Eco-Structured Adsorptive Removal of Tigecycline from Wastewater: Date Pits’ Biochar versus the Magnetic Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Reagents, Equipment, and Software
2.2. Preparation of the Biochar (BCDP)
2.3. Preparation of the Magnetic Biochar (MBC-DP)
2.4. Sorption Equilibrium and Kinetic Studies
2.5. Box–Behnken (BB) Design
3. Results and Discussion
3.1. Adsorbents’ Characterization
3.1.1. FT-IR and Raman Spectroscopic Analyses
3.1.2. SEM, EDX, and TEM Analyses
3.1.3. Brunauer–Emmett–Teller (BET) Analysis
3.2. Response Surface Methodology (RSM)
3.3. Response Modelling and Statistical Analysis
− 0.003643 [TIGC] × pH − 0.000549 [TIGC] × AD + 0.001939 CT × pH + 0.001994 pH × AD,
− 0.000223 [TIGC]2 + 0.000049 CT2 + 0.01328 pH2 + 0.000177 AD2
− 0.000035 [TIGC] × CT − 0.000499 [TIGC] × pH − 0.000356 [TIGC] × AD + 0.000774 CT × pH − 0.000027 CT × AD + 0.000470 pH × AD,
− 0.1113 pH2 − 0.000104 AD2 + 0.000004 [TIGC] × CT
+ 0.001835 [TIGC] × pH + 0.000128 [TIGC] × AD − 0.001038 CT × pH − 0.000045 CT × AD
+ 0.000580 pH × AD,
− 0.000127 [TIGC]2 + 0.000034 CT2 − 0.03805 pH2 + 0.000131 AD2
− 0.000001 [TIGC] × CT + 0.002082 [TIGC] × pH − 0.000083 [TIGC] × AD − 0.000328 CT × pH − 0.000017 CT × AD − 0.001153 pH × AD,
3.4. Response Optimzation
3.5. Equilibrium and Kinetics Studies
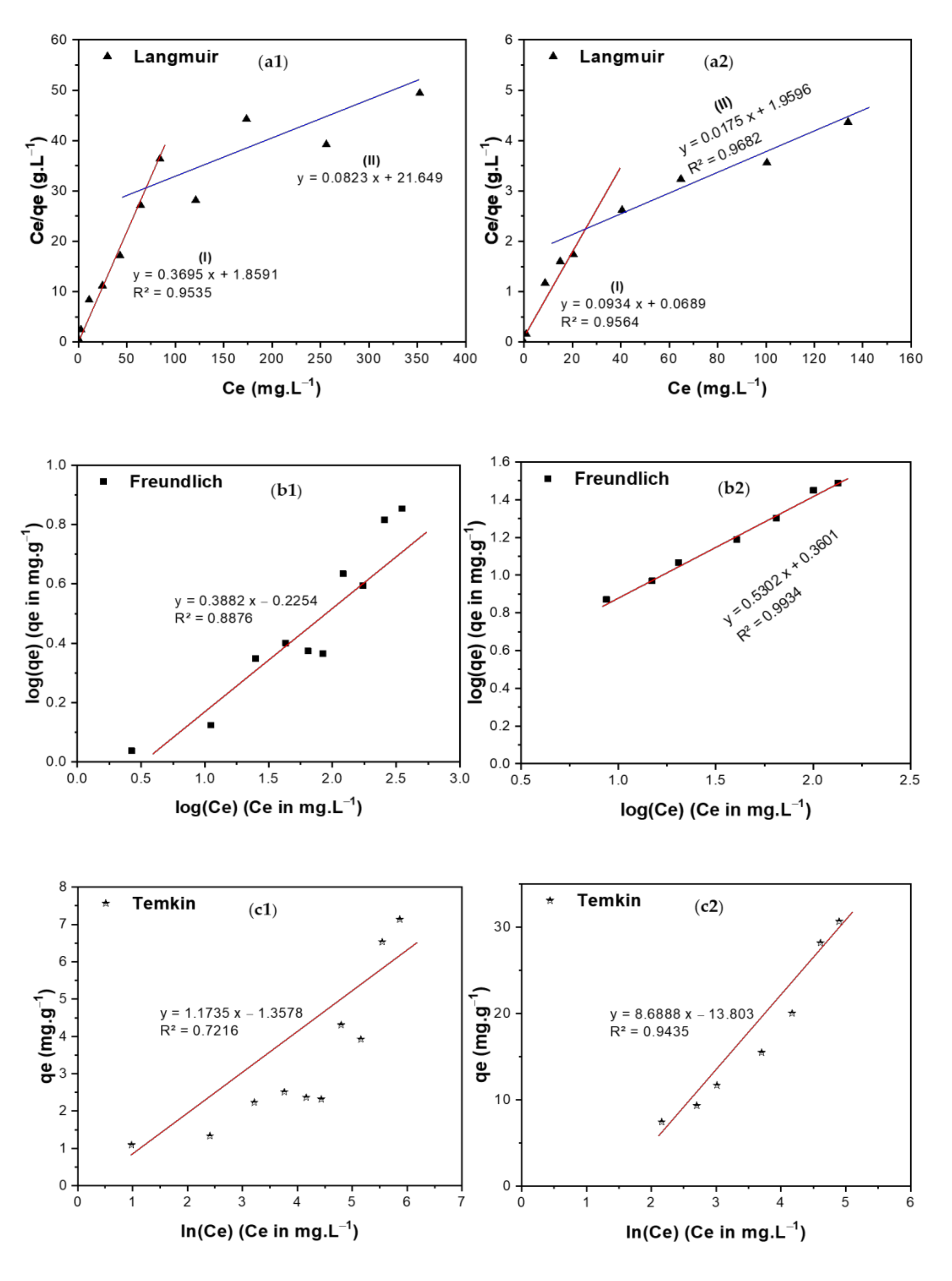
3.5.1. Equilibrium Isotherms
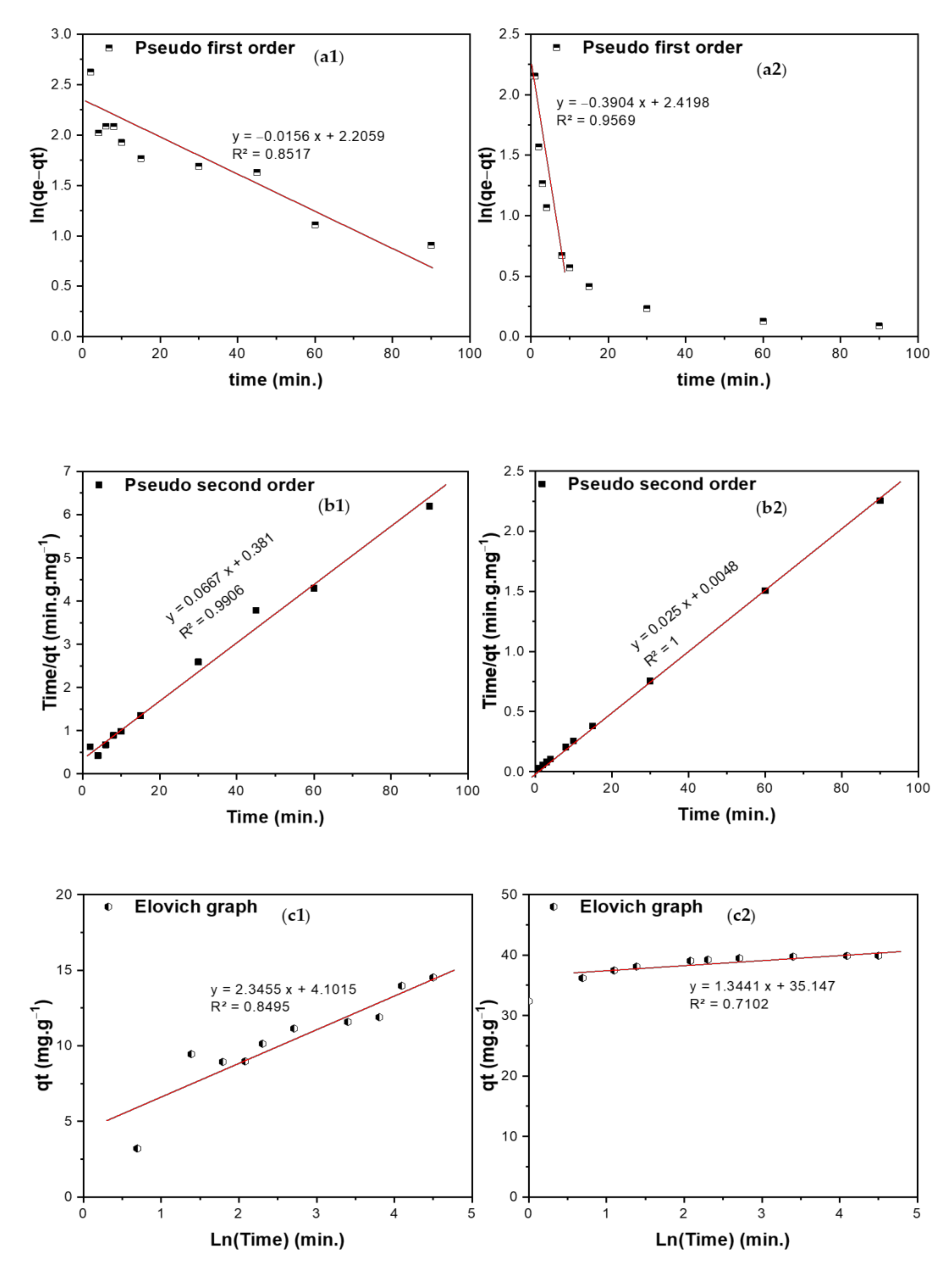
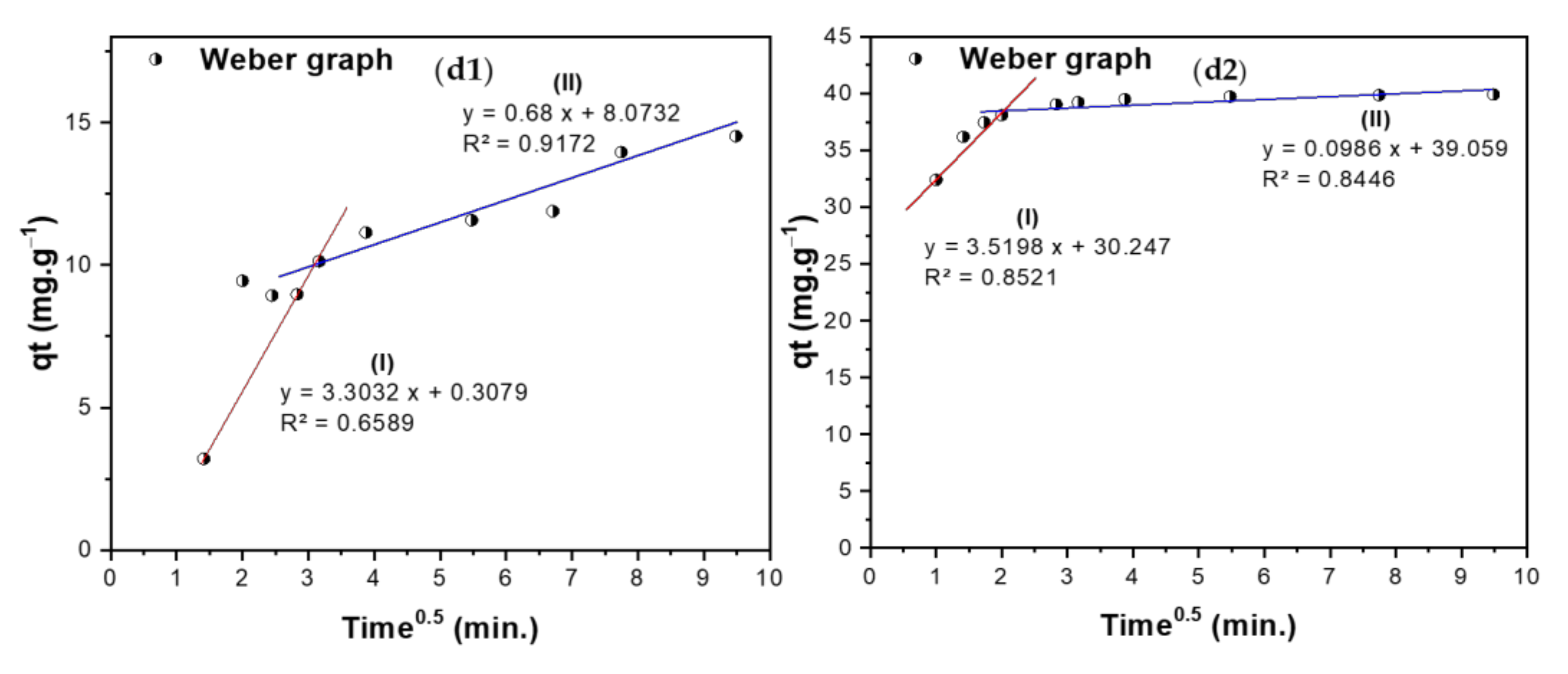
3.5.2. Kinetic Studies
3.6. Proposed Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Gendy, A.; El-Shafie, A.S.; Issa, A.; Al-Meer, S.; Al-Saad, K.; El-Azazy, M. Carbon-Based Materials (CBMS) for Determination and Remediation of Antimicrobials in Different Substrates: Wastewater and Infant Foods as Examples. In Carbon-Based Material for Environmental Protection and Remediation; Bartoli, M., Frediani, M., Rosi, L., Eds.; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/books/carbon-based-material-for-environmental-protection-and-remediation/carbon-based-materials-cbms-for-determination-and-remediation-of-antimicrobials-in-different-substrates (accessed on 10 November 2020). [CrossRef]
- Di Marcantonio, C.; Chiavola, A.; Dossi, S.; Cecchini, G.; Leoni, S.; Frugis, A.; Spizzirri, M.; Boni, M.R. Occurrence, Seasonal Variations and Removal of Organic Micropollutants in 76 Wastewater Treatment Plants. Process Saf. Environ. Prot. 2020, 141, 61–72. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of Pharmaceutical Compounds in Urban Wastewater: Removal, Mass Load and Environmental Risk after a Secondary Treatment—A Review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Rangel, A.; Sánchez-Polo, M.; Polo, A.M.S.; Rivera-Utrilla, J.; Berber-Mendoza, M.S. Sulfonamides Degradation Assisted by UV, UV/H2O2 and UV/K2S2O8: Efficiency, Mechanism and Byproducts Cytotoxicity. J. Environ. Manag. 2018, 225, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, A.J.; Murby, E.J.; Kolpin, D.W.; Costanzo, S.D. The Occurrence of Antibiotics in an Urban Watershed: From Wastewater to Drinking Water. Sci. Total Environ. 2009, 407, 2711–2723. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. MMBR 2001, 65, 232–260. [Google Scholar] [CrossRef] [Green Version]
- Elazazy, M.S. Interaction of Tetracycline Hydrochloride with Iron: Kinetic Spectrophotometric and Conductometric Investigations. Int. J. Pharm. Sci. Res. 2015, 6, 1022–1032. [Google Scholar]
- Priya, S.S.; Radha, K.V. A Review on the Adsorption Studies of Tetracycline onto Various Types of Adsorbents. Chem. Eng. Commun. 2017, 204, 8–821. [Google Scholar] [CrossRef]
- Kim, S.; Jensen, J.N.; Aga, D.S.; Weber, A.S. Tetracycline as a Selector for Resistant Bacteria in Activated Sludge. Chemosphere 2007, 66, 1643–1651. [Google Scholar] [CrossRef]
- WHO. Global Strategy for Containment of Antimicrobial Resistance, Executive Summary 2001; World Health Organization (WHO): Geneva, Switzerland, 2001. [Google Scholar]
- Chiavola, A.; Tedesco, P.; Boni, M.R. Fate of Selected Drugs in the Wastewater Treatment Plants (WWTPs) for Domestic Sewage. Environ. Sci. Pollut. Res. 2019, 26, 1113–1123. [Google Scholar] [CrossRef] [Green Version]
- Dorn, C.; Kratzer, A.; Liebchen, U.; Schleibinger, M.; Murschhauser, A.; Schlossmann, J.; Kees, F.; Simon, P.; Kees, M.G. Impact of Experimental Variables on the Protein Binding of Tigecycline in Human Plasma as Determined by Ultrafiltration. J. Pharm. Sci. 2018, 107, 739–744. [Google Scholar] [CrossRef]
- Drug Approval Package: Tygacil (Tigecycline) NDA #021821. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/21-821_Tygacil.cfm (accessed on 3 November 2020).
- Rose, W.; Rybak, M. Tigecycline: First of a New Class of Antimicrobial Agents. Pharmacotherapy 2006, 26, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Critically Important Antimicrobials for Human Medicine (6th Revision ed.); World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- TYGACIL U.S. Physician Prescribing Information, Pfizer. Available online: https://www.pfizer.com/products/product-detail/tygacil (accessed on 31 October 2020).
- U.S. Food and Drug Administration (FDA). FDA Drug Safety Communication: Increased Risk of Death with Tygacil (Tigecycline) Compared to Other Antibiotics Used to Treat Similar Infections. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-increased-risk-death-tygacil-tigecycline-compared-other-antibiotics (accessed on 2 November 2020).
- Fang, L.-X.; Chen, C.; Cui, C.-Y.; Li, X.-P.; Zhang, Y.; Liao, X.-P.; Sun, J.; Liu, Y.-H. Emerging High-Level Tigecycline Resistance: Novel Tetracycline Destructases Spread via the Mobile Tet(X). BioEssays 2020, 42, e2000014. [Google Scholar] [CrossRef] [PubMed]
- Tiana, Z.; Liu, R.; Zhang, H.; Yanga, M.; Zhang, Y. Developmental Dynamics of Antibiotic Resistome in Aerobic Biofilm Microbiota Treating Wastewater under Stepwise Increasing Tigecycline Concentrations. Environm. Int. 2019, 131, 105008. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.R.C.; Féris, L.A. Use of Functionalized Adsorbents for Tetracycline Removal in Wastewater: Adsorption Mechanism and Comparison with Activated Carbon. J. Environ. Sci. Health A 2020, 1–11. [Google Scholar] [CrossRef]
- Zhua, X.; Wana, Z.; Daniel, C.W.; Hea, T.M.; Houb, D.; Suc, Z.; Shang, J. Machine Learning for the Selection of Carbon-Based Materials for Tetracycline and Sulfamethoxazole Adsorption. Chem. Eng. J. 2021, 406, 126782. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Feng, M.; Tu, W.; Xiao, T.; Xiong, T.; Ang, H.; Yuan, X.; Chew, J.W. Visible-Light-Driven Removal of Tetracycline Antibiotics and Reclamation of Hydrogen Energy from Natural Water Matrices and Wastewater by Polymeric Carbon Nitride Foam. Water Res. 2018, 144, 215–225. [Google Scholar] [CrossRef]
- Maavia, A.; Aslam, I.; Tanveer, M.; Rizwan, M.; Iqbal, M.W.; Tahir, M.; Hussain, H.; Boddula, R.; Yousuf, M. Facile Synthesis of g-C3N4/CdWO4 with Excellent Photocatalytic Performance for the Degradation of Minocycline. Mater. Sci. Energy Technol. 2019, 2, 258–266. [Google Scholar] [CrossRef]
- Shao, S.; Wu, X. Microbial Degradation of Tetracycline in the Aquatic Environment: A Review. Crit. Rev. Biotechnol. 2020, 40, 1010–1018. [Google Scholar] [CrossRef]
- Xu, L.; Sun, Y.; Du, L.; Zhang, J. Removal of Tetracycline Hydrochloride from Wastewater by Nanofiltration Enhanced by Electro-Catalytic Oxidation. Desalination 2014, 352, 58–65. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Kumar, P.S.; Jaikumar, V.; Rajan, P.S. Removal of Colorants from Wastewater: A Review on Sources and Treatment Strategies. J. Ind. Eng. Chem. 2019, 75, 1–19. [Google Scholar] [CrossRef]
- Elazazy, M.S. Factorial Design and Machine Learning Strategies: Impacts on Pharmaceutical Analysis. In Spectroscopic Analyses; Zafar, F., Ed.; IntechOpen: London, UK, 2017; Available online: https://www.intechopen.com/books/spectroscopic-analyses-developments-and-applications/factorial-design-and-machine-learning-strategies-impacts-on-pharmaceutical-analysis (accessed on 30 October 2020).
- Elazazy, M.S.; El-Hamshary, M.; Sakr, M.; Al-Easa, H.S. Plackett-Burman and Box-Behnken Designs as Chemometric Tools for Micro-Determination of L-Ornithine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 193, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Elazazy, M.S.; Issa, A.A.; Al-Mashreky, M.; Al-Sulaiti, M.; Al-Saad, K.; Al-Sulaiti, M. Application of Fractional Factorial Design for Green Synthesis of Cyano-Modified Silica Nanoparticles: Chemometrics and Multifarious Response Optimization. Adv. Powder Technol. 2018, 29, 1204–1215. [Google Scholar] [CrossRef]
- Aly, H.; El-Shafie, A.S.; El-Azazy, M. Utilization of 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole (NBD-Cl) for Spectrochemical Determination of L-Ornithine: A Multivariate Optimization-Assisted Approach. RSC Adv. 2019, 9, 22106–22115. [Google Scholar] [CrossRef] [Green Version]
- Al-Saad, K.; El-Azazy, M.; Issa, A.A.; Al-Yafie, A.; El-Shafie, A.S.; Al-Sulaiti, M.; Shomar, B. Recycling of Date Pits into a Green Adsorbent for Removal of Heavy Metals: A Fractional Factorial Design-Based Approach. Front. Chem. 2019, 7, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Azazy, M.; El-Shafie, A.S.; Ashraf, A.; Issa, A.A. Eco-Structured Biosorptive Removal of Basic Fuchsin Using Pistachio Nutshells: A Definitive Screening Design—Based Approach. Appl. Sci. 2019, 9, 4855. [Google Scholar] [CrossRef] [Green Version]
- El-Azazy, M.; Dimassi, S.; El-Shafie, A.S.; Issa, A. Bio-waste Aloe vera leaves as an efficient adsorbent for titan yellow from wastewater: Structuring of a novel adsorbent using Plackett-Burman factorial design. Appl. Sci. 2019, 9, 4856. [Google Scholar] [CrossRef] [Green Version]
- El-Azazy, M.; El-Shafie, A.S.; Issa, A.A.; Al-Sulaiti, M.; Al-Yafie, J.; Shomar, B.; Al-Saad, K. Potato Peels as an Adsorbent for Heavy Metals from Aqueous Solutions: Eco-Structuring of a Green Adsorbent Operating Plackett-Burman Design. J. Chem. 2019, 2019, 4926240. [Google Scholar] [CrossRef] [Green Version]
- El-Azazy, M.; Kalla, R.N.; Issa, A.A.; Al-Sulaiti, M.; El-Shafie, A.S.; Shomar, B.; Al-Saad, K. Pomegranate Peels as Versatile Adsorbents for Water Purification: Application of Box-Behnken Design as a Methodological Optimization Approach. Environ. Prog. Sustain. Energy 2019, 38, 13223. [Google Scholar] [CrossRef]
- Hassan, S.S.; El-Shafie, A.S.; Zaher, N.; El-Azazy, M. Application of Pineapple Leaves as Adsorbents for Removal of Rose Bengal from Wastewater: Process Optimization Operating Face-Centered Central Composite Design (FCCCD). Molecules 2020, 25, 3752. [Google Scholar] [CrossRef]
- Al-Saad, K.; Issa, A.A.; Idoudi, S.; Shomar, B.; Al-Ghouti, M.A.; Al-Hashimi, N.; El-Azazy, M. Smart Synthesis of Trimethyl Ethoxysilane (TMS) Functionalized Core-Shell Magnetic Nanosorbents Fe3O4@SiO2: Process Optimization and Application for Extraction of Pesticides. Molecules 2020, 25, 4827. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.S.; El-Gendy, A.; Issa, A.; Al-Meer, S.; Al-Saad, K. A Comparison between Different Agro-Wastes and Carbon Nanotubes for Removal of Sarafloxacin from Wastewater: Kinetics and Equilibrium Studies. Molecules 2020, 25, 5429. [Google Scholar] [CrossRef] [PubMed]
- Gholamiyan, S.; Hamzehloo, M.; Farrokhnia, A. RSM Optimized Adsorptive Removal of Erythromycin Using Magnetic Activated Carbon: Adsorption Isotherm, Kinetic Modeling and Thermodynamic Studies. Sustain. Chem. Pharm. 2020, 17, 100309. [Google Scholar] [CrossRef]
- D’Cruz, B.; Madkour, M.; Amin, M.O.; Al-Hetlani, E. Efficient and Recoverable Magnetic AC-Fe3O4 Nanocomposite for Rapid Removal of Promazine from Wastewater. Mater. Chem. Phys. 2020, 240, 122109. [Google Scholar] [CrossRef]
- Liyanage, A.S.; Canaday, S.; Pittman, C.U., Jr.; Mlsna, T. Rapid Remediation of Pharmaceuticals from Wastewater Using Magnetic Fe3O4/Douglas Fir Biochar Adsorbents. Chemosphere 2020, 258, 127336. [Google Scholar] [CrossRef]
- Shahri, F.B.; Niazi, A. Synthesis of Modified Maghemite Nanoparticles and Its Application for Removal of Acridine Orange from Aqueous Solutions by Using Box-Behnken Design. J. Magn. Magn. Mater. 2015, 396, 318–326. [Google Scholar] [CrossRef]
- Karunanayake, A.G.; Todd, O.A.; Crowley, M.L.; Ricchetti, L.B.; Pittman, C.U., Jr.; Anderson, R.; Mlsna, T.E. Rapid Removal of Salicylic Acid, 4-Nitroaniline, Benzoic Acid and Phthalic Acid from Wastewater Using Magnetized Fast Pyrolysis Biochar from Waste Douglas Fir. Chem. Eng. J. 2017, 319, 75–88. [Google Scholar] [CrossRef]
- Zeng, X.; Xiao, Z.; Zhang, G.; Wang, A.; Li, Z.; Liu, Y.; Wang, H.; Zeng, Q.; Liang, Y.; Zou, D. Speciation and Bioavailability of Heavy Metals in Pyrolytic Biochar of Swine and Goat Manures. J. Anal. Appl. Pyrol. 2018, 132, 82–93. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Ghaedi, M.; Asfaram, A.; Bazrafshan, A.A.; Jannesar, R. Comparative Study on Ultrasonic Assisted Adsorption of Dyes from Single System onto Fe3O4 Magnetite Nanoparticles Loaded on Activated Carbon: Experimental Design Methodology. Ultrason. Sonochem. 2017, 34, 294–304. [Google Scholar] [CrossRef]
- Shahrashoub, M.; Bakhtiari, S. The Efficiency of Activated Carbon/Magnetite Nanoparticles Composites in Copper Removal: Industrial Waste Recovery, Green Synthesis, Characterization, and Adsorption-Desorption Studies. Microporous Mesoporous Mater. 2021, 311, 110692. [Google Scholar] [CrossRef]
- Rahmani, R.; Gharanfoli, M.; Gholamin, M.; Darroudi, M.; Chamani, J.; Sadri, K. Green synthesis of 99mTc-labeled-Fe3O4 nanoparticles using Quince seeds extract and evaluation of their cytotoxicity and biodistribution in rats. J. Mol. Struct. 2019, 1196, 394–402. [Google Scholar] [CrossRef]
- Nnadozie, E.C.; Ajibade, P.A. Green Synthesis and Characterization of Magnetite (Fe3O4) Nanoparticles Using Chromolaena odorata Root Extract for Smart Nanocomposite. Mater. Lett. 2020, 263, 127145. [Google Scholar] [CrossRef]
- Zhao, G.M.; Yang, Y.Q.; Zhang, W.; Luo, X.; Zhang, R.J.; Chen, Y. Raman Scattering Characterization of a Carbon Coating after Low-Energy Argon Ion Bombardment. Phys. B Condens. Matter. 2011, 406, 3876–3884. [Google Scholar] [CrossRef]
- Guo, C.; Hu, Y.; Qian, H.; Ning, J.; Xu, S. Magnetite (Fe3O4) Tetrakaidekahedral Microcrystals: Synthesis, Characterization, and Micro-Raman Study. Mater. Charact. 2011, 62, 148–151. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Lopes, F.N. Heated Goethite and Natural Hematite: Can Raman Spectroscopy Be Used to Differentiate Them? Vib. Spectrosc. 2007, 45, 117–121. [Google Scholar] [CrossRef]
- Ivashchenko, O.; Jurga-Stopa, J.; Coy, E.; Peplinska, B.; Pietralik, Z.; Jurga, S. Fourier Transform Infrared and Raman Spectroscopy Studies on Magnetite/Ag/Antibiotic Nanocomposites. Appl. Surf. Sci. 2016, 364, 400–409. [Google Scholar] [CrossRef]
- Huizar-Félix, A.M.; Aguilar-Flores, C.; Martinez-de-la Cruz, A.M.; Barandiarán, J.M.; Sepulveda-Guzmán, S.; Cruz-Silva, R. Removal of Tetracycline Pollutants by Adsorption and Magnetic Separation Using Reduced Graphene Oxide Decorated with α-Fe2O3 Nanoparticles. Nanomaterials 2019, 9, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidayat, D.; Purwanto, A.; Wang, W.-N.; Okuyama, K. Preparation of Size-Controlled Tungsten Oxide Nanoparticles and Evaluation of Their Adsorption Performance. Mater. Res. Bull. 2010, 45, 165–173. [Google Scholar] [CrossRef]
- Box, G.E.; Behnken, D.W. Some New Three Level Designs for the Study of Quantitative Variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Langmuir, I. Adsorption of Gases on Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Wang, J. Comparison of Linearization Methods for Modeling the Langmuir Adsorption Isotherm. J. Mol. Liq. 2019, 296, 111850. [Google Scholar] [CrossRef]
- Araújo, C.S.T.; Almeida, I.L.S.; Rezende, H.C.; Marcionilio, S.M.L.O.; Léon, J.J.L.; de Matos, T.N. Elucidation of Mechanism Involved in Adsorption of Pb (II) onto Lobeira Fruit (Solanum lycocarpum) Using Langmuir, Freundlich and Temkin Isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
- Moussavi, G.; Barikbin, B. Biosorption of Chromium (VI) from Industrial Wastewater onto Pistachio Hull Waste Biomass. Chem. Eng. J. 2010, 162, 893–900. [Google Scholar] [CrossRef]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.H.; Indraswati, N.; Ismadji, S. Equilibrium and Kinetic Studies in Adsorption of Heavy Metals Using Biosorbent: A Summary of Recent Studies. J. Hazard Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, Z.; Yu, Q.J.; El Hanandeh, A. Competitive Adsorption of Heavy Metal Ions (Pb2+, Cu2+, and Ni2+) onto Date SEED biochar: Batch and Fixed Bed Experiments. Sep. Sci. Technol. 2019, 54, 888–901. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, R.; Nayak, A.; Saleh, T.A.; Barakat, M.A. Adsorptive Removal Dyes from aqueous Solution onto Carbon Nanotubes: A Review. Adv. Colloid Interface Sci. 2013, 193–194, 24–34. [Google Scholar] [CrossRef]
- Ahmed, M.J.K.; Ahmaruzzaman, M. A Facile Synthesis of Fe3O4-Charcoal Composite for the Sorption of a Hazardous Dye from Aquatic Environment. J. Environ. Manag. 2015, 163, 163–173. [Google Scholar] [CrossRef]
- Joshi, S.; Garg, V.K.; Kataria, N.; Kadirvelu, K. Applications of Fe3O4@AC Nanoparticles for Dye Removal from Simulated Wastewater. Chemosphere 2019, 236, 124280. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of Arsenic by Magnetic Biochar Prepared from Pinewood and Natural Hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef]
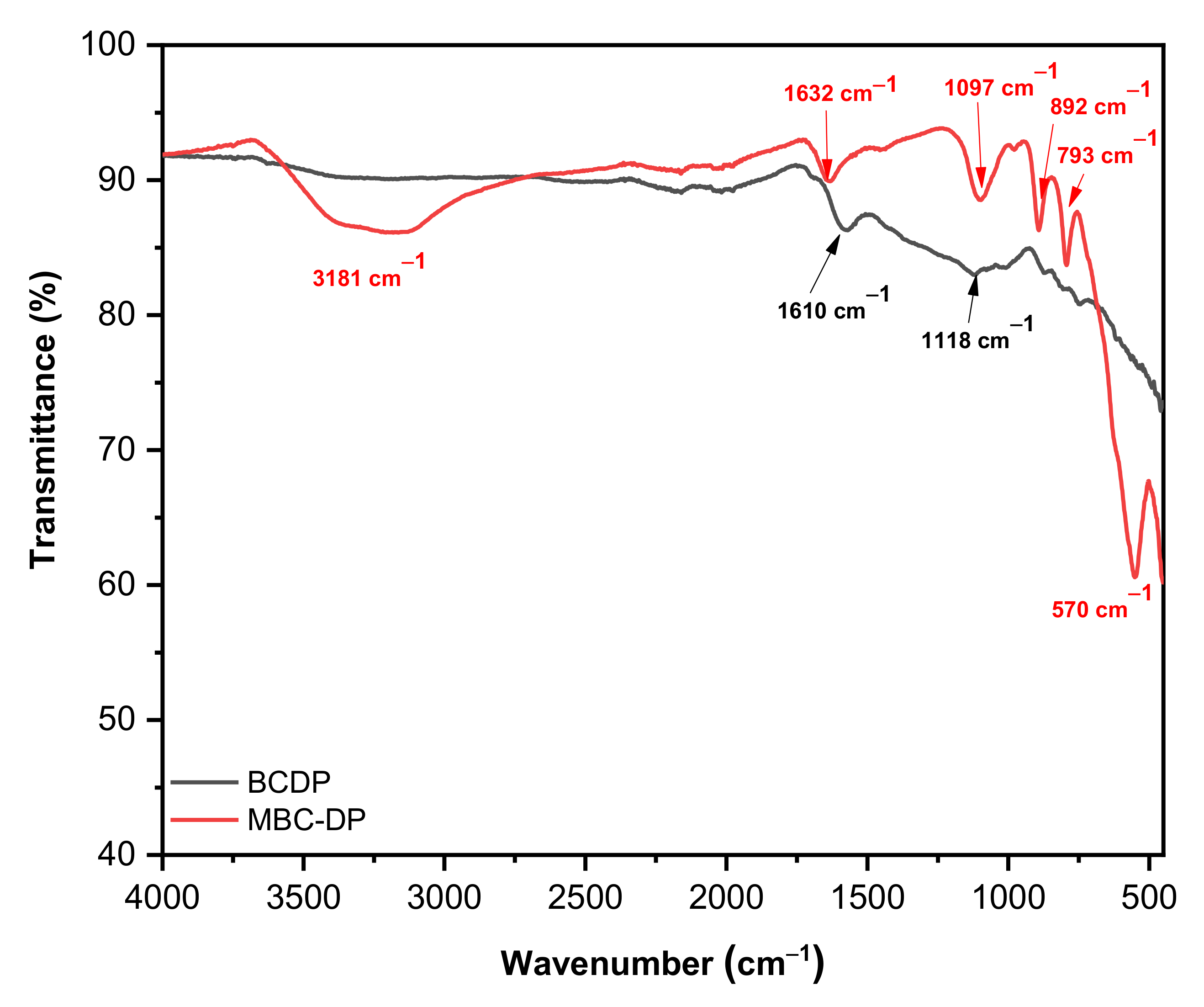



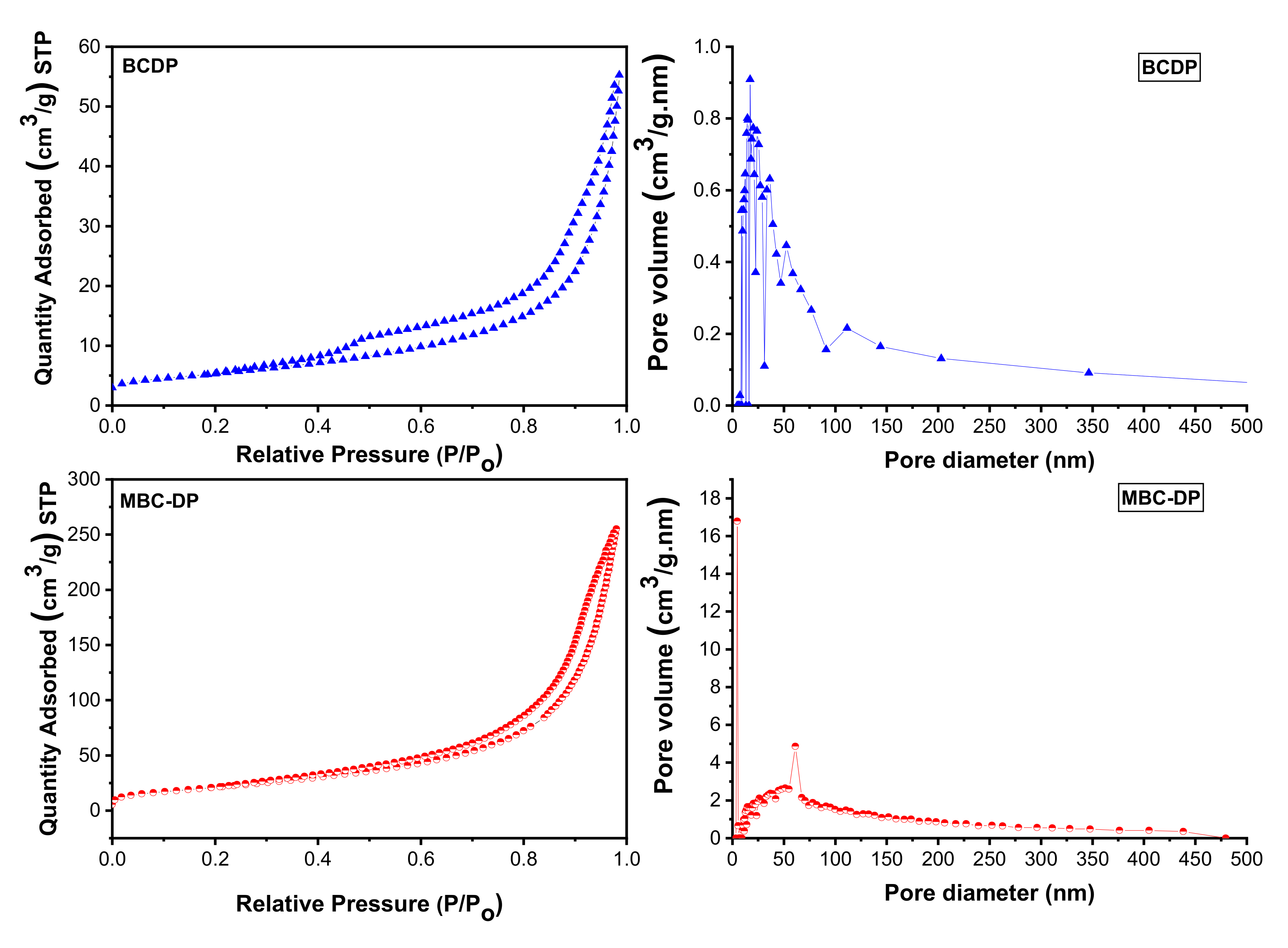
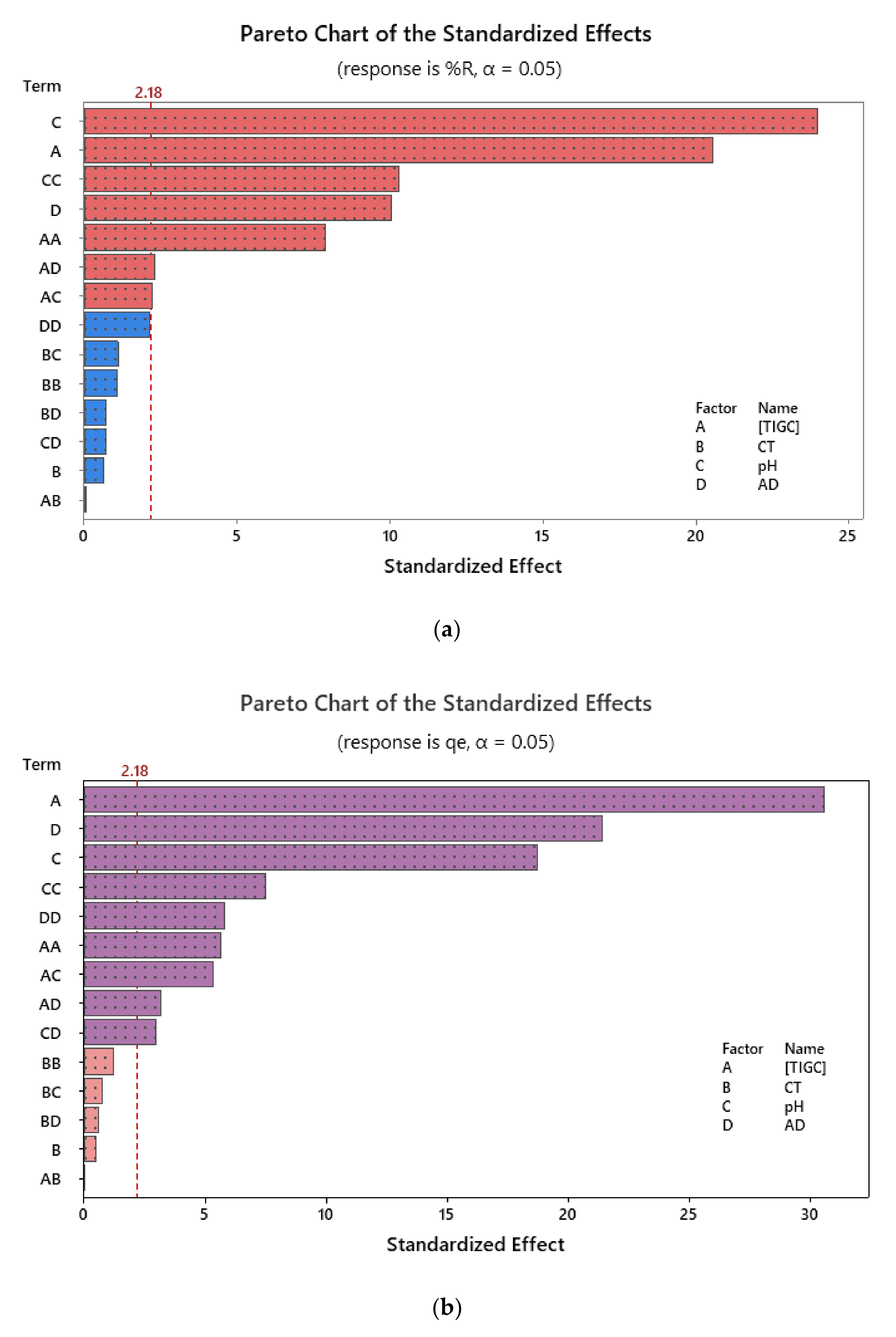
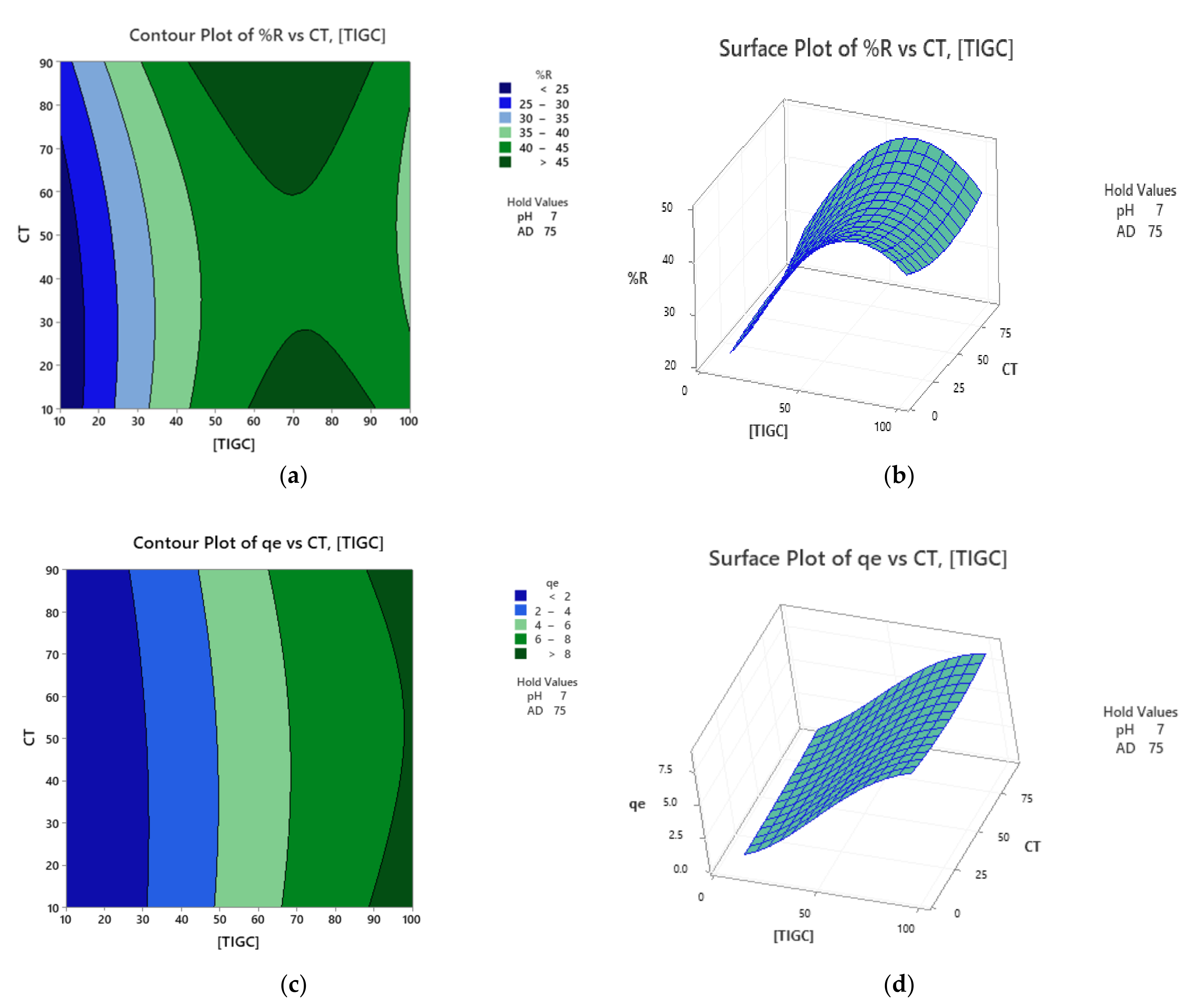

| Independent Variables | Code | Units | −1 | 0 | +1 |
|---|---|---|---|---|---|
| Initial Drug Concentration ([TIGC]) | A | mg/L | 10.0 | 55.0 | 100.0 |
| Contact Time (CT) | B | min | 10.0 | 50.0 | 90.0 |
| pH | C | pH unit | 4.0 | 7.0 | 10.0 |
| Adsorbent Dose (AD) | D | mg/15 mL | 30.0 | 75.0 | 120.0 |
| Responses | Percentage Removal (%R) | ||||
| Adsorption Capacity (qe, mg/g) | |||||
| Variables | BCDP | MBC-DP | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial No | [TIGC] | CT | pH | AD | %R Obs. * | %R Pred. ** | RE *** | qe Obs.* | qe Pred. ** | RE *** | %R Obs. * | %R Pred.** | RE *** | qe Obs. * | qe Pred. ** | RE *** |
| 01 | 55 (0) | 10 (−) | 10 (+) | 75 (0) | 47.79 | 49.31 | 0.03 | 5.24 | 5.23 | 0.00 | 74.58 | 72.77 | 0.02 | 8.15 | 8.29 | 0.02 |
| 02 | 55 (0) | 10 (−) | 7 (0) | 30 (−) | 37.32 | 37.82 | 0.01 | 10.00 | 10.14 | 0.01 | 48.08 | 47.7 | 0.01 | 13.13 | 12.24 | 0.07 |
| 03 | 55 (0) | 90 (+) | 7 (0) | 30 (−) | 40.80 | 41.5 | 0.02 | 11.31 | 11.44 | 0.01 | 50.48 | 51.15 | 0.01 | 13.70 | 12.88 | 0.06 |
| 04 | 55 (0) | 50 (0) | 4 (−) | 120 (+) | 46.47 | 45.05 | 0.03 | 3.18 | 3.23 | 0.01 | 34.21 | 31.25 | 0.09 | 2.34 | 2.06 | 0.14 |
| 05 | 55 (0) | 50 (0) | 7 (0) | 75 (0) | 42.45 | 42.81 | 0.01 | 4.64 | 4.64 | 0.00 | 61.11 | 60.63 | 0.09 | 6.67 | 6.63 | 0.01 |
| 06 | 100 (+) | 50 (0) | 7 (0) | 120 (+) | 30.40 | 31.42 | 0.03 | 3.31 | 3.19 | 0.04 | 59.52 | 62.77 | 0.05 | 7.42 | 7.26 | 0.02 |
| 07 | 100 (+) | 50 (0) | 7 (0) | 30 (−) | 47.25 | 46.83 | 0.01 | 21.72 | 21.28 | 0.02 | 38.11 | 37.21 | 0.026 | 19.12 | 18.72 | 0.02 |
| 08 | 55 (0) | 90 (+) | 10 (+) | 75 (0) | 62.01 | 60.53 | 0.02 | 6.81 | 6.61 | 0.03 | 74.19 | 69.99 | 0.06 | 8.14 | 8.01 | 0.02 |
| 09 | 55 (0) | 90 (+) | 4 (−) | 75 (0) | 45.15 | 44.19 | 0.02 | 4.95 | 4.97 | 0.00 | 29.08 | 30.23 | 0.048 | 3.19 | 3.14 | 0.01 |
| 10 | 10 (−) | 50 (0) | 7 (0) | 120 (+) | 39.36 | 39.85 | 0.01 | 0.81 | 0.90 | 0.10 | 99.99 | 101.5 | 0.01 | 1.24 | 1.37 | 0.09 |
| 11 | 55 (0) | 50 (0) | 7 (0) | 75 (0) | 43.83 | 42.81 | 0.02 | 4.76 | 4.64 | 0.02 | 63.87 | 60.64 | 0.05 | 6.96 | 6.62 | 0.05 |
| 12 | 100 (+) | 50 (0) | 4 (−) | 75 (0) | 44.27 | 44.38 | 0.00 | 8.75 | 8.7 | 0.01 | 20.5 | 19.48 | 0.05 | 4.06 | 4.22 | 0.04 |
| 13 | 100 (+) | 50 (0) | 10 (+) | 75 (0) | 40.86 | 40.26 | 0.01 | 8.56 | 8.82 | 0.03 | 61.76 | 64.41 | 0.04 | 13.68 | 14.08 | 0.03 |
| 14 | 55 (0) | 90 (+) | 7 (0) | 120 (+) | 53.71 | 55.17 | 0.03 | 3.67 | 3.60 | 0.02 | 66.87 | 68.82 | 0.03 | 4.57 | 4.97 | 0.08 |
| 15 | 10 (−) | 50 (0) | 10 (+) | 75 (0) | 33.32 | 33.79 | 0.01 | 0.66 | 0.68 | 0.03 | 99.89 | 104.0 | 0.04 | 1.98 | 1.78 | 0.11 |
| 16 | 55 (0) | 50 (0) | 4 (−) | 30 (−) | 40.00 | 39.25 | 0.02 | 11.01 | 10.96 | 0.00 | 18.9 | 19.73 | 0.04 | 5.27 | 5.87 | 0.10 |
| 17 | 55 (0) | 10 (−) | 4 (−) | 75 (0) | 44.73 | 46.52 | 0.04 | 5.18 | 5.37 | 0.03 | 24.17 | 26.68 | 0.09 | 2.66 | 2.77 | 0.04 |
| 18 | 55 (0) | 10 (−) | 7 (0) | 120 (+) | 53.22 | 50.92 | 0.05 | 3.65 | 3.60 | 0.01 | 69.36 | 70.10 | 0.01 | 4.75 | 5.10 | 0.07 |
| 19 | 10 (−) | 10 (−) | 7 (0) | 75 (0) | 22.03 | 21.30 | 0.03 | 0.33 | 0.28 | 0.18 | 99.85 | 99.21 | 0.01 | 1.99 | 2.03 | 0.02 |
| 20 | 55 (0) | 50 (0) | 10 (+) | 30 (−) | 40.64 | 40.87 | 0.01 | 11.27 | 11.15 | 0.01 | 52.35 | 54.79 | 0.04 | 14.06 | 14.97 | 0.06 |
| 21 | 55 (0) | 50 (0) | 10 (+) | 120 (+) | 63.11 | 62.69 | 0.01 | 4.31 | 4.32 | 0.00 | 81.91 | 78.57 | 0.04 | 5.57 | 5.10 | 0.09 |
| 22 | 100 (+) | 10 (−) | 7 (0) | 75 (0) | 43.07 | 42.48 | 0.01 | 8.58 | 8.59 | 0.00 | 54.84 | 52.97 | 0.03 | 10.97 | 10.8 | 0.01 |
| 23 | 10 (−) | 50 (0) | 4 (−) | 75 (0) | 16.54 | 17.32 | 0.04 | 0.33 | 0.29 | 0.14 | 58.6 | 57.43 | 0.02 | 0.72 | 0.58 | 0.24 |
| 24 | 55 (0) | 50 (0) | 7 (0) | 75 (0) | 41.37 | 42.81 | 0.03 | 4.50 | 4.63 | 0.03 | 57.03 | 60.64 | 0.06 | 6.26 | 6.62 | 0.05 |
| 25 | 10 (−) | 90 (+) | 7 (0) | 75 (0) | 28.14 | 28.03 | 0.00 | 0.57 | 0.56 | 0.02 | 99.91 | 100.63 | 0.01 | 1.99 | 2.14 | 0.07 |
| 26 | 100 (+) | 90 (+) | 7 (0) | 75 (0) | 40.97 | 41.26 | 0.01 | 8.19 | 8.44 | 0.03 | 55.28 | 54.40 | 0.02 | 10.92 | 10.98 | 0.00 |
| 27 | 10 (−) | 50 (0) | 7 (0) | 30 (−) | 10.17 | 9.64 | 0.05 | 0.74 | 0.79 | 0.06 | 90.13 | 86.25 | 0.04 | 4.39 | 4.56 | 0.04 |
| Parameters | BCDP | MBC-DP |
|---|---|---|
| Langmuir surface area (SA) (m2/g) | 30.45 | 86.06 |
| Total pore volume (cm3/g) | 0.0337 | 0.3967 |
| Average pore radius (°A) | 36.2 | 80.0 |
| Response | R2% | R2–adj % | R2–pred % |
|---|---|---|---|
| %R (BCDP) | 99.44 | 98.95 | 97.74 |
| qe(BCDP) | 99.92 | 99.82 | 99.55 |
| %R (MBC-DP) | 99.13 | 98.11 | 95.48 |
| qe(MBC-DP) | 99.39 | 98.69 | 96.66 |
| BCDP | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Response | %R | qe | ||||||||
| Source | DF * | Adj SS * | Adj MS * | F-Value | p-Value | DF * | Adj SS * | Adj MS * | F-Value | p-Value |
| Model | 12 | 27.9332 | 2.32777 | 205.59 | 0.000 | 14 | 26.4377 | 1.8884 | 1036.44 | 0.000 |
| Linear | 4 | 11.3998 | 2.84995 | 251.71 | 0.000 | 4 | 21.4541 | 5.3635 | 2943.74 | 0.000 |
| [TIGC] | 1 | 6.8935 | 6.89353 | 608.85 | 0.000 | 1 | 15.5857 | 15.5857 | 8554.14 | 0.000 |
| CT | 1 | 0.2567 | 0.25669 | 22.67 | 0.000 | 1 | 0.0291 | 0.0291 | 15.98 | 0.002 |
| pH | 1 | 1.3349 | 1.33487 | 117.90 | 0.000 | 1 | 0.0724 | 0.0724 | 39.75 | 0.000 |
| AD | 1 | 2.9147 | 2.91472 | 257.43 | 0.000 | 1 | 5.7669 | 5.7669 | 3165.11 | 0.000 |
| Square | 3 | 9.9691 | 3.32304 | 293.50 | 0.000 | 4 | 2.8157 | 0.7039 | 386.35 | 0.000 |
| [TIGC]2 | 1 | 6.9513 | 6.95132 | 613.95 | 0.000 | 1 | 1.0900 | 1.0900 | 598.21 | 0.000 |
| CT2 | 1 | 0.3630 | 0.36302 | 32.06 | 0.000 | 1 | 0.0326 | 0.0326 | 17.91 | 0.001 |
| pH2 | 1 | 0.4666 | 0.46665 | 41.22 | 0.000 | 1 | 0.0762 | 0.0762 | 41.82 | 0.000 |
| AD2 | 1 | 0.6882 | 0.6882 | 377.69 | 0.000 | |||||
| 2-Way Interactions | 5 | 6.5643 | 1.31286 | 115.95 | 0.000 | 6 | 2.1678 | 0.3613 | 198.30 | 0.000 |
| [TIGC] × CT | 1 | 0.1491 | 0.14908 | 13.17 | 0.003 | 1 | 0.0156 | 0.0156 | 8.58 | 0.013 |
| [TIGC] × pH | 1 | 0.9675 | 0.96753 | 85.45 | 0.000 | 1 | 0.0182 | 0.0182 | 9.97 | 0.008 |
| [TIGC] × AD | 1 | 4.9413 | 4.94129 | 436.42 | 0.000 | 1 | 2.0737 | 2.0737 | 1138.12 | 0.000 |
| CT × pH | 1 | 0.2165 | 0.21648 | 19.12 | 0.001 | 1 | 0.0345 | 0.0345 | 18.96 | 0.001 |
| CT × AD | 1 | 0.0097 | 0.0097 | 5.33 | 0.040 | |||||
| pH × AD | 1 | 0.2899 | 0.28991 | 25.61 | 0.000 | 1 | 0.0161 | 0.0161 | 8.84 | 0.012 |
| Error | 14 | 0.1585 | 0.01132 | 12 | 0.0219 | 0.0018 | ||||
| Lack-of-Fit | 12 | 0.1407 | 0.01172 | 1.32 | 0.511 | 10 | 0.0199 | 0.0020 | 2.04 | 0.374 |
| Pure Error | 2 | 0.0178 | 0.00891 | 2 | 0.0020 | 0.0010 | ||||
| Total | 26 | 28.0917 | 26 | 26.4595 | ||||||
| MBC-DP | ||||||||||
| Response | %R | qe | ||||||||
| Source | DF * | Adj SS * | Adj MS * | F-Value | p-Value | DF * | Adj SS * | Adj MS * | F-Value | p-Value |
| Model | 14 | 69.3253 | 4.9518 | 97.62 | 0.000 | 14 | 21.7529 | 1.5538 | 140.51 | 0.000 |
| Linear | 4 | 55.6423 | 13.9106 | 274.25 | 0.000 | 4 | 19.2720 | 4.8180 | 435.70 | 0.000 |
| [TIGC] | 1 | 21.3729 | 21.3729 | 421.37 | 0.000 | 1 | 10.3384 | 10.3384 | 934.92 | 0.000 |
| CT | 1 | 0.0213 | 0.0213 | 0.42 | 0.529 | 1 | 0.0028 | 0.0028 | 0.26 | 0.622 |
| pH | 1 | 29.1387 | 29.1387 | 574.47 | 0.000 | 1 | 3.8713 | 3.8713 | 350.09 | 0.000 |
| AD | 1 | 5.1093 | 5.1093 | 100.73 | 0.000 | 1 | 5.0595 | 5.0595 | 457.54 | 0.000 |
| Square | 4 | 13.0565 | 3.2641 | 64.35 | 0.000 | 4 | 1.9467 | 0.4867 | 44.01 | 0.000 |
| [TIGC]2 | 1 | 3.1572 | 3.1572 | 62.24 | 0.000 | 1 | 0.3511 | 0.3511 | 31.75 | 0.000 |
| CT2 | 1 | 0.0589 | 0.0589 | 1.16 | 0.302 | 1 | 0.0162 | 0.0162 | 1.47 | 0.249 |
| pH2 | 1 | 5.3537 | 5.3537 | 105.55 | 0.000 | 1 | 0.6255 | 0.6255 | 56.57 | 0.000 |
| AD2 | 1 | 0.2360 | 0.2360 | 4.65 | 0.052 | 1 | 0.3742 | 0.3742 | 33.84 | 0.000 |
| 2-Way Interactions | 6 | 0.6265 | 0.1044 | 2.06 | 0.135 | 6 | 0.5343 | 0.0890 | 8.05 | 0.001 |
| [TIGC] × CT | 1 | 0.0002 | 0.0002 | 0.00 | 0.954 | 1 | 0.0000 | 0.0000 | 0.00 | 0.966 |
| [TIGC] × pH | 1 | 0.2454 | 0.2454 | 4.84 | 0.048 | 1 | 0.3160 | 0.3160 | 28.57 | 0.000 |
| [TIGC] × AD | 1 | 0.2683 | 0.2683 | 5.29 | 0.040 | 1 | 0.1117 | 0.1117 | 10.10 | 0.008 |
| CT × pH | 1 | 0.0621 | 0.0621 | 1.22 | 0.290 | 1 | 0.0062 | 0.0062 | 0.56 | 0.469 |
| CT × AD | 1 | 0.0260 | 0.0260 | 0.51 | 0.488 | 1 | 0.0036 | 0.0036 | 0.32 | 0.581 |
| pH × AD | 1 | 0.0245 | 0.0245 | 0.48 | 0.500 | 1 | 0.0969 | 0.0969 | 8.76 | 0.012 |
| Error | 12 | 0.6087 | 0.0507 | 12 | 0.1327 | 0.0111 | ||||
| Lack-of-Fit | 10 | 0.5105 | 0.0510 | 1.04 | 0.585 | 10 | 0.1232 | 0.0123 | 2.60 | 0.310 |
| Pure Error | 2 | 0.0982 | 0.0491 | 2 | 0.0095 | 0.0047 | ||||
| Total | 26 | 69.9340 | 26 | 21.8856 | ||||||
| Isotherm | Equations (Generalized/Linearized Forms) | Parameters | BCDP | MBC-DP | ||
|---|---|---|---|---|---|---|
| (I) (II) | (I) (II) | |||||
| Langmuir | qm (mg/g) | 2.71 | 12.15 | 10.71 | 57.14 | |
| KL (L·mole−1) | 0.19 | 0.004 | 1.35 | 0.090 | ||
| R2 | 0.9535 | 0.9564 | 0.9682 | |||
| Freundlich | 1/n | 0.3882 | 0.5302 | |||
| KF (mole/g) (L/mole)1/n | 0.595 | 2.29 | ||||
| R2 | 0.8876 | 0.9934 | ||||
| Temkin | bT (J/mole) | 2075.8 | 280.36 | |||
| AT (L/mole) | 0.314 | 0.2042 | ||||
| R2 | 0.7216 | 0.9435 | ||||
| DR | β | 6 × 10−9 | 4 × 10−8 | 5 × 10−10 | 2 × 10−8 | |
| E (kJ/mol) | 9.13 | 3.54 | 31.62 | 5.00 | ||
| qm (mg/g) | 3.07 | 8.72 | 7.83 | 40.15 | ||
| R2 | 0.8565 | 0.8952 | 0.9409 | 0.9524 | ||
| Models | Parameter | BCDP | MBC-DP | ||
|---|---|---|---|---|---|
| Pseudo-first order (PFO) | K1 (min−1) | 0.0156 | 0.3904 | ||
| qe (mg/g) | 9.078 | 11.24 | |||
| R2 | 0.8517 | 0.9569 | |||
| Pseudo-second order (PSO) t Where K2 is rate constant (g·mg−1·min−1) | K2 (g·mg−1·min−1) | 0.0117 | 0.130 | ||
| qe (mg/g) | 14.99 | 40.00 | |||
| R2 | 0.9906 | 1.0000 | |||
| Elovich equation is is used to predict the sorption mechanism, where qt is adsorbed quantity at time t; while α and β are initial sorption concentration rate (mg·g−1·min−1), and desorption constant (g/mg), respectively. | α | 13.49 | 3.05 × 1011 | ||
| β | 0.426 | 0.744 | |||
| R2 | 0.8495 | 0.7102 | |||
| Weber–Morris intraparticle diffusion model is used to study the formed layers around the adsorbent and rate-controlling step, which is expressed as , where KI is intraparticle diffusion rate constant (mg·g−1·min−0.5), and C is the boundary thickness effect. | KI | 3.303 | 0.680 | 3.519 | 0.0986 |
| C | 0.308 | 8.073 | 30.25 | 39.059 | |
| R2 | 0.6589 | 0.9172 | 0.8521 | 0.8446 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Azazy, M.; El-Shafie, A.S.; Al-Meer, S.; Al-Saad, K.A. Eco-Structured Adsorptive Removal of Tigecycline from Wastewater: Date Pits’ Biochar versus the Magnetic Biochar. Nanomaterials 2021, 11, 30. https://doi.org/10.3390/nano11010030
El-Azazy M, El-Shafie AS, Al-Meer S, Al-Saad KA. Eco-Structured Adsorptive Removal of Tigecycline from Wastewater: Date Pits’ Biochar versus the Magnetic Biochar. Nanomaterials. 2021; 11(1):30. https://doi.org/10.3390/nano11010030
Chicago/Turabian StyleEl-Azazy, Marwa, Ahmed S. El-Shafie, Saeed Al-Meer, and Khalid A. Al-Saad. 2021. "Eco-Structured Adsorptive Removal of Tigecycline from Wastewater: Date Pits’ Biochar versus the Magnetic Biochar" Nanomaterials 11, no. 1: 30. https://doi.org/10.3390/nano11010030
APA StyleEl-Azazy, M., El-Shafie, A. S., Al-Meer, S., & Al-Saad, K. A. (2021). Eco-Structured Adsorptive Removal of Tigecycline from Wastewater: Date Pits’ Biochar versus the Magnetic Biochar. Nanomaterials, 11(1), 30. https://doi.org/10.3390/nano11010030









