Aqueous Dilution of Noble NPs Bulk Dispersions: Modeling Instability due to Dissolution by AF4 and Stablishing Considerations for Plasmonic Assays
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instrumentation
2.3. TMB Assay
2.4. Plasmonic Assays
3. Results and Discussion
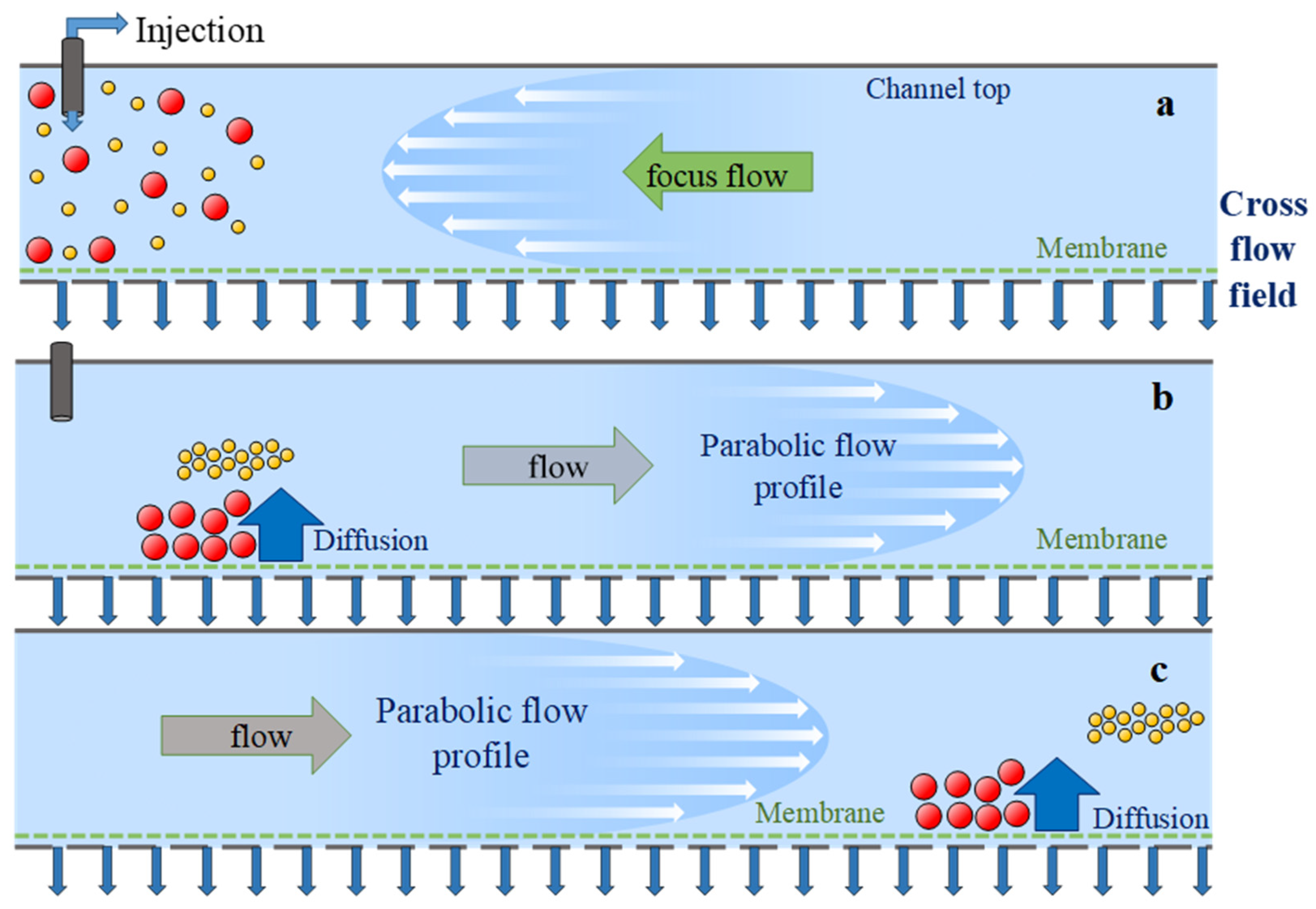
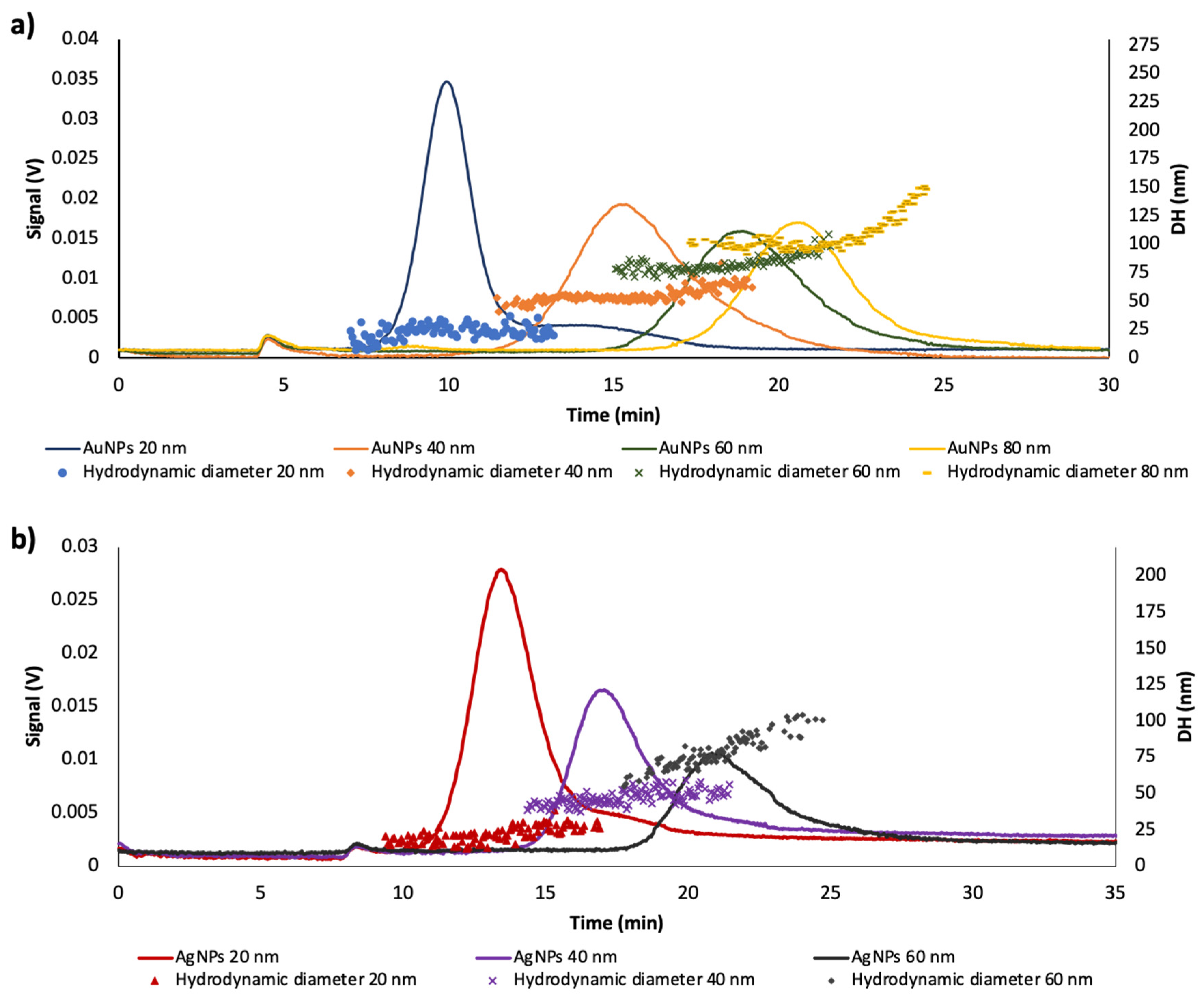
3.1. Characterization of Citrate-Capped—AuNPs and AgNPs Dispersions
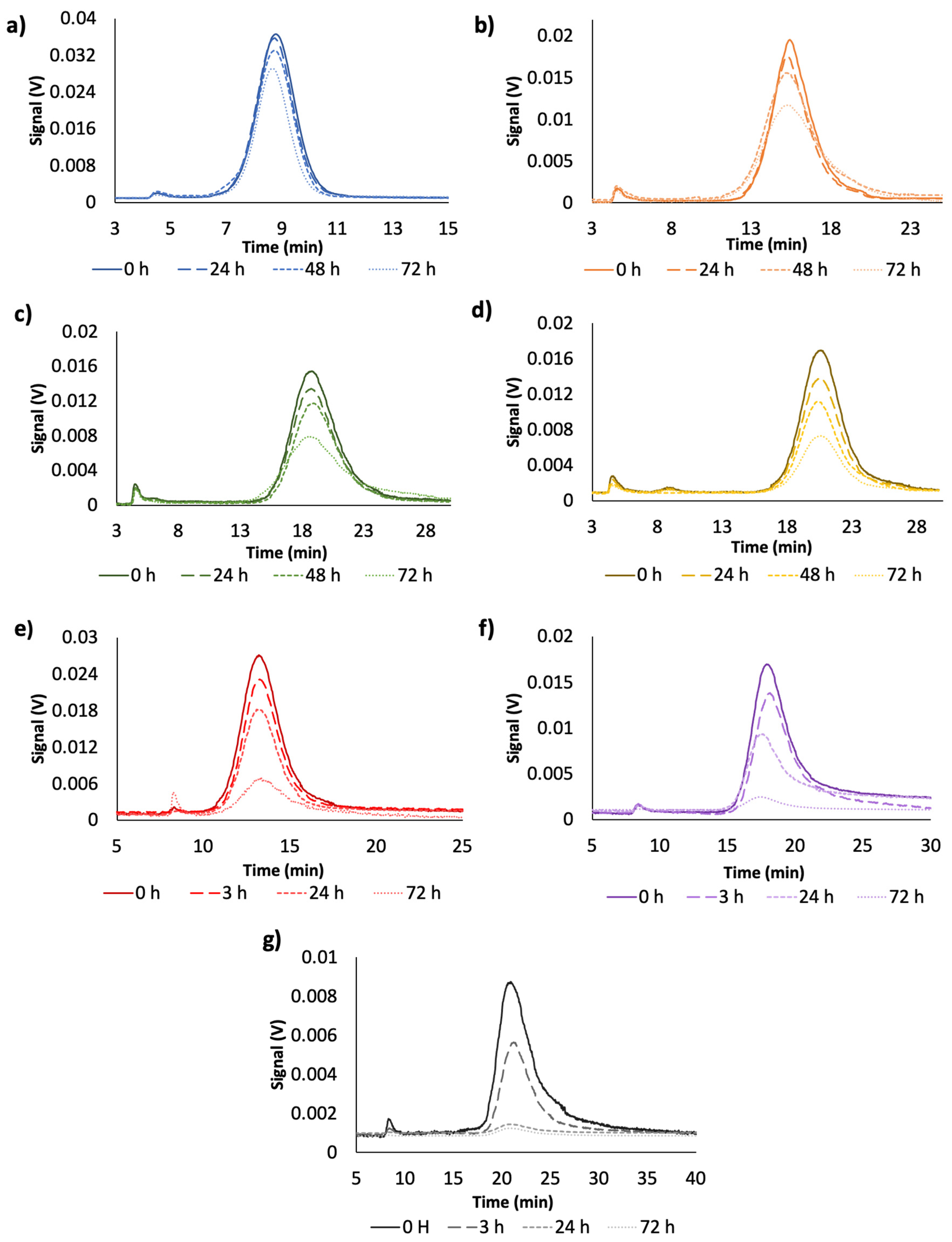
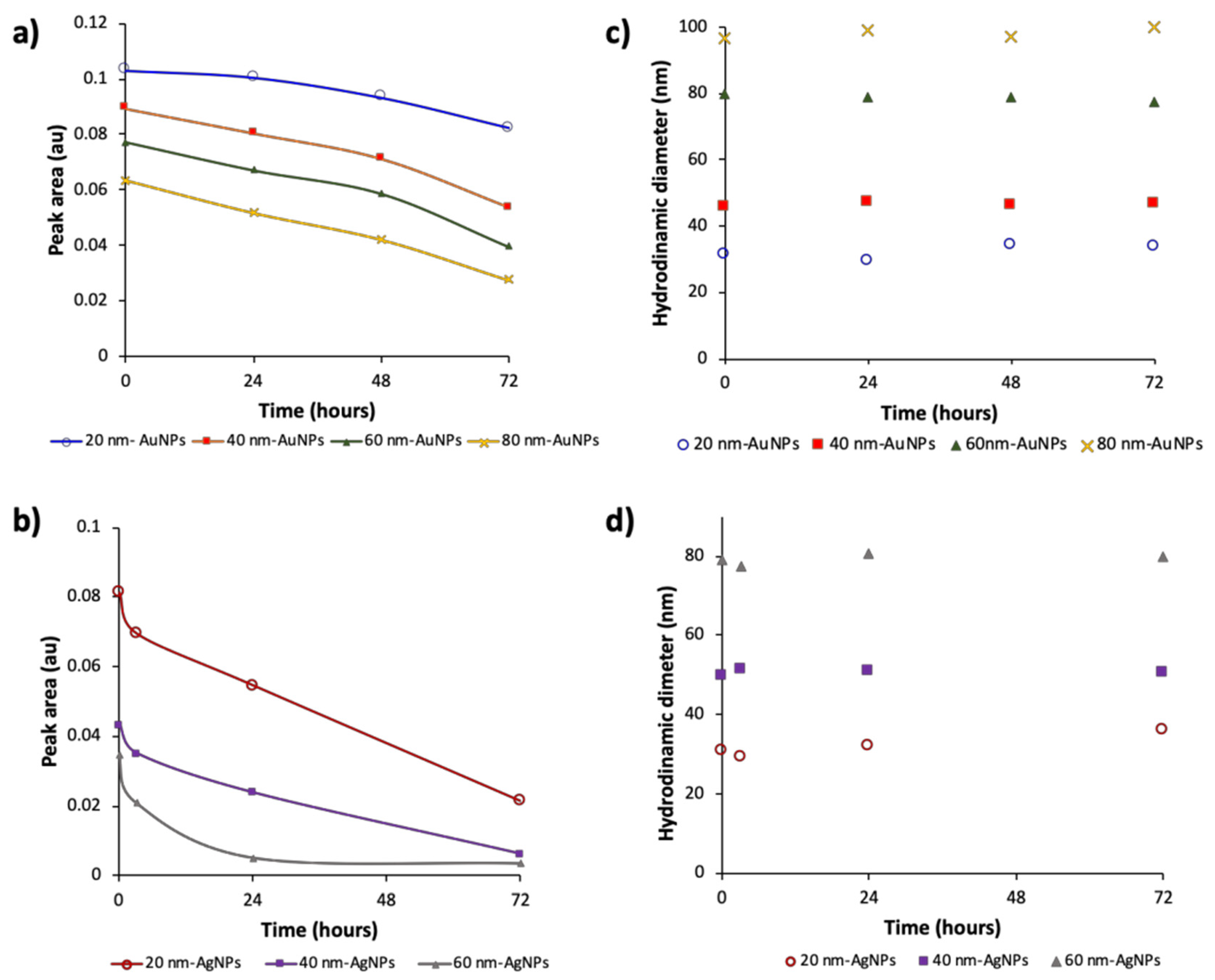
3.2. Effect of Time in the Analytical Responses of Diluted Dispersions of NPs
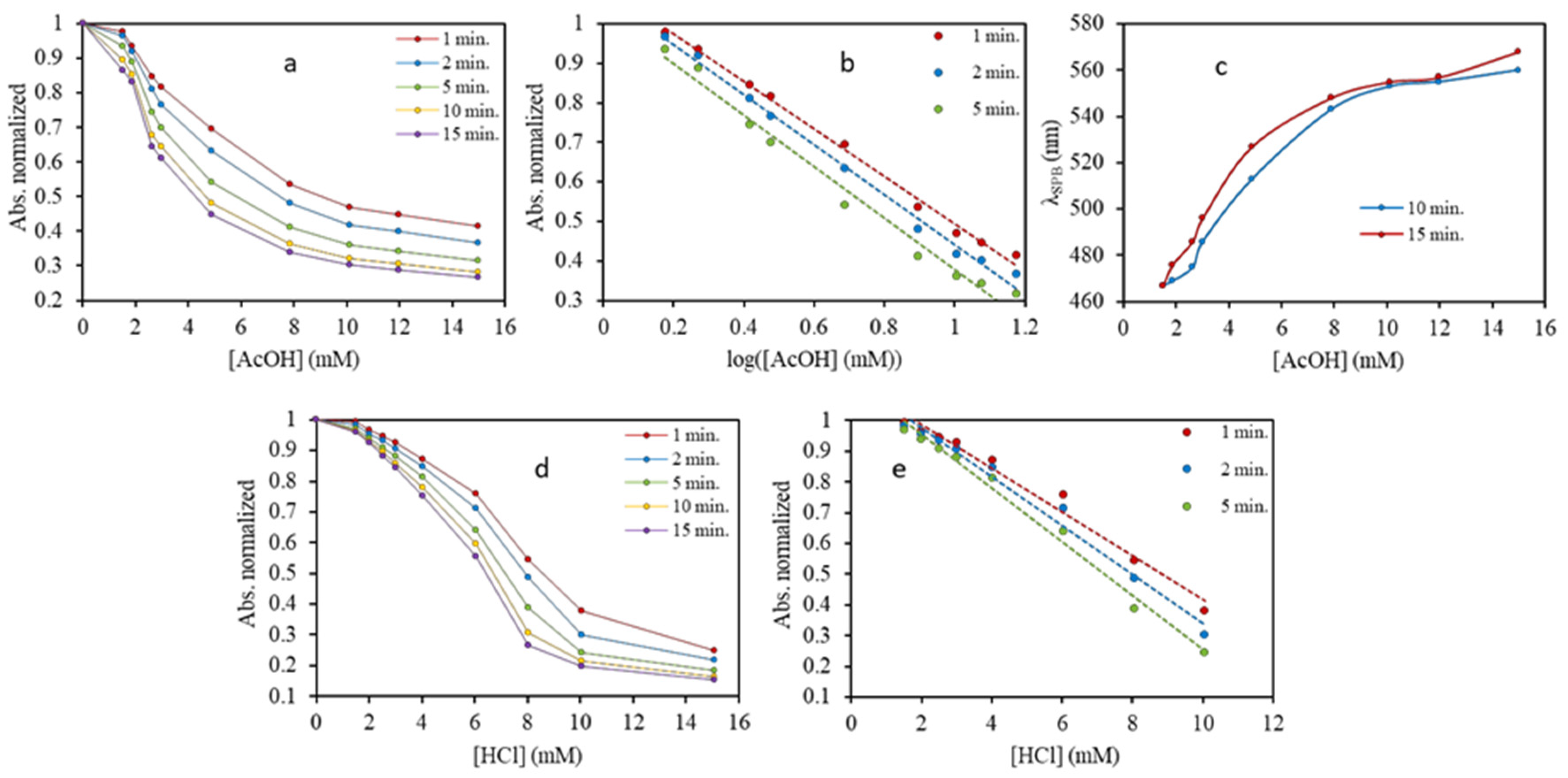
3.3. Plasmonic Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hinterwirth, H.; Wiedmer, S.K.; Moilanen, M.; Lehner, A.; Allmaier, G.; Waitz, T.; Linder, W.; Lämmerhofer, M. Comparative method evaluation for size and size-distribution analysis of gold nanoparticles. J. Sep. Sci. 2013, 36, 2952–2961. [Google Scholar] [CrossRef]
- Hone, D.C.; Walker, P.I.; Evans-Gowing, R.; FitzGerlad, S.; Beeby, A.; Chambrier, I. Generation of cytotoxic singlet oxygen via phthalocyanine-stabilized gold nanoparticles: A potential delivery vehicle for photodynamic therapy. Langmuir 2002, 18, 2985–2987. [Google Scholar] [CrossRef]
- Hutter, E.; Maysinger, D. Gold nanoparticles and quantum dots for bioimaging. Microsc. Res. Techniq. 2011, 74, 592–604. [Google Scholar] [CrossRef]
- Kneipp, J.; Kneipp, H.; Rice, W.L.; Kneipp, K. Optical probes for biological applications based on surface-enhanced raman scattering from indocyanine green on gold nanoparticles. Anal. Chem. 2005, 77, 2381–2385. [Google Scholar] [CrossRef]
- Saenmuangchin, R.; Siripinyanond, A. Flow field-flow fractionation for hydrodynamic diameter estimation of gold nanoparticles with various types of surface coatings. Anal. Bioanal. Chem. 2018, 410, 6845–6859. [Google Scholar] [CrossRef]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef]
- Chugh, H.; Sood, D.; Chandra, I.; Tomar, V.; Dhawan, G.; Chandra, R. Role of gold and silver nanoparticles in cancer nano-medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1210–1220. [Google Scholar] [CrossRef]
- González-Fuenzalida, R.A.; Sanjuan-Navarro, L.; Moliner-Martínez, Y.; Campíns-Falcó, P. Quantitative study of the capture of silver nanoparticles by several kinds of soils. Sci. Total Environ. 2018, 630, 1226–1236. [Google Scholar] [CrossRef]
- Dueñas-Mas, M.J.; Soriano, M.L.; Ruiz-Palomero, C.; Valcárcel, M. Modified nanocellulose as promising material for the extraction of gold nanoparticles. Microchem. J. 2018, 138, 379–383. [Google Scholar] [CrossRef]
- González-Fuenzalida, R.A.; Moliner-Martínez, Y.; Molins-Legua, C.; Campíns-Falcó, P. Miniaturized liquid chromatography coupled on-line to in-tube solid-pase microextraction for characterization of metallic nanoparticles using plasmonic measurements. A tutorial. Anal. Chim. Acta 2019, 1045, 23–41. [Google Scholar] [CrossRef]
- Meisterjahn, B.; Wagner, S.; Kammer, F.; Hennecke, D.; Hofmann, T. Silver and gold nanoparticles separation using asymmetrical flow-field flow fractionation: Influence of run conditions and of particle membrane charges. J. Chromatogr. A 2016, 1440, 150–159. [Google Scholar] [CrossRef]
- Malik, M.I.; Pasch, H. Field-flow fractionation: New and exciting perspectives in polymer analysis. Prog. Polym. Sci. 2016, 63, 42–85. [Google Scholar] [CrossRef]
- Irfan, M.; Moniruzzaman, M.; Ahmad, T.; Osman, O.Y.; Mandal, P.C.; Bhattacharjee, S.; Hussain, M. Stability, interparticle interactions and catalytic performance of gold nanoparticles synthesized through ionic liquid mediated oil palm leaves extract. J. Environ. Chem. Eng. 2018, 6, 5024–5031. [Google Scholar] [CrossRef]
- Rouhaba, L.L.; Jaber, J.A.; Schlenoff, J.B. Aggregation-Resistant Water-Soluble Gold Nanoparticles. Langmuir 2007, 23, 12799–12801. [Google Scholar]
- Baalousha, M.; Arkill, K.P.; Romer, I.; Palmer, R.E.; Lead, J.R. Transformations of citrate and Tween coated silver nanoparticles reacted with Na2S. Sci. Total Environ. 2015, 502, 344–353. [Google Scholar] [CrossRef]
- González-Fuenzalida, R.A.; Moliner-Martínez, Y.; González-Béjar, M.; Molins-Legua, C.; Verdú-Andrés, J.; Pérez-Prieto, J.; Campíns-Falcó, P. In Situ Colorimetric Quantification of Silver Cations in the Presence of Silver Nanoparticles. Anal. Chem. 2013, 85, 10013–10016. [Google Scholar] [CrossRef]
- Calzolai, L.; Gilliland, D.; Pascual Garcia, C.; Rossi, F. Separation and characterization of gold nanoparticle mixtures by flow-field-flow fractionation. J. Chromatogr. A 2011, 1218, 4234–4239. [Google Scholar] [CrossRef]
- Jochem, A.R.; Ankah, G.N.; Meyer, L.A.; Elsenberg, S.; Johann, C.; Kraus, T. Colloidal Mechanisms of Gold Nanoparticles Loss in Asymmetric Flow Field-Flow Fractionation. Anal. Chem. 2016, 88, 10065–10073. [Google Scholar] [CrossRef]
- Chang, Y.J.; Shih, Y.H.; Su, C.H.; Ho, H.C. Comparison of three analytical methods to measure the size of silver nanoparticles in real environmental water and wastewater samples. J. Hazard. Mater. 2017, 322, 95–104. [Google Scholar] [CrossRef]
- Yang, Y.; Long, C.L.; Li, H.P.; Wang, Q.; Yang, Z.G. Analysis of silver and gold nanoparticles in environmental water using single particle-inductively coupled plasma-mass spectrometry. Sci. Total Environ. 2016, 563, 996–1007. [Google Scholar] [CrossRef]
- Baiee, R.; Liu, Z.; Li, L. Understanding the stability and durability of laser-generated Ag nanoparticles and effects on their antibacterial activities. Adv. Nat. Sci Nanosci. 2019, 10, 035001. [Google Scholar] [CrossRef]
- Espinoza, M.G.; Hinks, M.L.; Mendoza, A.M.; Pullman, D.P.; Peterson, K.I. Kinetics of Halide-Induced Decomposition and Aggregation of Silver Nanoparticles. J. Phys. Chem. C 2012, 116, 8305–8313. [Google Scholar] [CrossRef]
- Li, X.; Lenhart, J.J.; Walker, H.W. Dissolution-Accompanied Aggregation Kinetics of Silver Nanoparticles. Langmuir 2010, 26, 16690–16698. [Google Scholar] [CrossRef] [PubMed]
- Henglein, A. Colloidal Silver Nanoparticles: Photochemical Preparation and Interaction with O2, CCl4, and Some Metal Ions. Chem. Mater. 1998, 10, 444–450. [Google Scholar] [CrossRef]
- Yin, Y.; Li, Z.; Zhong, Z.; Gates, B.; Xia, Y.; Venkateswaran, S. Synthesis and characterization of stable aqueous dispersions of silver nanoparticles through the Tollens process. J. Mater. Chem. 2002, 12, 522–527. [Google Scholar] [CrossRef]
- Axson, J.L.; Stark, D.I.; Bondy, A.L.; Capracotta, S.S.; Maynard, A.D.; Philbert, M.A.; Bergin, I.L.; Ault, A.P. Rapid Kinetics of Size and pH-Dependent Dissolution and Aggregation of Silver Nanoparticles in Simulated Gastric Fluid. J. Phys. Chem. C 2015, 119, 20632–20641. [Google Scholar] [CrossRef]
- Prathna, T.C.; Chandrasekaran, N.; Mukherjee, A. Studies on aggregation behaviour of silver nanoparticles in aqueous matrices: Effect of surface functionalization and matrix composition. Colloids Surf. A Physicochem. Eng. Asp. 2011, 390, 216–224. [Google Scholar] [CrossRef]
- El-Badawy, A.M.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of Environmental Conditions (pH, Ionic Strength, and Electrolyte Type) on the Surface Charge and Aggregation of Silver Nanoparticles Suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef]
- Rogers, K.R.; Bradham, K.; Tolaymat, T.; Thomas, D.J.; Hartmann, T.; Ma, L.; Williams, A. Alterations in physical state of silver nanoparticles exposed to synthetic human stomach fluid. Sci. Total Environ. 2012, 420, 334–339. [Google Scholar] [CrossRef]
- Mwilu, S.K.; El-Badawy, A.M.; Bradham, K.; Nelson, C.; Thomas, D.; Scheckel, K.G.; Tolaymat, T.; Ma, L.; Rogers, K.R. Changes in silver nanoparticles exposed to human synthetic stomach fluid: Effects of particle size and surface chemistry. Sci. Total Environ. 2013, 447, 90–98. [Google Scholar] [CrossRef]
- Liu, J.; Hurt, R.H. Ion Release Kinetics and Particle Persistence in Aqueous Nano-Silver Colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Jornet-Martínez, N.; González-Béjar, M.; Moliner-Martínez, Y.; Campins-Falcó, P.; Pérez-Prieto, J. Sensitive and Selective Plasmonic Assay for Spermine as Biomarker in Human Urine. Anal. Chem. 2014, 86, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Jornet-Martínez, N.; Hakobyan, L.; Argente-García, A.I.; Molins-Legua, C.; Campins-Falcó, P. Nylon-Supported Plasmonic Assay Based on the Aggregation of Silver Nanoparticles: In Situ Determination of Hydrogen Sulfide-like Compounds in Breath Samples as a Proof of Concept. ACS Sens. 2019, 4, 2164–2172. [Google Scholar] [CrossRef] [PubMed]

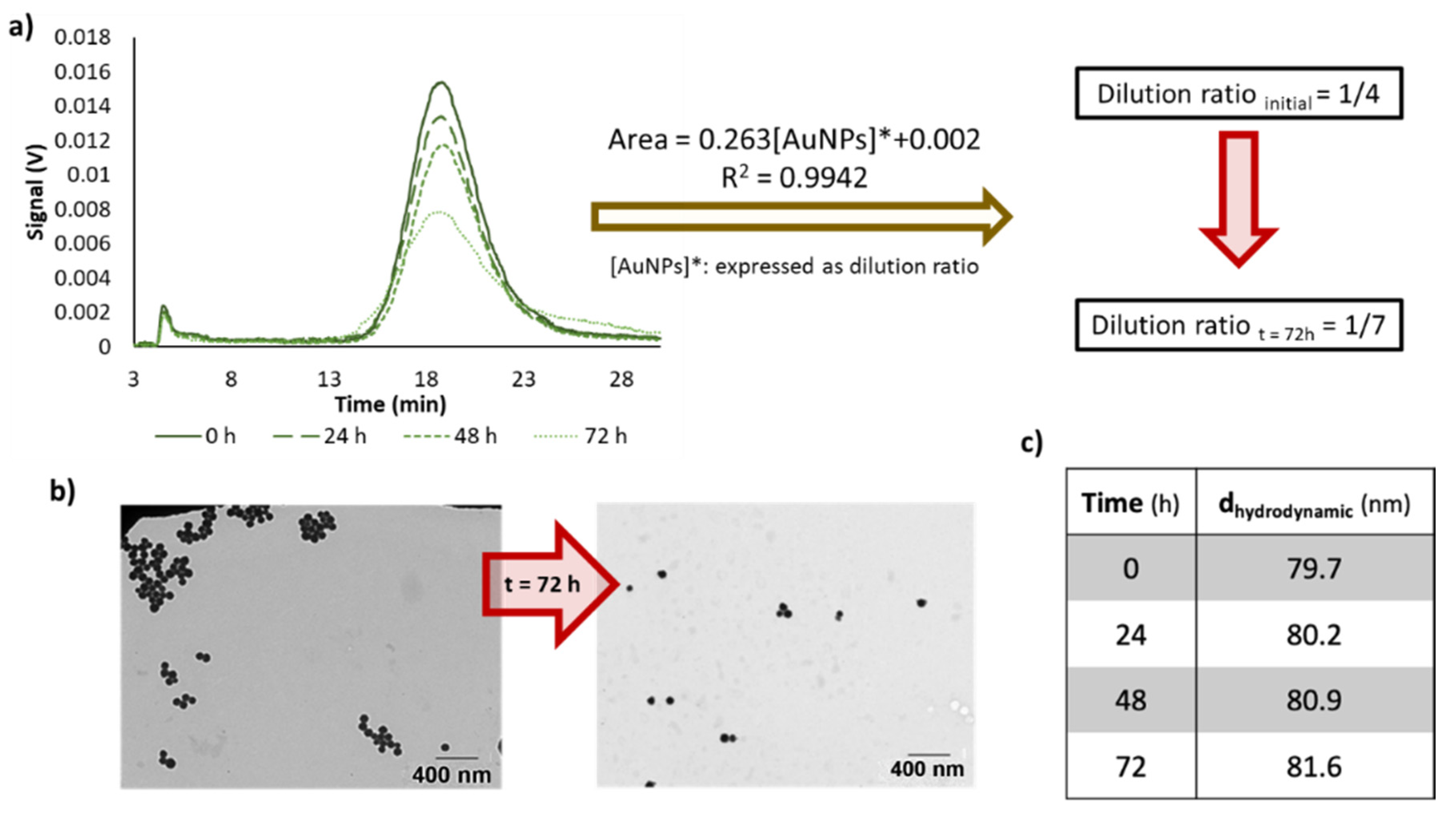
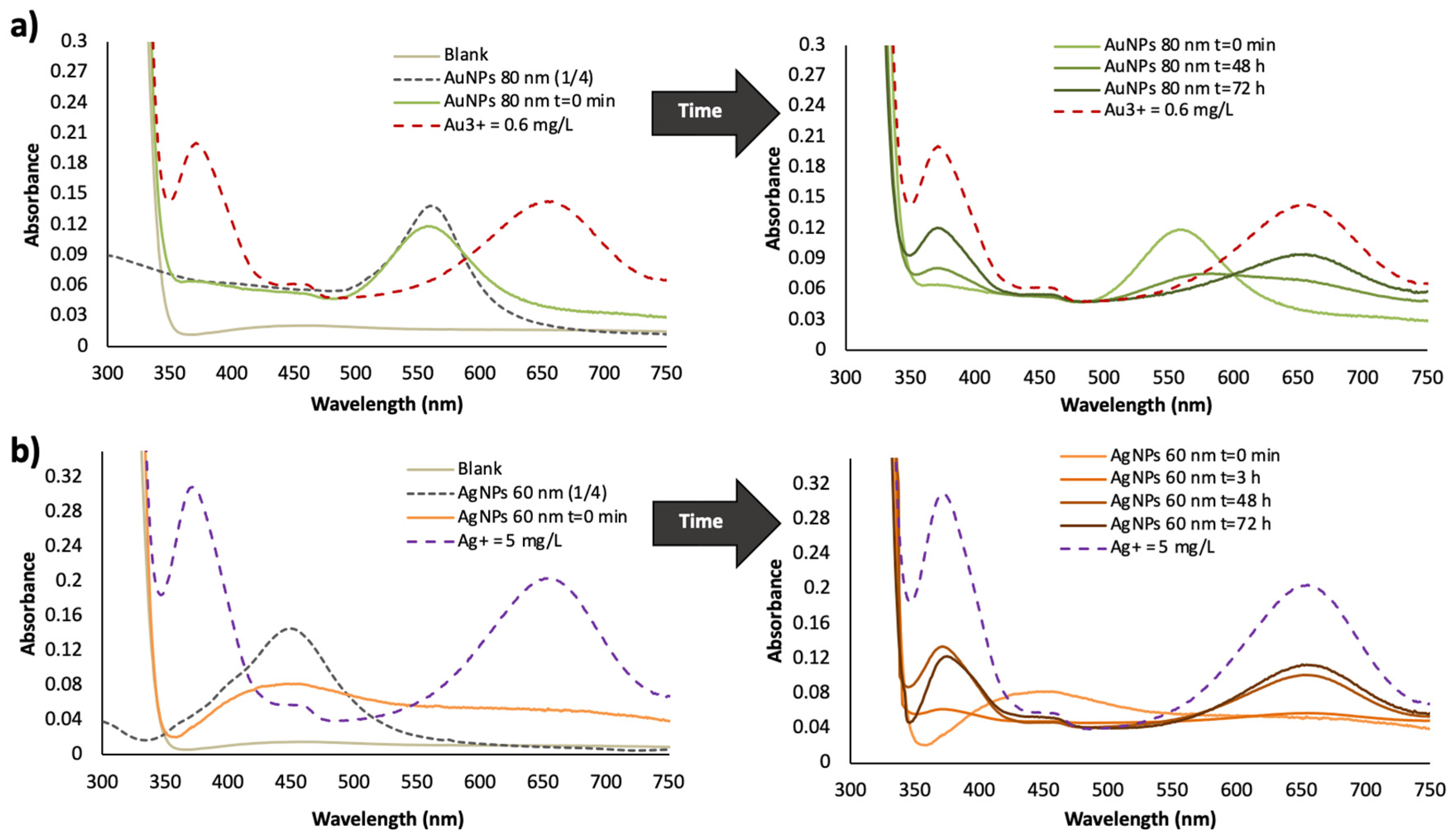
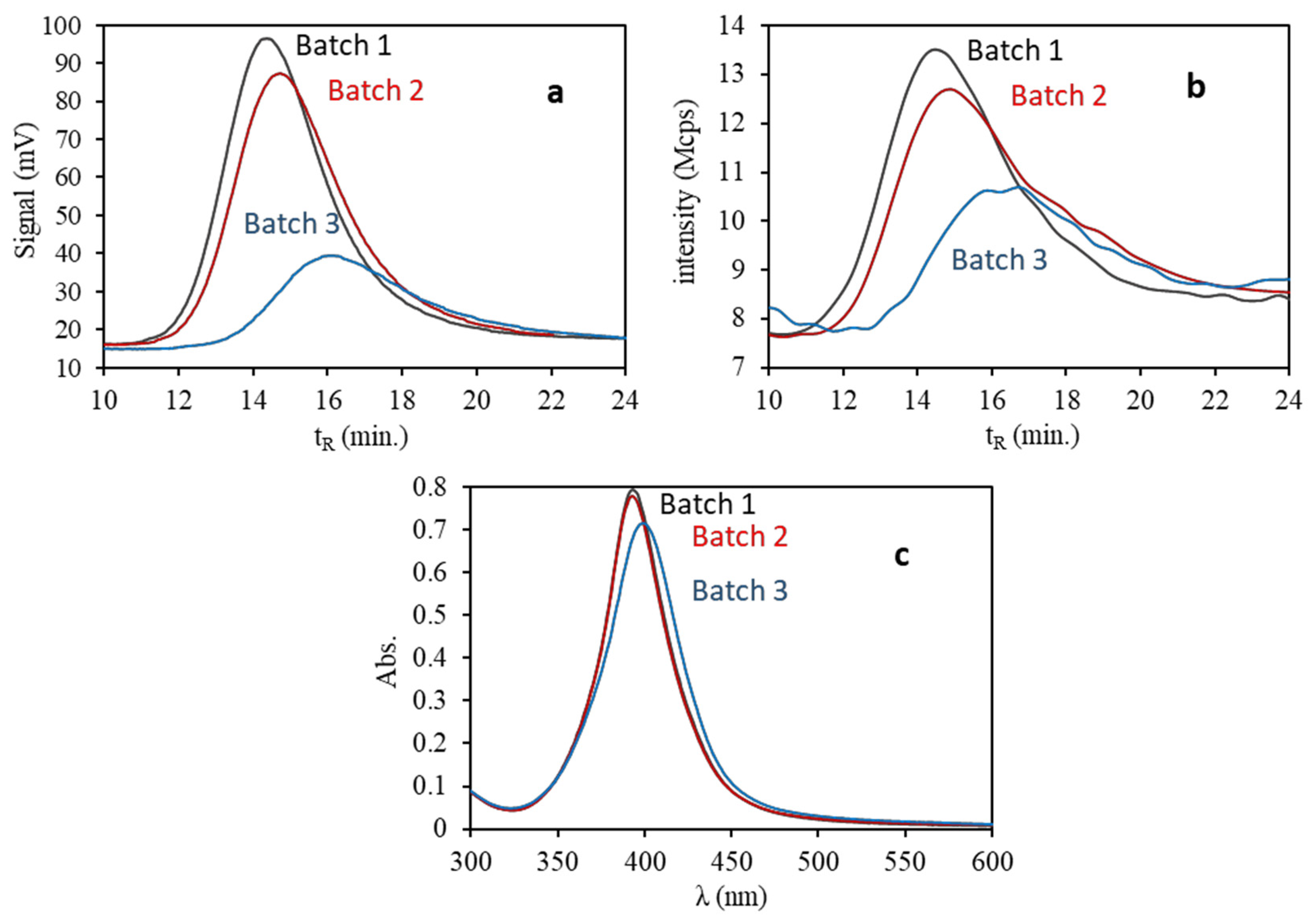
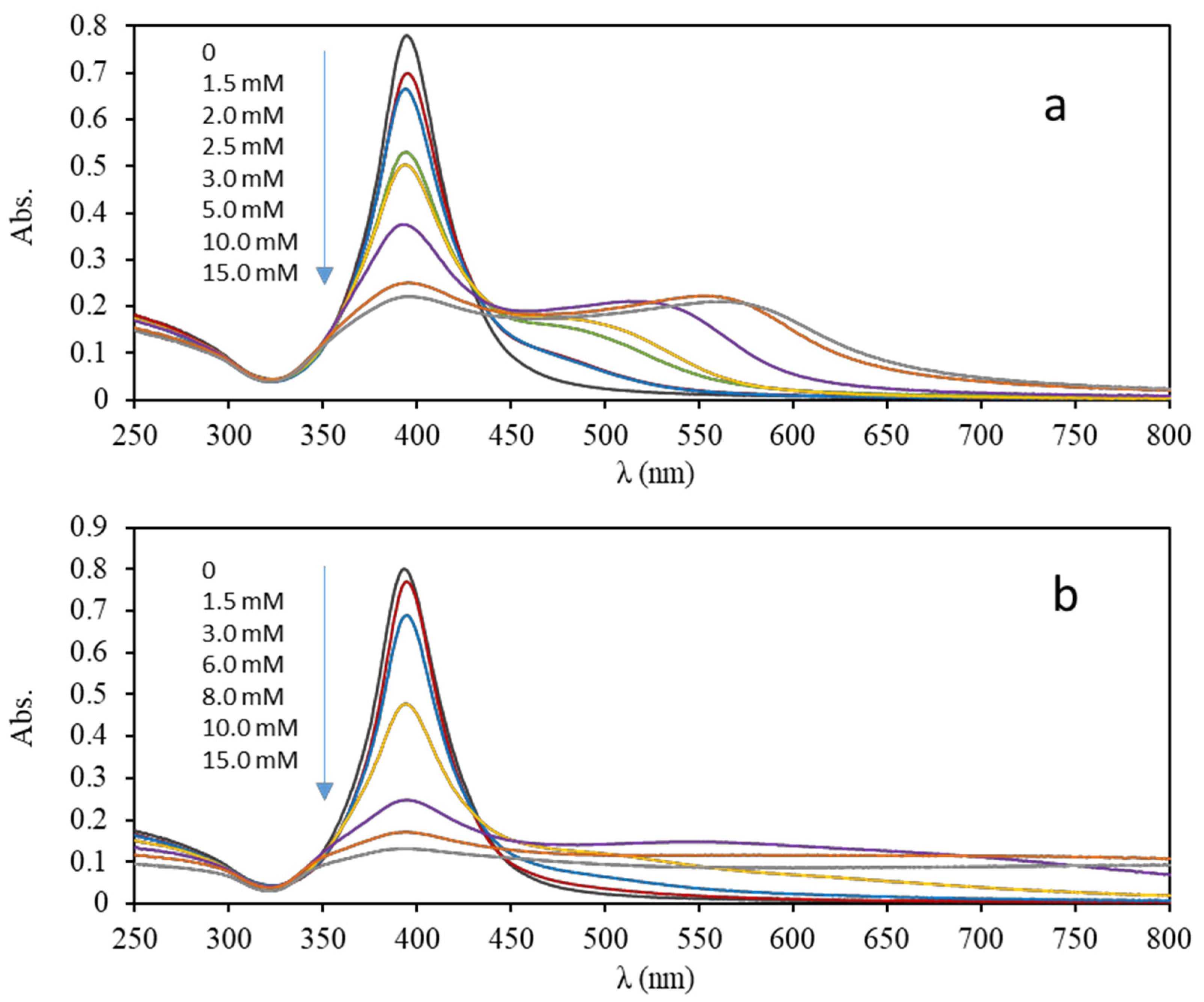

| Dilution Ratio | Concentration 102 (mg/mL) | A ± SA | B ± SB (%−1) | R2 | RSD (%) | |
|---|---|---|---|---|---|---|
| AuNPs 20 nm | 1/2 to 1/10 | 2.66 to 0.53 | 0.002 ± 0.004 | 0.409 ± 0.015 | 0.9961 | 2.2 |
| AuNPs 40 nm | 1/2 to 1/8 | 2.33 to 0.58 | 0.002 ± 0.003 | 0.338 ± 0.008 | 0.9988 | 5.3 |
| AuNPs 60 nm | 1/2 to 1/8 | 2.15 to 0.54 | 0.002 ± 0.005 | 0.262 ± 0.014 | 0.9942 | 6.3 |
| AuNPs 80 nm | 1/2 to 1/8 | 2.03 to 0.51 | 0.000 ± 0.005 | 0.233 ± 0.016 | 0.9911 | 6.4 |
| AgNPs 20 nm | 1/2 to 1/8 | 1.00 to 0.25 | 0.001 ± 0.005 | 0.339 ± 0.019 | 0.9904 | 3.1 |
| AgNPs 40 nm | 1/2 to 1/6 | 1.00 to 0.33 | 0.001 ± 0.004 | 0.171 ± 0.013 | 0.9883 | 9.9 |
| AgNPs 60 nm | 1/2 to 1/6 | 1.00 to 0.33 | 0.002 ± 0.003 | 0.119 ± 0.009 | 0.9890 | 10.1 |
| Co (mg/mL) | LOD (µg/mL) t = 0 min | LOD (µg/mL) t = 72 | ||
|---|---|---|---|---|
| AuNPs | 20 nm | 0.053 | 0.22 | 0.26 |
| 40 nm | 0.047 | 0.12 | 0.17 | |
| 60 nm | 0.043 | 0.14 | 0.21 | |
| 80 nm | 0.041 | 0.06 | 0.09 | |
| AgNPs | 20 nm | 0.020 | 0.10 | 0.18 |
| 40 nm | 0.020 | 0.23 | 0.43 | |
| 60 nm | 0.020 | 0.47 | 0.89 |
| Batch | λ (nm) | Width1/2 (nm) | εmax (mM−1·cm−1) | DH a (nm) |
|---|---|---|---|---|
| 3 | 397.6 ± 2.4 | 50.2 ± 0.1 | 15.43 ± 0.02 | 22.3 ± 2.4 |
| 2 | 393.4 ± 2.0 | 42.7 ± 0.2 | 16.83 ± 0.05 | 20.5 ± 3.1 |
| 1 | 393.1 ± 2.2 | 42.8 ± 0.2 | 17.05 ± 0.03 | 20.1 ± 3.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanjuan-Navarro, L.; Boughbina-Portolés, A.; Moliner-Martínez, Y.; Campíns-Falcó, P. Aqueous Dilution of Noble NPs Bulk Dispersions: Modeling Instability due to Dissolution by AF4 and Stablishing Considerations for Plasmonic Assays. Nanomaterials 2020, 10, 1802. https://doi.org/10.3390/nano10091802
Sanjuan-Navarro L, Boughbina-Portolés A, Moliner-Martínez Y, Campíns-Falcó P. Aqueous Dilution of Noble NPs Bulk Dispersions: Modeling Instability due to Dissolution by AF4 and Stablishing Considerations for Plasmonic Assays. Nanomaterials. 2020; 10(9):1802. https://doi.org/10.3390/nano10091802
Chicago/Turabian StyleSanjuan-Navarro, Lorenzo, Aaron Boughbina-Portolés, Yolanda Moliner-Martínez, and Pilar Campíns-Falcó. 2020. "Aqueous Dilution of Noble NPs Bulk Dispersions: Modeling Instability due to Dissolution by AF4 and Stablishing Considerations for Plasmonic Assays" Nanomaterials 10, no. 9: 1802. https://doi.org/10.3390/nano10091802
APA StyleSanjuan-Navarro, L., Boughbina-Portolés, A., Moliner-Martínez, Y., & Campíns-Falcó, P. (2020). Aqueous Dilution of Noble NPs Bulk Dispersions: Modeling Instability due to Dissolution by AF4 and Stablishing Considerations for Plasmonic Assays. Nanomaterials, 10(9), 1802. https://doi.org/10.3390/nano10091802






