Realizing Hydrogen De/Absorption Under Low Temperature for MgH2 by Doping Mn-Based Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
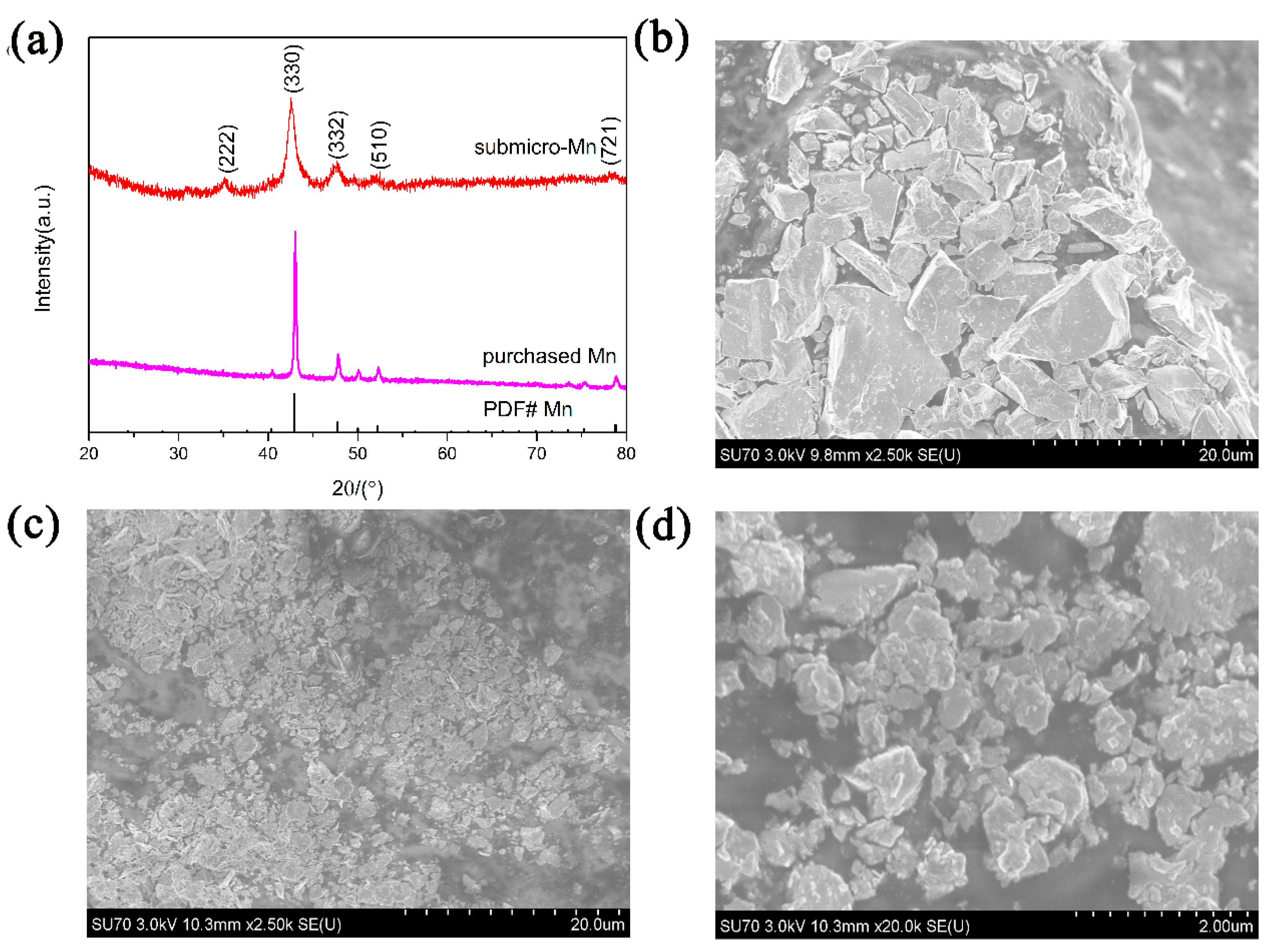
2.2. Sample Characterization
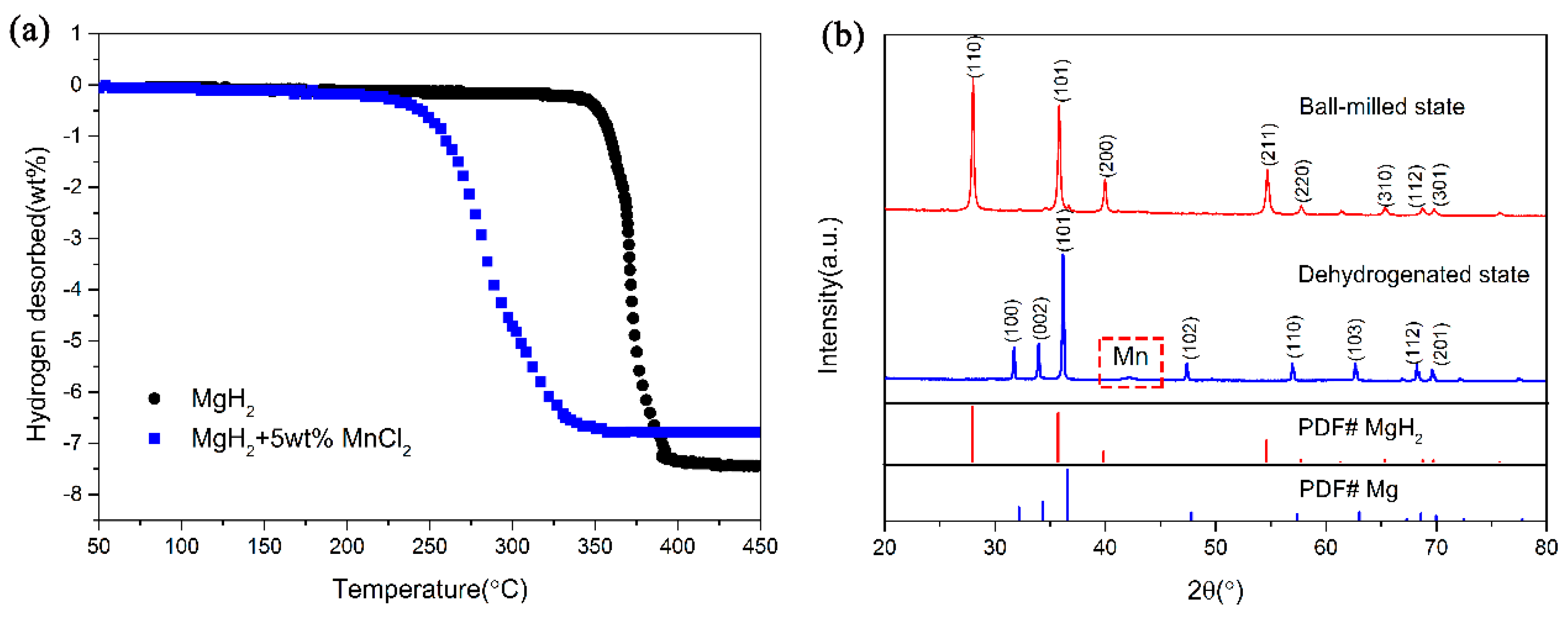
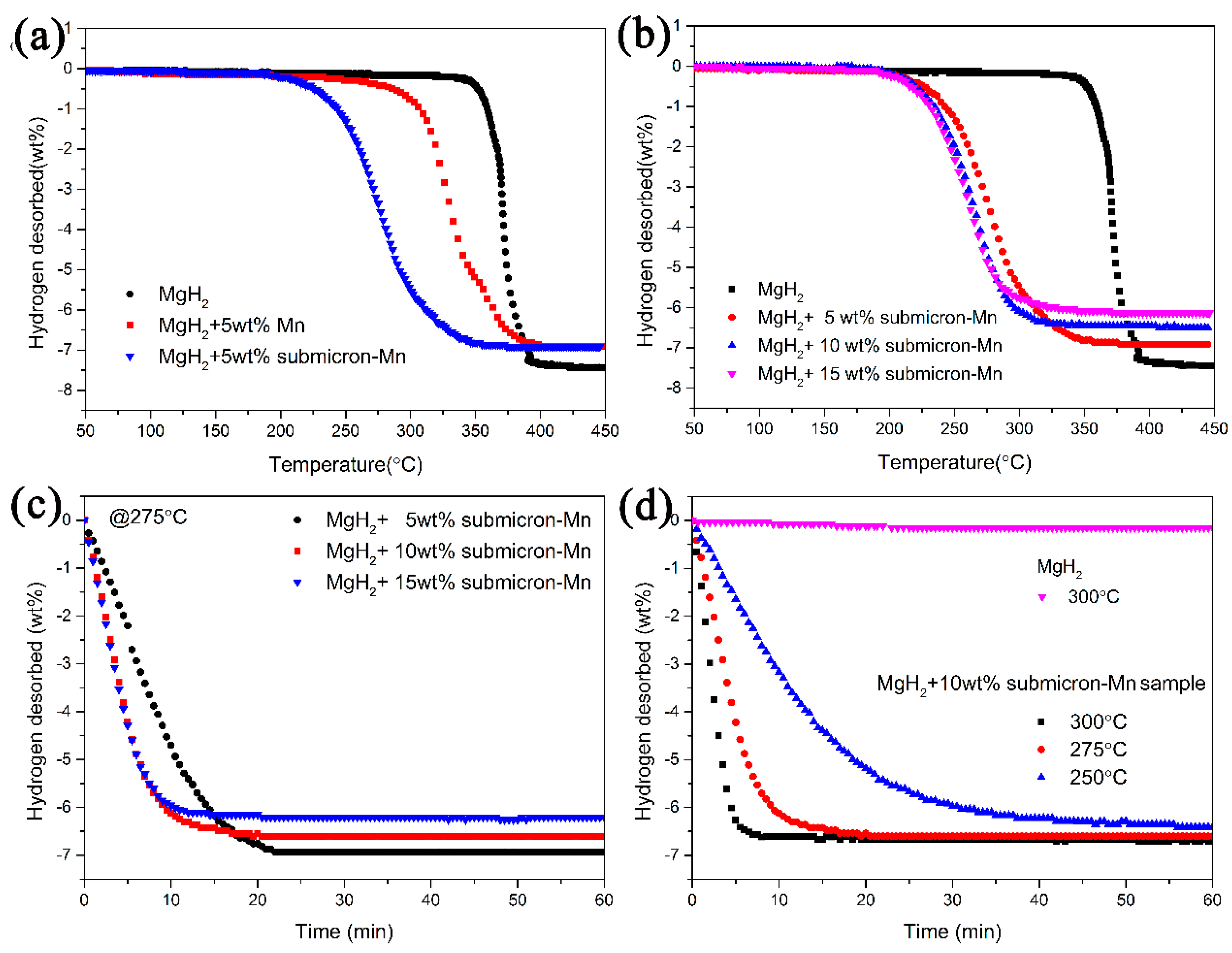
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wan, C.; Zhou, L.; Sun, L.; Xu, L.; Cheng, D.-G.; Chen, F.; Zhan, X.; Yang, Y. Boosting visible-light-driven hydrogen evolution from formic acid over AgPd/2D g-C3N4 nanosheets Mott-Schottky photocatalyst. Chem. Eng. J. 2020, 396, 125229. [Google Scholar] [CrossRef]
- Jain, I.P. Hydrogen the fuel for 21st century. Int. J. Hydrogen Energy 2009, 34, 7368–7378. [Google Scholar] [CrossRef]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Reddy, S.N.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of biomass for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 6912–6926. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Yao, L.; Xu, C.; Liu, Y.; Li, L. State of the art multi-strategy improvement of Mg-based hydrides for hydrogen storage. J. Alloys Compd. 2019, 782, 796–823. [Google Scholar] [CrossRef]
- Yu, X.; Tang, Z.; Sun, D.; Ouyang, L.; Zhu, M. Recent advances and remaining challenges of nanostructured materials for hydrogen storage applications. Prog. Mater. Sci. 2017, 88, 1–48. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, H.; Liu, J.W.; Sun, D.L.; Fang, F.; Zhang, Q.A.; Ouyang, L.Z.; Zhu, M. Enhanced hydrolysis properties and energy efficiency of MgH2-base hydrides. J. Alloys Compd. 2016, 680, 419–426. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Zhu, Y.; Zhao, Y.; Lin, H.; Zhang, Y.; Li, H.; Zhang, J.; Liu, Y.; Gao, W.; et al. Crystal-facet-dependent catalysis of anatase TiO2 on hydrogen storage of MgH2. J. Alloys Compd. 2020, 822, 153553. [Google Scholar] [CrossRef]
- Liu, M.; Xiao, X.; Zhao, S.; Saremi-Yarahmadi, S.; Chen, M.; Zheng, J.; Li, S.; Chen, L. ZIF-67 derived Co@CNTs nanoparticles: Remarkably improved hydrogen storage properties of MgH2 and synergetic catalysis mechanism. Int. J. Hydrogen Energy 2019, 44, 1059–1069. [Google Scholar] [CrossRef]
- Gao, S.; Wang, X.; Liu, H.; He, T.; Wang, Y.; Li, S.; Yan, M. Effects of nano-composites (FeB, FeB/CNTs) on hydrogen storage properties of MgH2. J. Power Source 2019, 438, 227006. [Google Scholar] [CrossRef]
- Zhou, C.; Bowman, R.C., Jr.; Fang, Z.Z.; Lu, J.; Xu, L.; Sun, P.; Liu, H.; Wu, H.; Liu, Y. Amorphous TiCu-Based Additives for Improving Hydrogen Storage Properties of Magnesium Hydride. ACS Appl. Mater. Interfaces 2019, 11, 38868–38879. [Google Scholar] [CrossRef] [PubMed]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Jefferson, M. Sustainable energy development: Performance and prospects. Renew. Energy 2006, 31, 571–582. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, X.; Nyahuma, F.M.; Yan, N.; Xiao, J.; Su, S.; Zhang, L. Enhancing Hydrogen Storage Properties of MgH2 by Transition Metals and Carbon Materials: A Brief Review. Front. Chem. 2020, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Shen, N.; Chen, L.; Chen, Y.; Zhang, W. Effect of BiVO4 additive on the hydrogen storage properties of MgH2. Mater. Res. Bull. 2017, 89, 197–203. [Google Scholar] [CrossRef]
- Ouyang, L.Z.; Yang, X.S.; Zhu, M.; Liu, J.W.; Dong, H.W.; Sun, D.L.; Zou, J.; Yao, X.D. Enhanced Hydrogen Storage Kinetics and Stability by Synergistic Effects of in Situ Formed CeH2.73 and Ni in CeH2.73-MgH2-Ni Nanocomposites. J. Phys. Chem. C 2014, 118, 7808–7820. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, Y.F.; Zhang, X.; Hu, J.J.; Gao, M.X.; Pan, H.G. Empowering hydrogen storage performance of MgH2 by nanoengineering and nanocatalysis. Mater. Today Nano 2020, 9, 100064. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Z.; Yao, Z.; Yang, L.; Yan, N.; Lu, X.; Xiao, B.; Zhu, X.; Chen, L. Excellent catalysis of Mn3O4 nanoparticles on the hydrogen storage properties of MgH2: An experimental and theoretical study. Nanoscale Adv. 2020, 2, 1666–1675. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.C.; Wang, H.; Wang, X.H.; Liu, H.Z.; He, T.; Wang, Y.Y.; Wu, C.; Li, S.Q.; Yan, M. MoSe2 hollow nanospheres decorated with FeNi3 nanoparticles for enhancing the hydrogen storage properties of MgH2. J. Alloys Compd. 2020, 830, 154631. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Chen, J.; Guo, X.; Zhu, Y.; Li, L. Enhancing hydrogen storage performances of MgH2 by Ni nano-particles over mesoporous carbon CMK-3. Nanotechnology 2018, 29, 265705. [Google Scholar] [CrossRef]
- He, D.; Wang, Y.; Wu, C.; Li, Q.; Ding, W.; Sun, C. Enhanced hydrogen desorption properties of magnesium hydride by coupling non-metal doping and nano-confinement. Appl. Phys. Lett. 2015, 107, 243907. [Google Scholar] [CrossRef]
- Yu, H.; Bennici, S.; Auroux, A. Hydrogen storage and release: Kinetic and thermodynamic studies of MgH2 activated by transition metal nanoparticles. Int. J. Hydrogen Energy 2014, 39, 11633–11641. [Google Scholar] [CrossRef]
- Lu, C.; Ma, Y.; Li, F.; Zhu, H.; Zeng, X.; Ding, W.; Deng, T.; Wu, J.; Zou, J. Visualization of fast “hydrogen pump” in core-shell nanostructured Mg@Pt through hydrogen-stabilized Mg3Pt. J. Mater. Chem. A 2019, 7, 14629–14637. [Google Scholar] [CrossRef]
- Chen, M.; Xiao, X.; Zhang, M.; Liu, M.; Huang, X.; Zheng, J.; Zhang, Y.; Jiang, L.; Chen, L. Excellent synergistic catalytic mechanism of in-situ formed nanosized Mg2Ni and multiple valence titanium for improved hydrogen desorption properties of magnesium hydride. Int. J. Hydrogen Energy 2019, 44, 1750–1759. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, H.; Ouyang, L.; Zeng, M. Composite structure and hydrogen storage properties in Mg-base alloys. Int. J. Hydrogen Energy 2006, 31, 251–257. [Google Scholar] [CrossRef]
- Liao, B.; Lei, Y.Q.; Chen, L.X.; Lu, G.L.; Pan, H.G.; Wang, Q.D. Effect of the La/Mg ratio on the structure and electrochemical properties of LaxMg3-xNi9 (x = 1.6–2.2) hydrogen storage electrode alloys for nickel–metal hydride batteries. J. Power Source 2004, 129, 358–367. [Google Scholar] [CrossRef]
- Liang, G. Synthesis and hydrogen storage properties of Mg-based alloys. J. Alloys Compd. 2004, 370, 123–128. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, X.; Luo, B.; Liu, M.; Chen, M.; Chen, L. Superior de/hydrogenation performances of MgH2 catalyzed by 3D flower-like TiO2@C nanostructures. J. Energy Chem. 2020, 46, 191–198. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, L.; Yao, Z.; Yan, N.; Sun, Z.; Yang, X.; Zhu, X.; Hu, S.; Chen, L. Facile synthesized Fe nanosheets as superior active catalyst for hydrogen storage in MgH2. Int. J. Hydrogen Energy 2019, 44, 21955–21964. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, Z.; Yao, Z.; Ji, L.; Sun, Z.; Yan, N.; Zhang, B.; Xiao, B.; Du, J.; Zhu, X.; et al. A striking catalytic effect of facile synthesized ZrMn2 nanoparticles on the de/rehydrogenation properties of MgH2. J. Mater. Chem. A 2019, 7, 5626–5634. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Ren, Z.; Liu, Y.; Hu, J.; Li, H.; Gao, M.; Pan, H.; Liu, Y. In situ formed ultrafine NbTi nanocrystals from a NbTiC solid-solution MXene for hydrogen storage in MgH2. J. Mater. Chem. A 2019, 7, 14244–14252. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, G.; Zhang, Y.; Zhu, Y.; Li, L. Boosting low-temperature de/re-hydrogenation performances of MgH2 with Pd-Ni bimetallic nanoparticles supported by mesoporous carbon. Int. J. Hydrogen Energy 2019, 44, 10777–10787. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, R.; Zhu, Y.; Liu, Y.; Zhang, Y.; Li, S.; Li, L. Remarkable Synergistic Catalysis of Ni-Doped Ultrafine TiO2 on Hydrogen Sorption Kinetics of MgH2. ACS Appl. Mater. Interfaces 2018, 10, 24975–24980. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, G.; Tian, H.; Wang, Y. Effect of the hierarchical Co@C nanoflowers on the hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2017, 42, 28464–28472. [Google Scholar] [CrossRef]
- Su, W.; Zhu, Y.; Zhang, J.; Liu, Y.; Yang, Y.; Mao, Q.; Li, L. Effect of multi-wall carbon nanotubes supported nano-nickel and TiF3 addition on hydrogen storage properties of magnesium hydride. J. Alloys Compd. 2016, 669, 8–18. [Google Scholar] [CrossRef]
- Plerdsranoy, P.; Thiangviriya, S.; Dansirima, P.; Thongtan, P.; Kaewsuwan, D.; Chanlek, N.; Utke, R. Synergistic effects of transition metal halides and activated carbon nanofibers on kinetics and reversibility of MgH2. J. Phys. Chem. Solids 2019, 124, 81–88. [Google Scholar] [CrossRef]
- Ismail, M.; Mustafa, N.S.; Juahir, N.; Yap, F.A.H. Catalytic effect of CeCl3 on the hydrogen storage properties of MgH2. Mater. Chem. Phys. 2016, 170, 77–82. [Google Scholar] [CrossRef]
- Cui, J.; Liu, J.; Wang, H.; Ouyang, L.; Sun, D.; Zhu, M.; Yao, X. Mg-TM (TM: Ti, Nb, V, Co, Mo or Ni) core-shell like nanostructures: Synthesis, hydrogen storage performance and catalytic mechanism. J. Mater. Chem. A 2014, 2, 9645–9655. [Google Scholar] [CrossRef]
- Grzech, A.; Lafont, U.; Magusin, P.C.M.M.; Mulder, F.M. Microscopic Study of TiF3 as Hydrogen Storage Catalyst for MgH2. J. Phys. Chem. C 2012, 116, 26027–26035. [Google Scholar] [CrossRef]
- Ma, L.P.; Kang, X.D.; Dai, H.B.; Liang, Y.; Fang, Z.Z.; Wang, P.J.; Wang, P.; Cheng, H.M. Superior catalytic effect of TiF3 over TiCl3 in improving the hydrogen sorption kinetics of MgH2: Catalytic role of fluorine anion. Acta Mater. 2009, 57, 2250–2258. [Google Scholar] [CrossRef]
- Kim, J.W.; Ahn, J.P.; Jin, S.A.; Lee, S.H.; Chung, H.-S.; Shim, J.H.; Cho, Y.W.; Oh, K.H. Microstructural evolution of NbF5-doped MgH2 exhibiting fast hydrogen sorption kinetics. J. Power Source 2008, 178, 373–378. [Google Scholar] [CrossRef]
- Jangir, M.; Jain, A.; Yamaguchi, S.; Ichikawa, T.; Lal, C.; Jain, I.P. Catalytic effect of TiF4 in improving hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2016, 41, 14178–14183. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, H.; Yan, S.; Yin, L.R.; Zhou, D.W. Dehydrogenation properties and mechanisms of MgH2-NiCl2 and MgH2-NiCl2-graphene hydrogen storage composites. Met. Mater. Int. 2017, 23, 831–837. [Google Scholar] [CrossRef]
- Ismail, M. Influence of different amounts of FeCl3 on decomposition and hydrogen sorption kinetics of MgH2. Int. J. Hydrogen Energy 2014, 39, 2567–2574. [Google Scholar] [CrossRef]
- Mao, J.; Guo, Z.; Yu, X.; Liu, H.; Wu, Z.; Ni, J. Enhanced hydrogen sorption properties of Ni and Co-catalyzed MgH2. Int. J. Hydrogen Energy 2010, 35, 4569–4575. [Google Scholar] [CrossRef]
- Yao, P.; Jiang, Y.; Liu, Y.; Wu, C.; Chou, K.C.; Lyu, T.; Li, Q. Catalytic effect of Ni@rGO on the hydrogen storage properties of MgH2. J. Magnes. Alloy. 2020, 8, 461–471. [Google Scholar] [CrossRef]
- Luo, Q.; Gu, Q.; Liu, B.; Zhang, T.F.; Liu, W.; Li, Q. Achieving superior cycling stability by in situ forming NdH2-Mg-Mg2Ni nanocomposites. J. Mater. Chem. A 2018, 6, 23308–23317. [Google Scholar] [CrossRef]
- Jensen, F. Activation energies and the arrhenius equation. Qual. Reliab. Eng. Int. 1985, 1, 13–17. [Google Scholar] [CrossRef]
- Xia, G.; Tan, Y.; Chen, X.; Sun, D.; Guo, Z.; Liu, H.; Ouyang, L.; Zhu, M.; Yu, X. Monodisperse magnesium hydride nanoparticles uniformly self-assembled on graphene. Adv. Mater. 2015, 27, 5981–5988. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Zhang, L.; Yan, N.; Zheng, J.; Bian, T.; Yang, Z.; Su, S. Realizing Hydrogen De/Absorption Under Low Temperature for MgH2 by Doping Mn-Based Catalysts. Nanomaterials 2020, 10, 1745. https://doi.org/10.3390/nano10091745
Sun Z, Zhang L, Yan N, Zheng J, Bian T, Yang Z, Su S. Realizing Hydrogen De/Absorption Under Low Temperature for MgH2 by Doping Mn-Based Catalysts. Nanomaterials. 2020; 10(9):1745. https://doi.org/10.3390/nano10091745
Chicago/Turabian StyleSun, Ze, Liuting Zhang, Nianhua Yan, Jiaguang Zheng, Ting Bian, Zongming Yang, and Shichuan Su. 2020. "Realizing Hydrogen De/Absorption Under Low Temperature for MgH2 by Doping Mn-Based Catalysts" Nanomaterials 10, no. 9: 1745. https://doi.org/10.3390/nano10091745



