Low-Temperature Processed TiOx Electron Transport Layer for Efficient Planar Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
2.3. Characterization
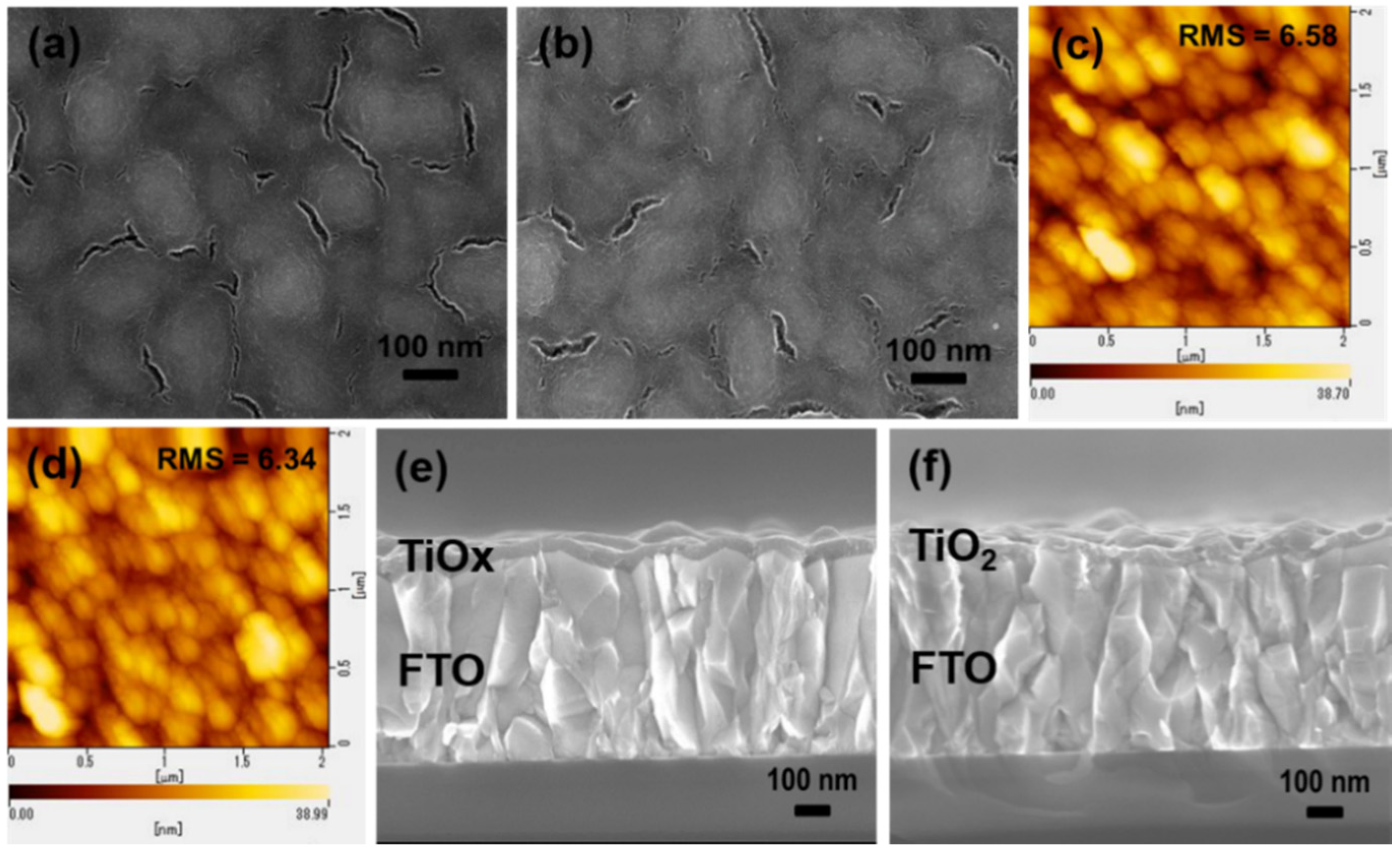
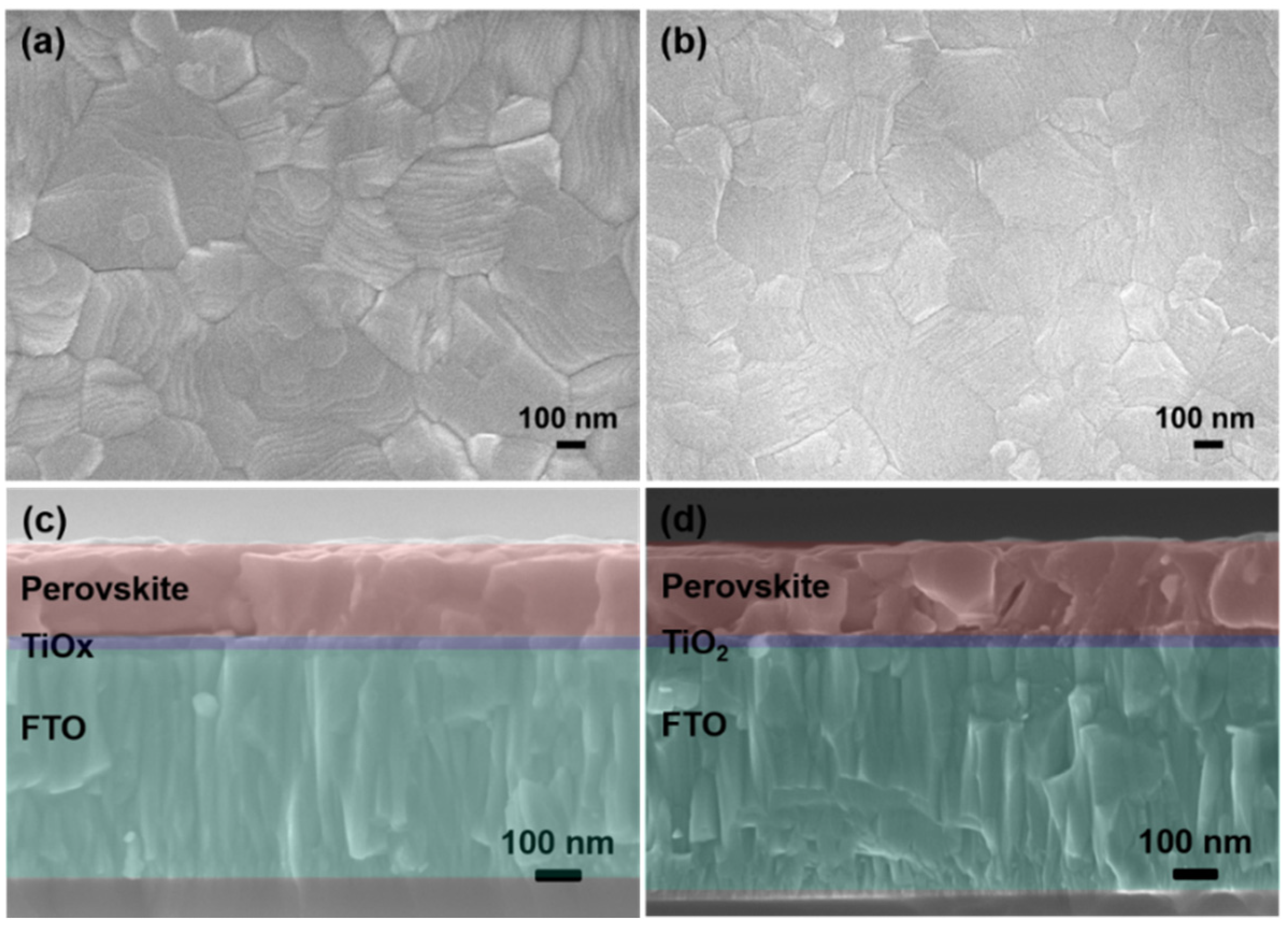
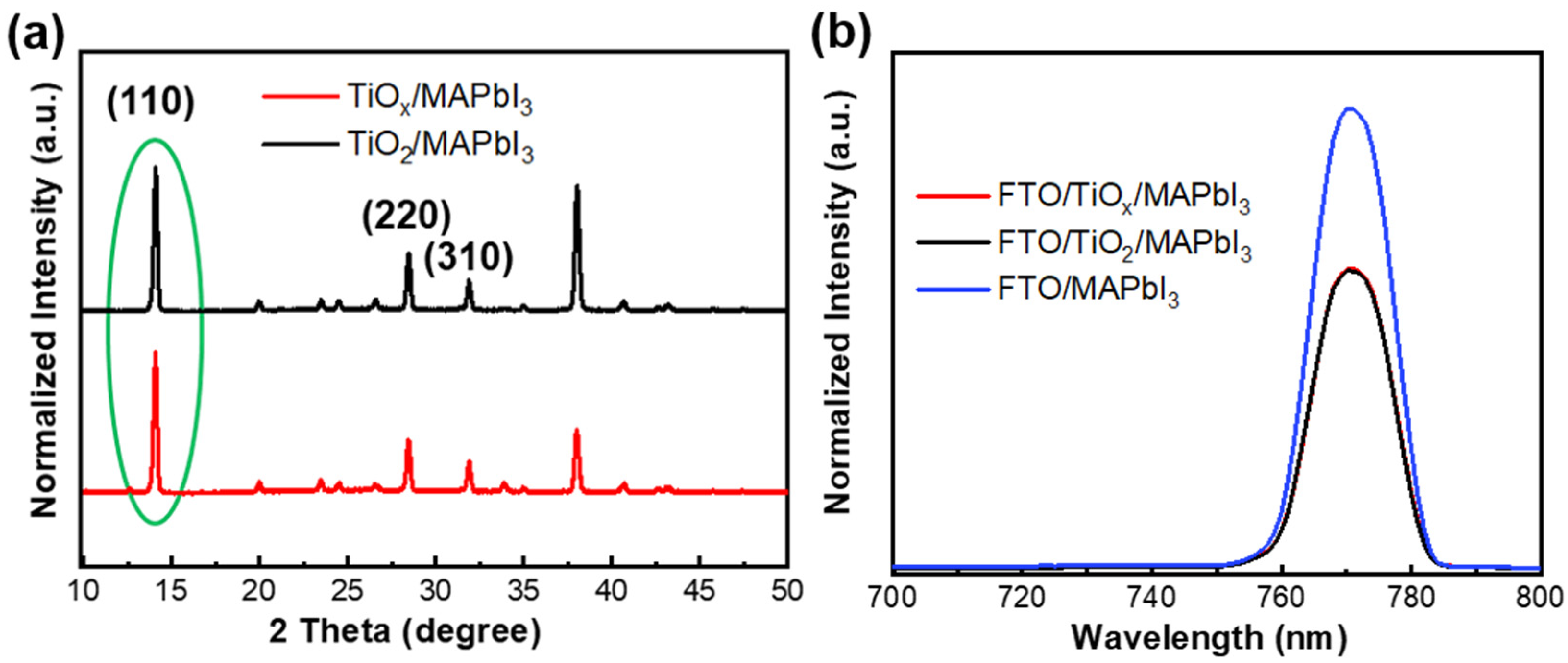
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical Management for Colorful, Efficient, and Stable Inorganic–Organic Hybrid Nanostructured Solar Cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef] [PubMed]
- Shahiduzzaman, M.; Fukaya, S.; Muslih, E.Y.; Wang, L.; Nakano, M.; Akhtaruzzaman, M.; Karakawa, M.; Takahashi, K.; Nunzi, J.-M.; Taima, T. Metal Oxide Compact Electron Transport Layer Modification for Efficient and Stable Perovskite Solar Cells. Materials 2020, 13, 2207. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 24 May 2020).
- Shalan, A.E.; Narra, S.; Oshikiri, T.; Ueno, K.; Shi, X.; Wu, H.-P.; Elshanawany Mahmoud, M.; Wei-Guang Diau, E.; Misawa, H. Optimization of a compact layer of TiO2 via atomic-layer deposition for high-performance perovskite solar cells. Sustain. Energy Fuels 2017, 1, 1533–1540. [Google Scholar] [CrossRef] [Green Version]
- Shahiduzzaman, M.; Visal, S.; Kuniyoshi, M.; Kaneko, T.; Umezu, S.; Katsumata, T.; Iwamori, S.; Kakihana, M.; Taima, T.; Isomura, M.; et al. Low-Temperature-Processed Brookite-Based TiO2 Heterophase Junction Enhances Performance of Planar Perovskite Solar Cells. Nano Lett. 2019, 19, 598–604. [Google Scholar] [CrossRef]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Yonezawa, K.; Yamamoto, K.; Ripolles, T.S.; Karakawa, M.; Kuwabara, T.; Takahashi, K.; Hayase, S.; Taima, T. Improved Reproducibility and Intercalation Control of Efficient Planar Inorganic Perovskite Solar Cells by Simple Alternate Vacuum Deposition of PbI2 and CsI. ACS Omega 2017, 2, 4464–4469. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Zhou, H.; Hong, Z.; Luo, S.; Duan, H.-S.; Wang, H.-H.; Liu, Y.; Li, G.; Yang, Y. Planar Heterojunction Perovskite Solar Cells via Vapor-Assisted Solution Process. J. Am. Chem. Soc. 2014, 136, 622–625. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-Performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Shahiduzzaman, M.; Hamada, K.; Yamamoto, K.; Nakano, M.; Karakawa, M.; Takahashi, K.; Taima, T. Thermal Control of PbI2 Film Growth for Two-Step Planar Perovskite Solar Cells. Cryst. Growth Des. 2019, 19, 5320–5325. [Google Scholar] [CrossRef]
- Ke, W.; Fang, G.; Wan, J.; Tao, H.; Liu, Q.; Xiong, L.; Qin, P.; Wang, J.; Lei, H.; Yang, G.; et al. Efficient hole-blocking layer-free planar halide perovskite thin-film solar cells. Nat. Commun. 2015, 6, 6700. [Google Scholar] [CrossRef]
- Liu, D.; Yang, J.; Kelly, T.L. Compact Layer Free Perovskite Solar Cells with 13.5% Efficiency. J. Am. Chem. Soc. 2014, 136, 17116–17122. [Google Scholar] [CrossRef] [PubMed]
- Shahiduzzaman, M.; Ashikawa, H.; Kuniyoshi, M.; Visal, S.; Sakakibara, S.; Kaneko, T.; Katsumata, T.; Taima, T.; Iwamori, S.; Isomura, M.; et al. Compact TiO2/Anatase TiO2 Single-Crystalline Nanoparticle Electron-Transport Bilayer for Efficient Planar Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2018, 6, 12070–12078. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Kulkarni, A.; Visal, S.; Wang, L.; Nakano, M.; Karakawa, M.; Takahashi, K.; Umezu, S.; Masuda, A.; Iwamori, S.; et al. A single-phase brookite TiO2 nanoparticle bridge enhances the stability of perovskite solar cells. Sustain. Energy Fuels 2020, 4, 2009–2017. [Google Scholar] [CrossRef]
- Choi, J.; Song, S.; Hörantner, M.T.; Snaith, H.J.; Park, T. Well-Defined Nanostructured, Single-Crystalline TiO2 Electron Transport Layer for Efficient Planar Perovskite Solar Cells. ACS Nano 2016, 10, 6029–6036. [Google Scholar] [CrossRef]
- Deng, X.; Wilkes, G.C.; Chen, A.Z.; Prasad, N.S.; Gupta, M.C.; Choi, J.J. Room-Temperature Processing of TiOx Electron Transporting Layer for Perovskite Solar Cells. J. Phys. Chem. Lett. 2017, 8, 3206–3210. [Google Scholar] [CrossRef]
- Yang, C.; Yu, M.; Chen, D.; Zhou, Y.; Wang, W.; Li, Y.; Lee, T.-C.; Yun, D. An annealing-free aqueous-processed anatase TiO2 compact layer for efficient planar heterojunction perovskite solar cells. Chem. Commun. 2017, 53, 10882–10885. [Google Scholar] [CrossRef] [Green Version]
- Visal, S.; Shahiduzzaman, M.; Kuniyoshi, M.; Kaneko, T.; Katsumata, T.; Iwamori, S.; Tomita, K.; Isomura, M. Efficient Planar Perovskite Solar Cells with Entire Low-Temperature Processes via Brookite TiO2 Nanoparticle Electron Transport Layer. In Proceedings of the 26th International Workshop on Active-Matrix Flatpanel Displays and Devices (AM-FPD), Kyoto, Japan, 2–5 July 2019; pp. 1–4. [Google Scholar]
- Möllmann, A.; Gedamu, D.; Vivo, P.; Frohnhoven, R.; Stadler, D.; Fischer, T.; Ka, I.; Steinhorst, M.; Nechache, R.; Rosei, F.; et al. Highly Compact TiO2 Films by Spray Pyrolysis and Application in Perovskite Solar Cells. Adv. Eng. Mater. 2019, 21, 1801196. [Google Scholar]
- Kavan, L. Electrochemistry and perovskite photovoltaics. Curr. Opin. Electrochem. 2018, 11, 122–129. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Sakuma, T.; Kaneko, T.; Tomita, K.; Isomura, M.; Taima, T.; Umezu, S.; Iwamori, S. Oblique Electrostatic Inkjet-Deposited TiO2 Electron Transport Layers for Efficient Planar Perovskite Solar Cells. Sci. Rep. 2019, 9, 19494. [Google Scholar] [CrossRef] [PubMed]
- Su, T.-S.; Hsieh, T.-Y.; Hong, C.-Y.; Wei, T.-C. Electrodeposited Ultrathin TiO2 Blocking Layers for Efficient Perovskite Solar Cells. Sci. Rep. 2015, 5, 16098. [Google Scholar] [CrossRef]
- Huckaba, A.J.; Lee, Y.; Xia, R.; Paek, S.; Bassetto, V.C.; Oveisi, E.; Lesch, A.; Kinge, S.; Dyson, P.J.; Girault, H.; et al. Inkjet-Printed Mesoporous TiO2 and Perovskite Layers for High Efficiency Perovskite Solar Cells. Energy Technol. 2019, 7, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Dong, B.; Hu, H.; Chen, F.; Kong, M.; Zhang, Q.; Luo, T.; Zhao, L.; Guo, Z.; Li, J.; et al. Atomic Layer Deposition of TiO2 for a High-Efficiency Hole-Blocking Layer in Hole-Conductor-Free Perovskite Solar Cells Processed in Ambient Air. ACS Appl. Mater. Interfaces 2016, 8, 17999–18007. [Google Scholar] [CrossRef]
- Alberti, A.; Smecca, E.; Sanzaro, S.; Bongiorno, C.; Giannazzo, F.; Mannino, G.; La Magna, A.; Liu, M.; Vivo, P.; Listorti, A.; et al. Nanostructured TiO2 Grown by Low-Temperature Reactive Sputtering for Planar Perovskite Solar Cells. ACS Appl. Energy Mater. 2019, 2, 6218–6229. [Google Scholar] [CrossRef]
- Ren, X.; Xie, L.; Kim, W.B.; Lee, D.G.; Jung, H.S.; Liu, S. Chemical Bath Deposition of Co-Doped TiO2 Electron Transport Layer for Hysteresis-Suppressed High-Efficiency Planar Perovskite Solar Cells. Solar RRL 2019, 3, 1900176. [Google Scholar] [CrossRef]
- Kuwabara, T.; Sugiyama, H.; Kuzuba, M.; Yamaguchi, T.; Takahashi, K. Inverted bulk-heterojunction organic solar cell using chemical bath deposited titanium oxide as electron collection layer. Org. Electron. 2010, 11, 1136–1140. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Tang, L.J.; Yang, S.; Hou, Y.; Yang, H.G. A low-temperature processed flower-like TiO2 array as an electron transport layer for high-performance perovskite solar cells. J. Mater. Chem. A 2016, 4, 6521–6526. [Google Scholar] [CrossRef]
- Yin, G.; Ma, J.; Jiang, H.; Li, J.; Yang, D.; Gao, F.; Zeng, J.; Liu, Z.; Liu, S.F. Enhancing Efficiency and Stability of Perovskite Solar Cells through Nb-Doping of TiO2 at Low Temperature. ACS Appl. Mater. Interfaces 2017, 9, 10752–10758. [Google Scholar] [CrossRef]
- Jung, H.S.; Han, G.S.; Park, N.-G.; Ko, M.J. Flexible Perovskite Solar Cells. Joule 2019, 3, 1850–1880. [Google Scholar] [CrossRef]
- Kim, D.H.; Whitaker, J.B.; Li, Z.; van Hest, M.F.A.M.; Zhu, K. Outlook and Challenges of Perovskite Solar Cells toward Terawatt-Scale Photovoltaic Module Technology. Joule 2018, 2, 1437–1451. [Google Scholar] [CrossRef] [Green Version]
- Wakamiya, A.; Endo, M.; Sasamori, T.; Tokitoh, N.; Ogomi, Y.; Hayase, S.; Murata, Y. Reproducible Fabrication of Efficient Perovskite-based Solar Cells: X-ray Crystallographic Studies on the Formation of CH3NH3PbI3 Layers. Chem. Lett. 2014, 43, 711–713. [Google Scholar] [CrossRef] [Green Version]
- Rossella, F.; Galinetto, P.; Mozzati, M.C.; Malavasi, L.; Diaz Fernandez, Y.; Drera, G.; Sangaletti, L. TiO2 thin films for spintronics application: A Raman study. J. Raman Spectrosc. 2010, 41, 558–565. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Yamamoto, K.; Furumoto, Y.; Kuwabara, T.; Takahashi, K.; Taima, T. Enhanced Photovoltaic Performance of Perovskite Solar Cells via Modification of Surface Characteristics Using a Fullerene Interlayer. Chem. Lett. 2015, 44, 1735–1737. [Google Scholar] [CrossRef]
- Kim, H.-S.; Park, N.-G. Parameters Affecting I–V Hysteresis of CH3NH3PbI3 Perovskite Solar Cells: Effects of Perovskite Crystal Size and Mesoporous TiO2 Layer. J. Phys. Chem. Lett. 2014, 5, 2927–2934. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, H.; Fang, Y.; Stieg, A.Z.; Song, T.-B.; Wang, H.-H.; Xu, X.; Liu, Y.; Lu, S.; You, J.; et al. The optoelectronic role of chlorine in CH3NH3PbI3(Cl)-based perovskite solar cells. Nat. Commun. 2015, 6, 7269. [Google Scholar] [CrossRef]
- Ma, S.; Ahn, J.; Oh, Y.; Kwon, H.-C.; Lee, E.; Kim, K.; Yun, S.-C.; Moon, J. Facile Sol–Gel-Derived Craterlike Dual-Functioning TiO2 Electron Transport Layer for High-Efficiency Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 14649–14658. [Google Scholar] [CrossRef]
- de Quilettes, D.W.; Vorpahl, S.M.; Stranks, S.D.; Nagaoka, H.; Eperon, G.E.; Ziffer, M.E.; Snaith, H.J.; Ginger, D.S. Impact of microstructure on local carrier lifetime in perovskite solar cells. Science 2015, 348, 683–686. [Google Scholar] [CrossRef] [Green Version]
- Jena, A.K.; Kulkarni, A.; Ikegami, M.; Miyasaka, T. Steady state performance, photo-induced performance degradation and their relation to transient hysteresis in perovskite solar cells. J. Power Sources 2016, 309, 1–10. [Google Scholar] [CrossRef] [Green Version]


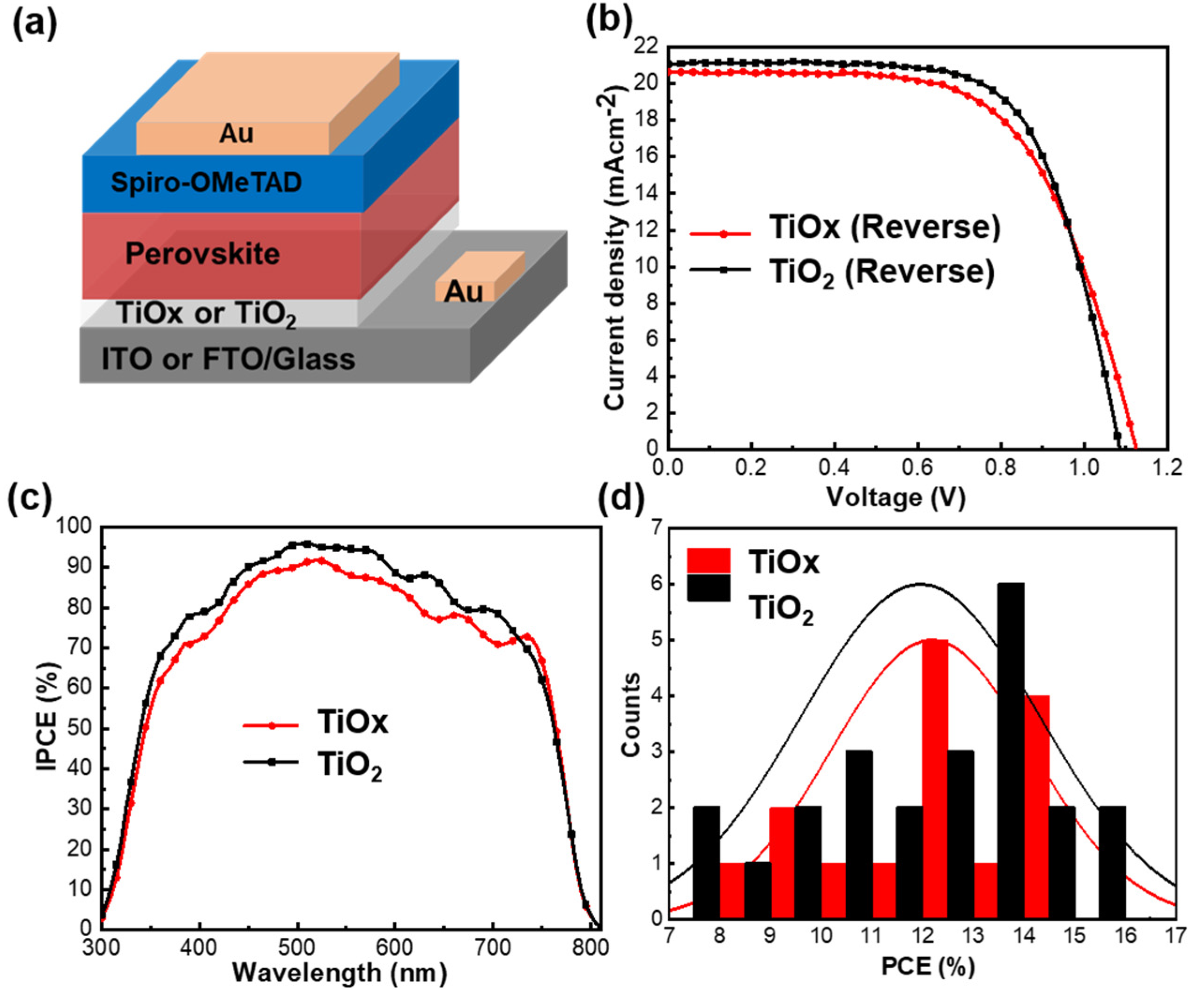
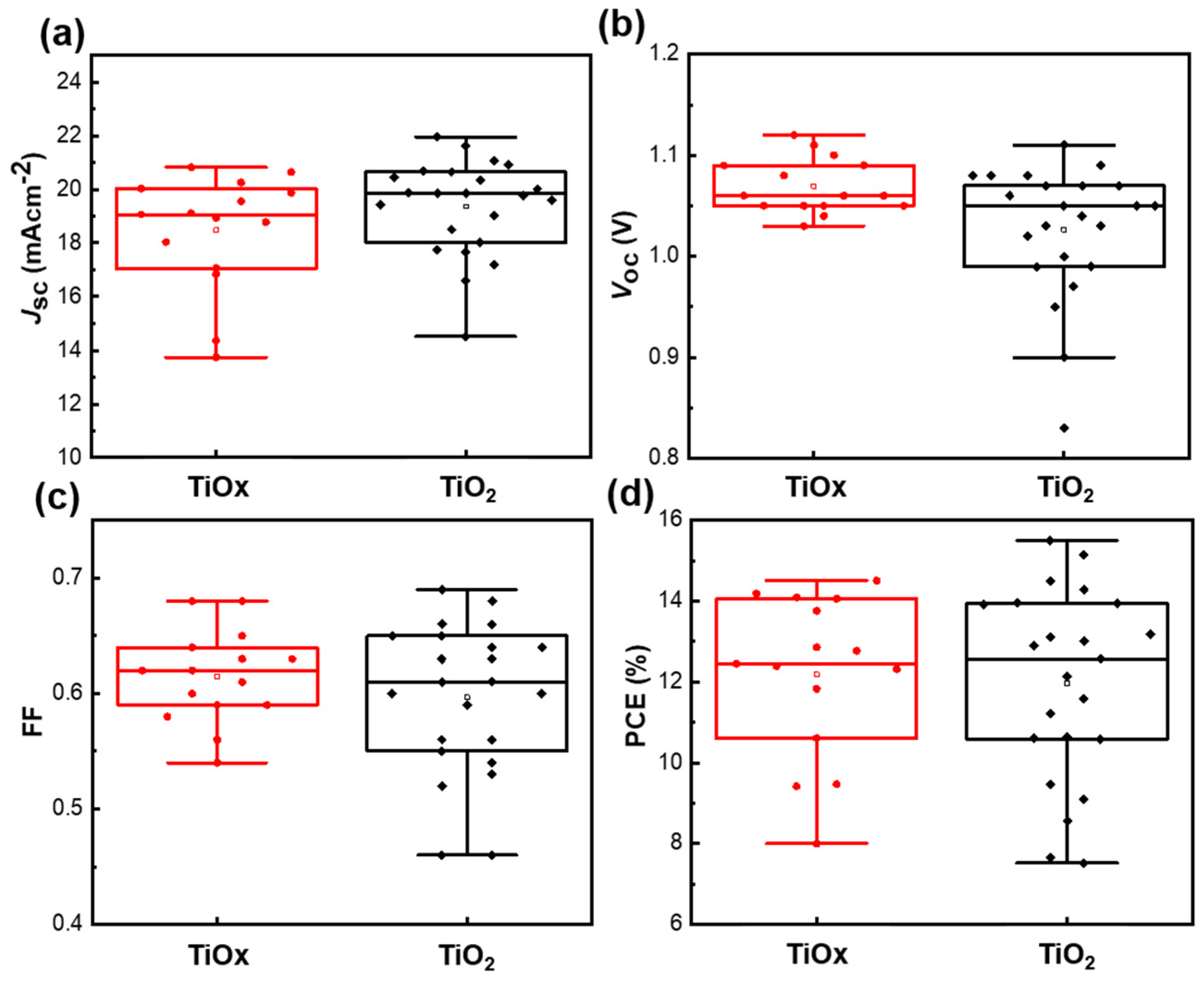
| ETLs Layer | JSC (mA/cm2) | Voc (V) | FF | PCE (%) | |
|---|---|---|---|---|---|
| TiOx | Champion | 20.64 | 1.12 | 0.63 | 14.51 |
| Average ± SD | 18.3 ± 2.3 | 1.07 ± 0.03 | 0.61 ± 0.04 | 12.1 ± 2.1 | |
| TiO2 | Champion | 21.06 | 1.08 | 0.68 | 15.50 |
| Average ± SD | 19.2 ± 1.9 | 1.02 ± 0.07 | 0.59 ± 0.07 | 11.9 ± 2.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahiduzzaman, M.; Kuwahara, D.; Nakano, M.; Karakawa, M.; Takahashi, K.; Nunzi, J.-M.; Taima, T. Low-Temperature Processed TiOx Electron Transport Layer for Efficient Planar Perovskite Solar Cells. Nanomaterials 2020, 10, 1676. https://doi.org/10.3390/nano10091676
Shahiduzzaman M, Kuwahara D, Nakano M, Karakawa M, Takahashi K, Nunzi J-M, Taima T. Low-Temperature Processed TiOx Electron Transport Layer for Efficient Planar Perovskite Solar Cells. Nanomaterials. 2020; 10(9):1676. https://doi.org/10.3390/nano10091676
Chicago/Turabian StyleShahiduzzaman, Md., Daiki Kuwahara, Masahiro Nakano, Makoto Karakawa, Kohshin Takahashi, Jean-Michel Nunzi, and Tetsuya Taima. 2020. "Low-Temperature Processed TiOx Electron Transport Layer for Efficient Planar Perovskite Solar Cells" Nanomaterials 10, no. 9: 1676. https://doi.org/10.3390/nano10091676








