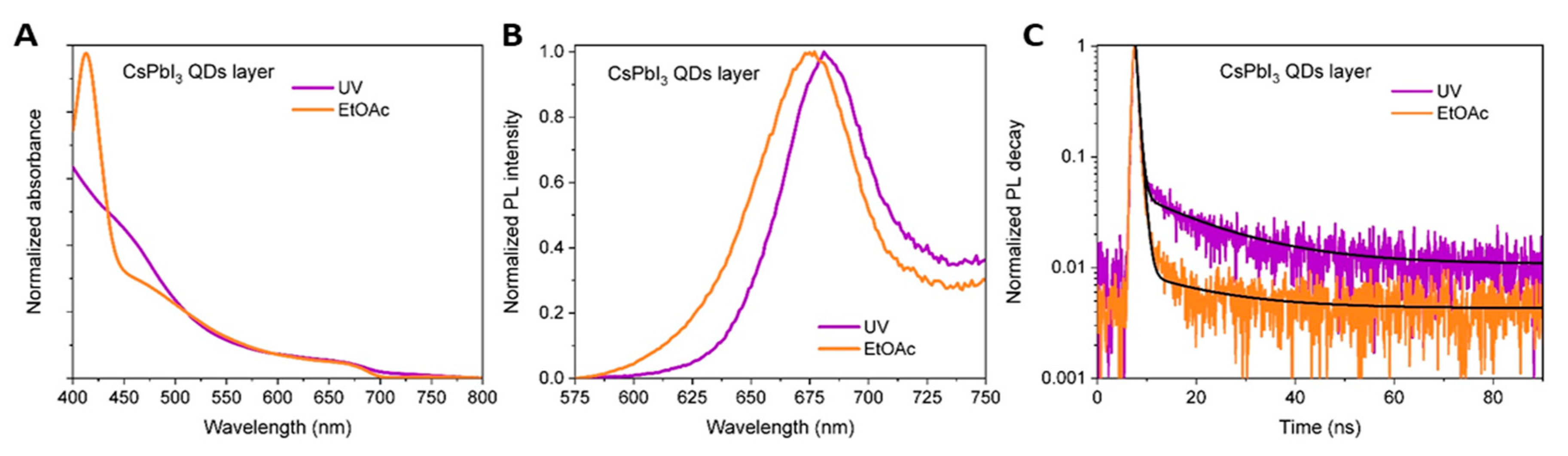
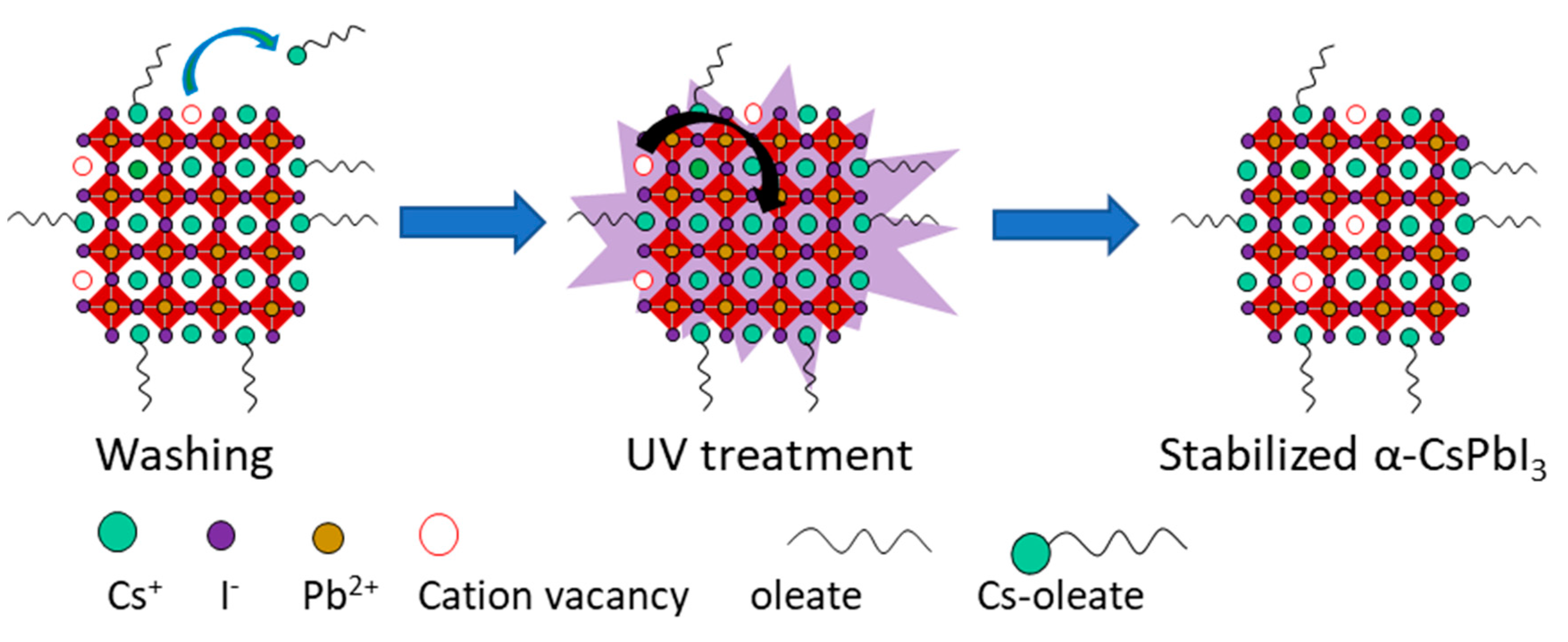
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- NREL Best Research-Cell Efficiencies. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.20200406.pdf (accessed on 10 July 2020).
- Masi, S.; Gualdrón-Reyes, A.F.; Mora-Seró, I. Stabilization of Black Perovskite Phase in FAPbI3 and CsPbI3. ACS Energy Lett. 2020, 1974–1985. [Google Scholar] [CrossRef]
- Becker, M.; Klüner, T.; Wark, M. Formation of hybrid ABX3 perovskite compounds for solar cell application: First-principles calculations of effective ionic radii and determination of tolerance factors. Dalt. Trans. 2017, 46, 3500–3509. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot-induced phase stabilization of -CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Jin, W.; Zhang, Y.; Yang, T.; Reiss, P.; Zhong, Q.; Bach, U.; Li, Q.; Wang, Y.; Zhang, H.; et al. High Efficiency Mesoscopic Solar Cells Using CsPbI3 Perovskite Quantum Dots Enabled by Chemical Interface Engineering. J. Am. Chem. Soc. 2020, 142, 3775–3783. [Google Scholar] [CrossRef] [Green Version]
- Hao, M.; Bai, Y.; Zeiske, S.; Ren, L.; Liu, J.; Yuan, Y.; Zarrabi, N.; Cheng, N.; Ghasemi, M.; Chen, P.; et al. Ligand-assisted cation-exchange engineering for high-efficiency colloidal Cs1−xFAxPbI3 quantum dot solar cells with reduced phase segregation. Nat. Energy 2020, 5, 79–88. [Google Scholar] [CrossRef]
- Sanehira, E.M.; Marshall, A.R.; Christians, J.A.; Harvey, S.P.; Ciesielski, P.N.; Wheeler, L.M.; Schulz, P.; Lin, L.Y.; Beard, M.C.; Luther, J.M. Enhanced mobility CsPbI3 quantum dot arrays for record-efficiency, high-voltage photovoltaic cells. Sci. Adv. 2017, 3, eaao4204. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Ling, X.; Yang, D.; Li, F.; Zhou, S.; Shi, J.; Qian, Y.; Hu, J.; Sun, Y.; Yang, Y.; et al. Band-Aligned Polymeric Hole Transport Materials for Extremely Low Energy Loss α-CsPbI3 Perovskite Nanocrystal Solar Cells. Joule 2018, 2, 2450–2463. [Google Scholar] [CrossRef] [Green Version]
- Draguta, S.; Sharia, O.; Yoon, S.J.; Brennan, M.C.; Morozov, Y.V.; Manser, J.S.; Kamat, P.V.; Schneider, W.F.; Kuno, M. Rationalizing the light-induced phase separation of mixed halide organic–inorganic perovskites. Nat. Commun. 2017, 8, 200. [Google Scholar] [CrossRef] [Green Version]
- Gualdrón-Reyes, A.F.; Yoon, S.J.; Mora-Seró, I. Recent insights for achieving mixed halide perovskites without halide segregation. Curr. Opin. Electrochem. 2018, 11, 84–90. [Google Scholar] [CrossRef]
- Gualdrón-Reyes, A.F.; Yoon, S.J.; Barea, E.M.; Agouram, S.; Muñoz-Sanjosé, V.; Meléndez, Á.M.; Niño-Gómez, M.E.; Mora-Seró, I. Controlling the Phase Segregation in Mixed Halide Perovskites through Nanocrystal Size. ACS Energy Lett. 2019, 4, 54–62. [Google Scholar] [CrossRef]
- Yuan, J.; Hazarika, A.; Zhao, Q.; Ling, X.; Moot, T.; Ma, W.; Luther, J.M. Metal Halide Perovskites in Quantum Dot Solar Cells: Progress and Prospects. Joule 2020, 4, 1160–1185. [Google Scholar] [CrossRef]
- Zhao, Q.; Hazarika, A.; Chen, X.; Harvey, S.P.; Larson, B.W.; Teeter, G.R.; Liu, J.; Song, T.; Xiao, C.; Shaw, L.; et al. High efficiency perovskite quantum dot solar cells with charge separating heterostructure. Nat. Commun. 2019, 10, 2842. [Google Scholar] [CrossRef]
- Wheeler, L.M.; Sanehira, E.M.; Marshall, A.R.; Schulz, P.; Suri, M.; Anderson, N.C.; Christians, J.A.; Nordlund, D.; Sokaras, D.; Kroll, T.; et al. Targeted Ligand-Exchange Chemistry on Cesium Lead Halide Perovskite Quantum Dots for High-Efficiency Photovoltaics. J. Am. Chem. Soc. 2018, 140, 10504–10513. [Google Scholar] [CrossRef]
- Fu, Y.; Rea, M.T.; Chen, J.; Morrow, D.J.; Hautzinger, M.P.; Zhao, Y.; Pan, D.; Manger, L.H.; Wright, J.C.; Goldsmith, R.H.; et al. Selective Stabilization and Photophysical Properties of Metastable Perovskite Polymorphs of CsPbI3 in Thin Films. Chem. Mater. 2017, 29, 8385–8394. [Google Scholar] [CrossRef]
- Ling, X.; Zhou, S.; Yuan, J.; Shi, J.; Qian, Y.; Larson, B.W.; Zhao, Q.; Qin, C.; Li, F.; Shi, G.; et al. 14.1% CsPbI3 Perovskite Quantum Dot Solar Cells via Cesium Cation Passivation. Adv. Energy Mater. 2019, 9, 1900721. [Google Scholar] [CrossRef]
- Chen, K.; Zhong, Q.; Chen, W.; Sang, B.; Wang, Y.; Yang, T.; Liu, Y.; Zhang, Y.; Zhang, H. Short-Chain Ligand-Passivated Stable α-CsPbI3 Quantum Dot for All-Inorganic Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1900991. [Google Scholar] [CrossRef]
- Jia, D.; Chen, J.; Yu, M.; Liu, J.; Johansson, E.M.J.; Hagfeldt, A.; Zhang, X. Dual Passivation of CsPbI3 Perovskite Nanocrystals with Amino Acid Ligands for Efficient Quantum Dot Solar Cells. Small 2020, 16, 2001772. [Google Scholar] [CrossRef]
- Pan, L.; Ye, T.; Qin, C.; Zhou, B.; Lei, N.; Chen, S.; Yan, P.; Wang, X. α-CsPbI3 Nanocrystals by Ultraviolet Light-Driven Oriented Attachment. J. Phys. Chem. Lett. 2020, 11, 913–919. [Google Scholar] [CrossRef]
- Liu, J.; Song, K.; Shin, Y.; Liu, X.; Chen, J.; Yao, K.X.; Pan, J.; Yang, C.; Yin, J.; Xu, L.-J.; et al. Light-Induced Self-Assembly of Cubic CsPbBr3 Perovskite Nanocrystals into Nanowires. Chem. Mater. 2019, 31, 6642–6649. [Google Scholar] [CrossRef]
- Thapa, S.; Bhardwaj, K.; Basel, S.; Pradhan, S.; Eling, C.J.; Adawi, A.M.; Bouillard, J.S.G.; Stasiuk, G.J.; Reiss, P.; Pariyar, A.; et al. Long-term ambient air-stable cubic CsPbBr3 perovskite quantum dots using molecular bromine. Nanoscale Adv. 2019, 1, 3388–3391. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, X.; Sreejith, S.; Cao, F.; Wang, Z.; Stuparu, M.C.; Zeng, H.; Sun, H. Photon Driven Transformation of Cesium Lead Halide Perovskites from Few-Monolayer Nanoplatelets to Bulk Phase. Adv. Mater. 2016, 28, 10637–10643. [Google Scholar] [CrossRef] [PubMed]
- Méndez, P.F.; Muhammed, S.K.M.; Barea, E.M.; Masi, S.; Mora-Seró, I. Analysis of the UV-Ozone-Treated SnO2 Electron Transporting Layer in Planar Perovskite Solar Cells for High Performance and Reduced Hysteresis. Sol. RRL 2019, 3, 1900191. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Ding, C.; Kobayashi, S.; Izuishi, T.; Nakazawa, N.; Toyoda, T.; Ohta, T.; Hayase, S.; Minemoto, T.; et al. Highly Luminescent Phase-Stable CsPbI3 Perovskite Quantum Dots Achieving Near 100% Absolute Photoluminescence Quantum Yield. ACS Nano 2017, 11, 10373–10383. [Google Scholar] [CrossRef] [PubMed]
- Moyen, E.; Kanwat, A.; Cho, S.; Jun, H.; Aad, R.; Jang, J. Ligand removal and photo-activation of CsPbBr3 quantum dots for enhanced optoelectronic devices. Nanoscale 2018, 10, 8591–8599. [Google Scholar] [CrossRef]
- Carrillo-Carrión, C.; Cárdenas, S.; Simonet, B.M.; Valcárcel, M. Quantum dots luminescence enhancement due to illumination with UV/V is light. Chem. Commun. 2009, 35, 5214. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Sun, B.; Zhou, Z.; Wu, Y.T.; Zhu, M.F. Size-controlled and large-scale synthesis of organic-soluble Ag nanocrystals in water and their formation mechanism. Prog. Nat. Sci. Mater. Int. 2011, 21, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Hassanabadi, E.; Latifi, M.; Gualdrón-Reyes, A.F.; Masi, S.; Yoon, S.J.; Poyatos, M.; Julián-López, B.; Mora-Seró, I. Ligand & band gap engineering: Tailoring the protocol synthesis for achieving high-quality CsPbI3 quantum dots. Nanoscale 2020. [Google Scholar] [CrossRef]
- Zhang, T.; Dar, M.I.; Li, G.; Xu, F.; Guo, N.; Grätzel, M.; Zhao, Y. Bication lead iodide 2D perovskite component to stabilize inorganic α-CsPbI3 perovskite phase for high-efficiency solar cells. Sci. Adv. 2017, 3, e1700841. [Google Scholar] [CrossRef] [Green Version]
- Quitsch, W.A.; Dequilettes, D.W.; Pfingsten, O.; Schmitz, A.; Ognjanovic, S.; Jariwala, S.; Koch, S.; Winterer, M.; Ginger, D.S.; Bacher, G. The Role of Excitation Energy in Photobrightening and Photodegradation of Halide Perovskite Thin Films. J. Phys. Chem. Lett. 2018, 9, 2062–2069. [Google Scholar] [CrossRef]
- Li, Q.; Ji, S.; Yuan, X.; Li, J.; Fan, Y.; Zhang, J.; Zhao, J.; Li, H. Ultraviolet Light-Induced Degradation of Luminescence in Mn-Doped CsPbCl3 Nanocrystals. J. Phys. Chem. C 2019, 123, 14849–14857. [Google Scholar] [CrossRef]
- Tai, Q.; Tang, K.-C.; Yan, F. Recent progress of inorganic perovskite solar cells. Energy Environ. Sci. 2019, 12, 2375–2405. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Baikie, T.; Boix, P.P.; Yantara, N.; Mathews, N.; Mhaisalkar, S. Band-gap tuning of lead halide perovskites using a sequential deposition process. J. Mater. Chem. A 2014, 2, 9221–9225. [Google Scholar] [CrossRef] [Green Version]
- Shen, T.; Tian, J.; Lv, L.; Fei, C.; Wang, Y.; Pullerits, T.; Cao, G. Investigation of the role of Mn dopant in CdS quantum dot sensitized solar cell. Electrochim. Acta 2016, 191, 62–69. [Google Scholar] [CrossRef]
- Nakane, A.; Tampo, H.; Tamakoshi, M.; Fujimoto, S.; Kim, K.M.; Kim, S.; Shibata, H.; Niki, S.; Fujiwara, H. Quantitative determination of optical and recombination losses in thin-film photovoltaic devices based on external quantum efficiency analysis. J. Appl. Phys. 2016, 120, 064505. [Google Scholar] [CrossRef]
- Ding, X.; Cai, M.; Liu, X.; Ding, Y.; Liu, X.; Wu, Y.; Hayat, T.; Alsaedi, A.; Dai, S. Enhancing the Phase Stability of Inorganic α-CsPbI3 by the Bication-Conjugated Organic Molecule for Efficient Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 37720–37725. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Xu, H.; Sha, W.E.I.; Zhao, Y.; Wang, Y.; Yang, X.; Tang, Q. Inorganic perovskite solar cells: An emerging member of the photovoltaic community. J. Mater. Chem. A 2019, 7, 21036–21068. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Zhao, Y. Inorganic CsPbI3 Perovskites toward High-Efficiency Photovoltaics. Energy Environ. Mater. 2019, 2, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, X.; Sui, N.; Hu, Y.; Colvin, V.L.; Yu, W.W.; Zhang, Y. Photoluminescence Loss and Recovery of α-CsPbI3 Quantum Dots Originated from Chemical Equilibrium Shift of Oleylammonium. ACS Appl. Mater. Interfaces 2020, 12, 11769–11777. [Google Scholar] [CrossRef]
- Kye, Y.-H.; Yu, C.-J.; Jong, U.-G.; Ri, K.-C.; Kim, J.-S.; Choe, S.-H.; Hong, S.-N.; Li, S.; Wilson, J.N.; Walsh, A. Vacancy-Driven Stabilization of the Cubic Perovskite Polymorph of CsPbI3. J. Phys. Chem. C 2019, 123, 9735–9744. [Google Scholar] [CrossRef] [Green Version]
- Domanski, K.; Roose, B.; Matsui, T.; Saliba, M.; Turren-Cruz, S.H.; Correa-Baena, J.P.; Carmona, C.R.; Richardson, G.; Foster, J.M.; De Angelis, F.; et al. Migration of cations induces reversible performance losses over day/night cycling in perovskite solar cells. Energy Environ. Sci. 2017, 10, 604–613. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Shi, Q.; Pradhan, B.; Mushtaq, A.; Acharya, S.; Karki, K.J.; Pullerits, T.; Pal, S.K. Light-Induced Defect Healing and Strong Many-Body Interactions in Formamidinium Lead Bromide Perovskite Nanocrystals. J. Phys. Chem. Lett. 2020, 11, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Cheng, Z.; Lin, J. An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs. Chem. Soc. Rev. 2019, 48, 310–350. [Google Scholar] [CrossRef] [PubMed]

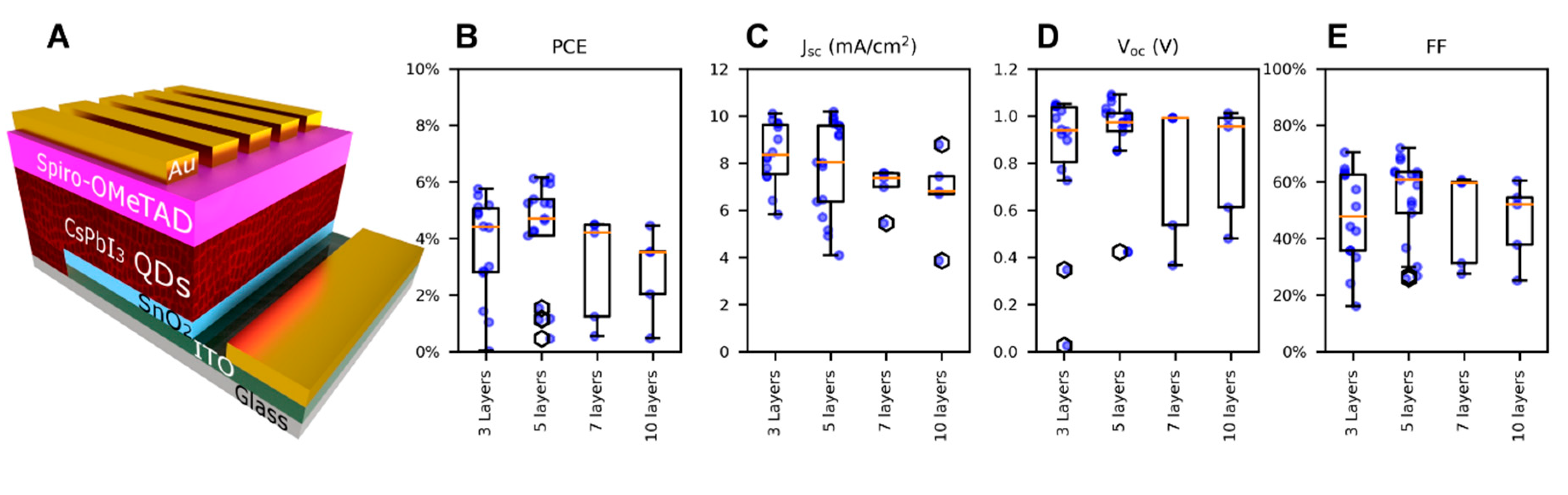
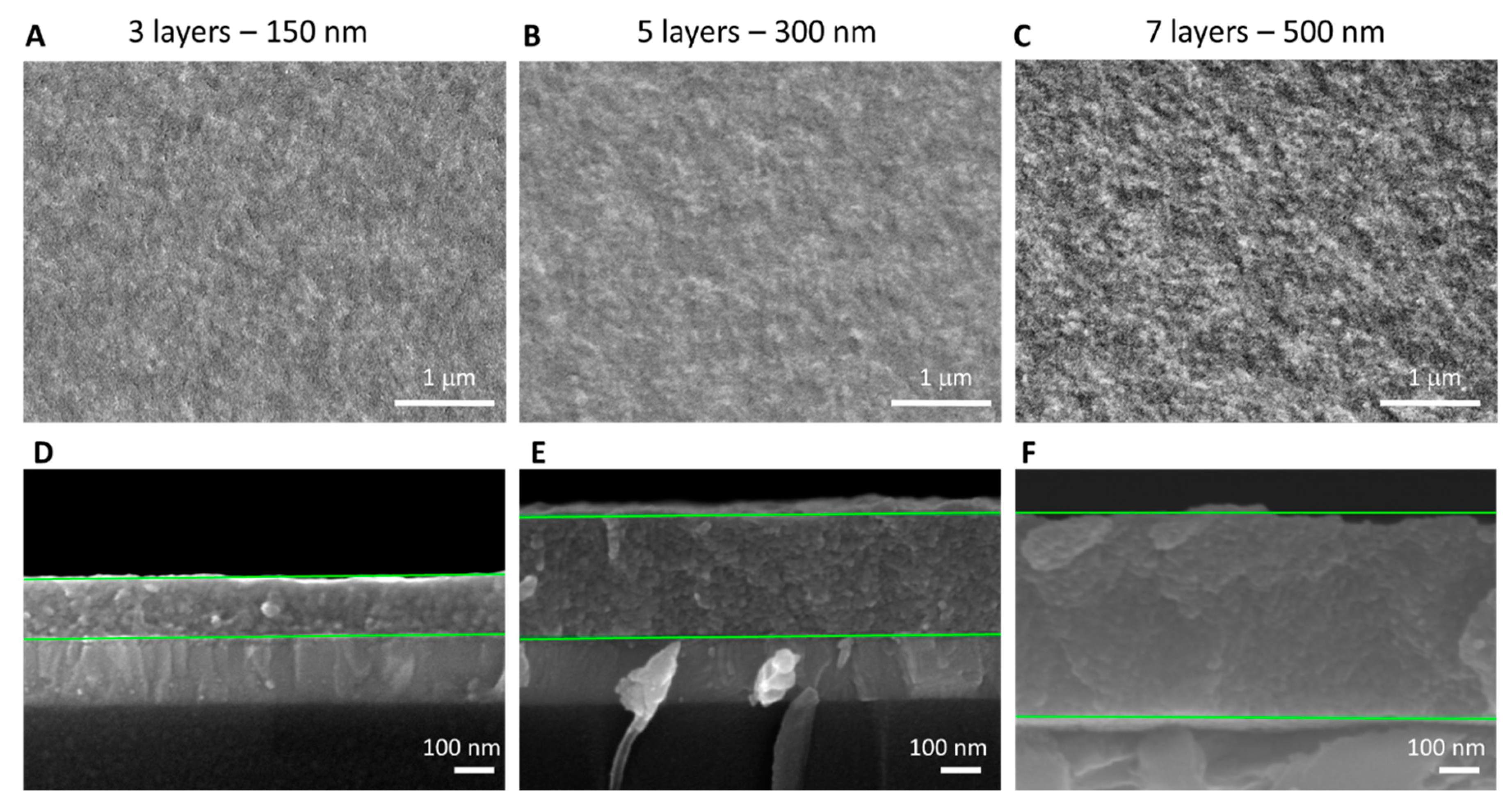
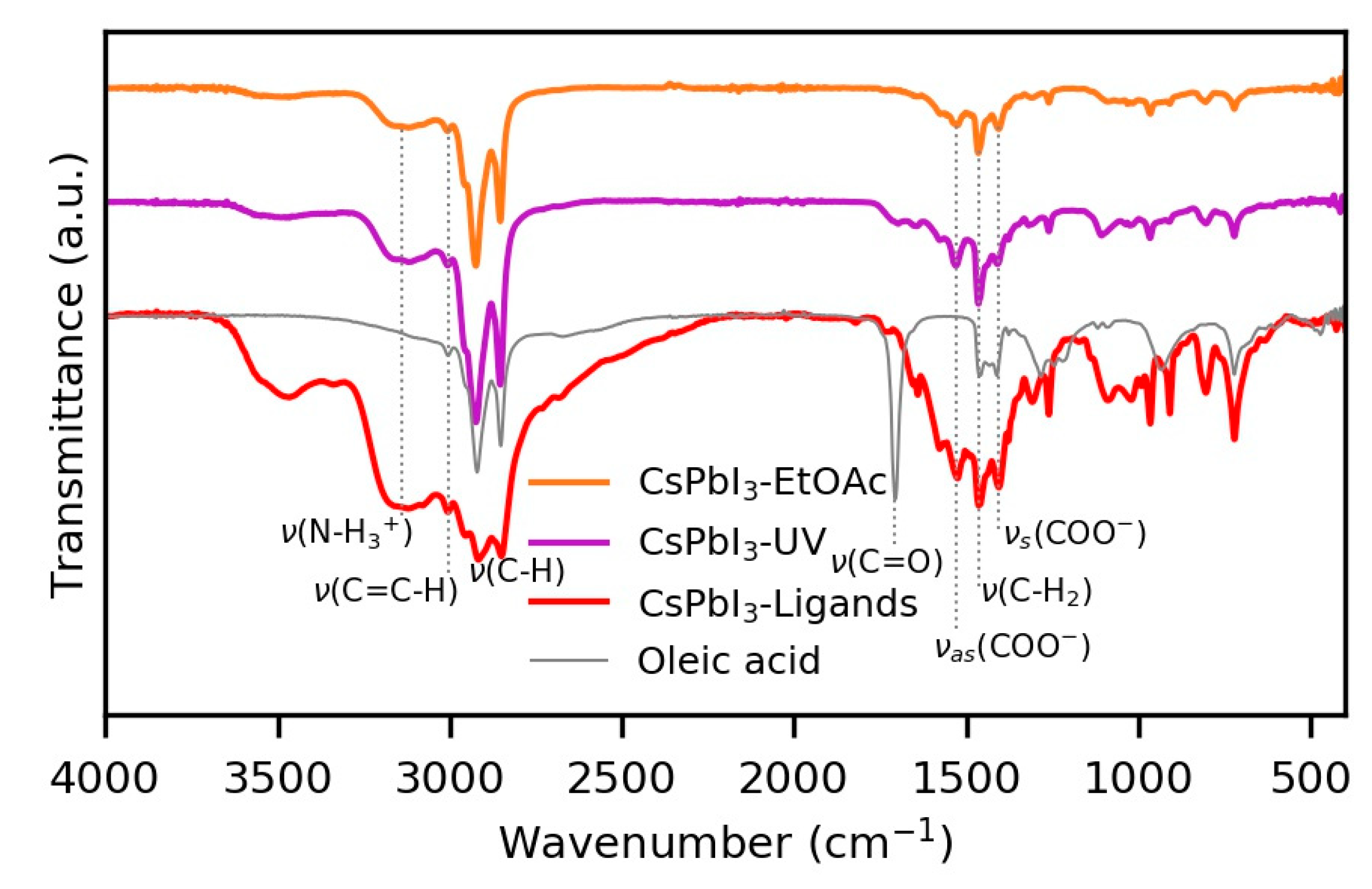

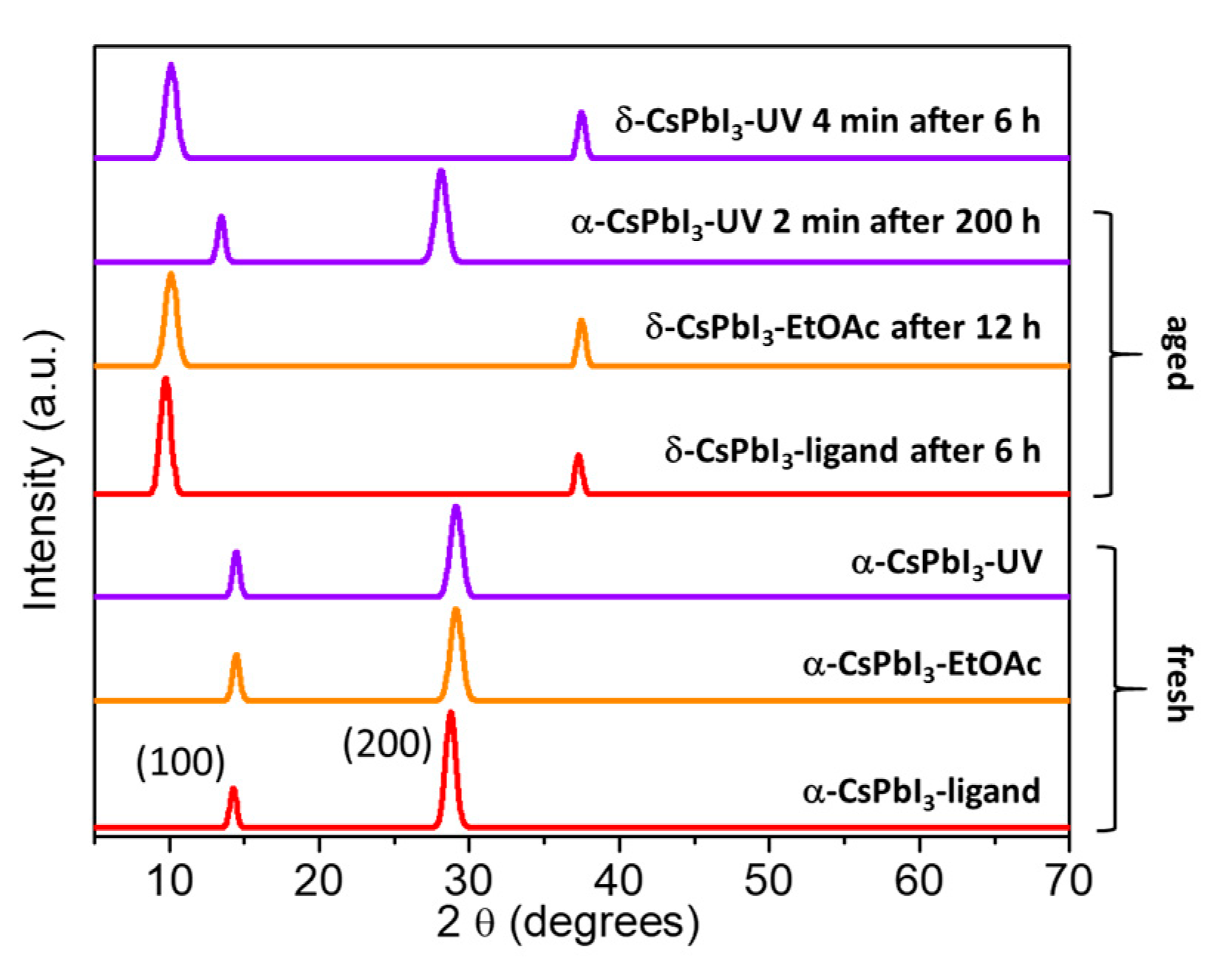
| Number of Layers | PCE (%) (Mean ± SD) | Jsc (mA/cm2) (Mean ± SD) | Voc (V) (Mean ± SD) | FF (%) (Mean ± SD) |
|---|---|---|---|---|
| 3 | 3.7 ± 1.8 | 8.4 ± 1.3 | 0.840 ± 0.290 | 47.4 ± 16.2 |
| 5 | 4.2 ± 1.9 | 7.9 ± 2.0 | 0.921 ± 0.192 | 53.9 ± 14.7 |
| 7 | 3.0 ± 1.7 | 7.0 ± 0.8 | 0.776 ± 0.270 | 47.9 ± 15.1 |
| 10 | 2.8 ± 1.4 | 6.7 ± 1.6 | 0.810 ± 0.220 | 46.0 ± 12.8 |
| Variation | PCE (%) (Mean ± SD) | Jsc (mA/cm2) (Mean ± SD) | Voc (V) (Mean ± SD) | FF (%) (Mean ± SD) | Best PCE (%) |
|---|---|---|---|---|---|
| EtOAc 3 L | 3.7 ± 1.8 | 8.4 ± 1.3 | 0.840 ± 0.290 | 47.4 ± 16.2 | 5.8 |
| EtOAc 5 L | 4.2 ± 1.9 | 7.9 ± 2.0 | 0.921 ± 0.192 | 53.9 ± 14.7 | 6.2 |
| UV 2 min 3 L | 6.7 ± 0.4 | 9.8 ± 0.4 | 1.025 ± 0.047 | 66.6 ± 3.5 | 7.4 |
| UV 2 min 5 L | 3.9 ± 1.2 | 6.8 ± 1.4 | 0.961 ± 0.066 | 57.7 ± 6.5 | 5.2 |
| UV 4 min 3 L | 3.4 ± 1.3 | 6.9 ± 1.0 | 0.864 ± 0.145 | 55.4 ± 12.7 | 5.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erazo, E.A.; Sánchez-Godoy, H.E.; Gualdrón-Reyes, A.F.; Masi, S.; Mora-Seró, I. Photo-Induced Black Phase Stabilization of CsPbI3 QDs Films. Nanomaterials 2020, 10, 1586. https://doi.org/10.3390/nano10081586
Erazo EA, Sánchez-Godoy HE, Gualdrón-Reyes AF, Masi S, Mora-Seró I. Photo-Induced Black Phase Stabilization of CsPbI3 QDs Films. Nanomaterials. 2020; 10(8):1586. https://doi.org/10.3390/nano10081586
Chicago/Turabian StyleErazo, Eider A., H.E. Sánchez-Godoy, Andrés F. Gualdrón-Reyes, Sofia Masi, and Iván Mora-Seró. 2020. "Photo-Induced Black Phase Stabilization of CsPbI3 QDs Films" Nanomaterials 10, no. 8: 1586. https://doi.org/10.3390/nano10081586
APA StyleErazo, E. A., Sánchez-Godoy, H. E., Gualdrón-Reyes, A. F., Masi, S., & Mora-Seró, I. (2020). Photo-Induced Black Phase Stabilization of CsPbI3 QDs Films. Nanomaterials, 10(8), 1586. https://doi.org/10.3390/nano10081586






