Characterization of Commercial Metal Oxide Nanomaterials: Crystalline Phase, Particle Size and Specific Surface Area
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. X-ray Diffraction
2.3. Transmission Electron Microscopy
2.4. Specific Surface Area
3. Results
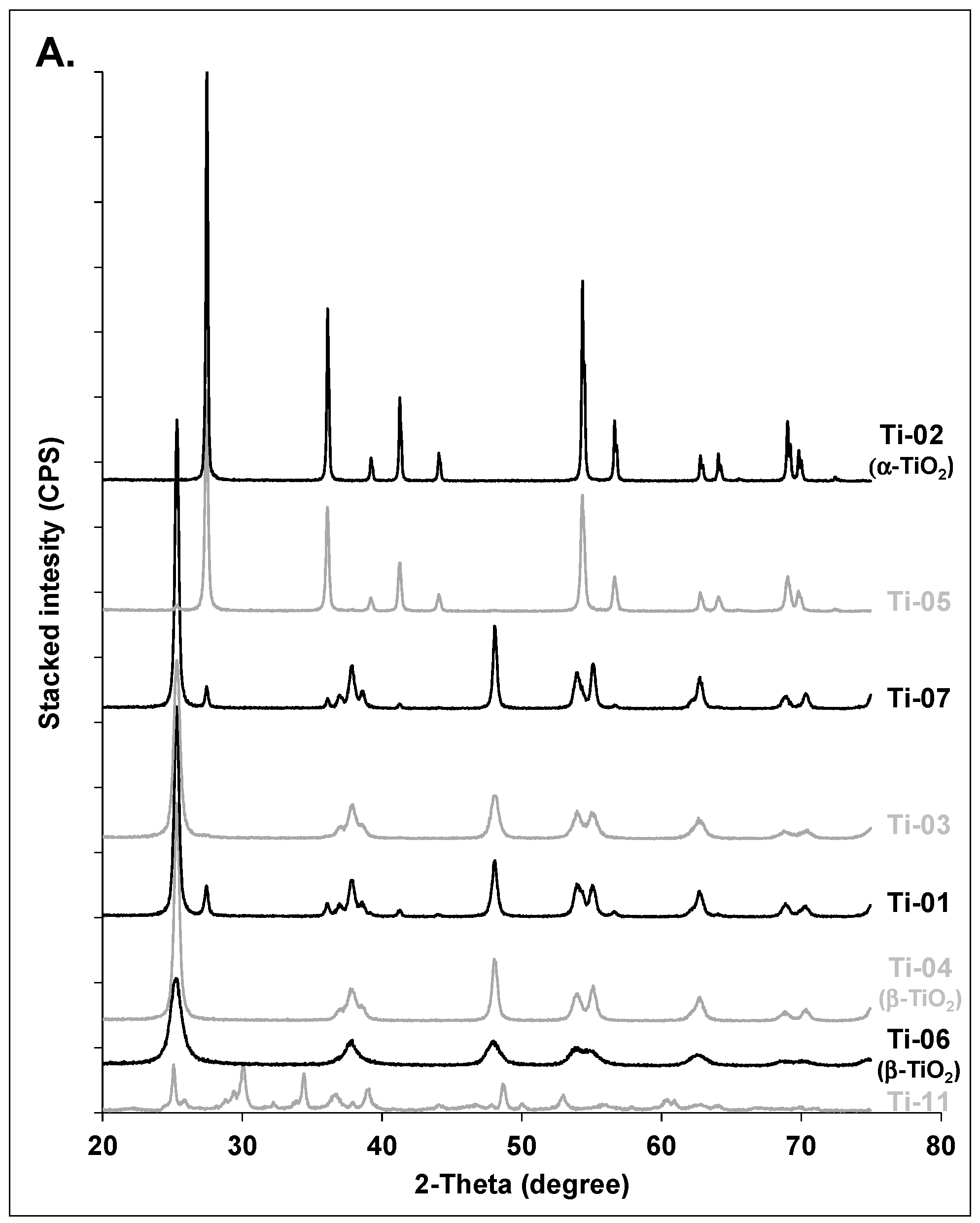
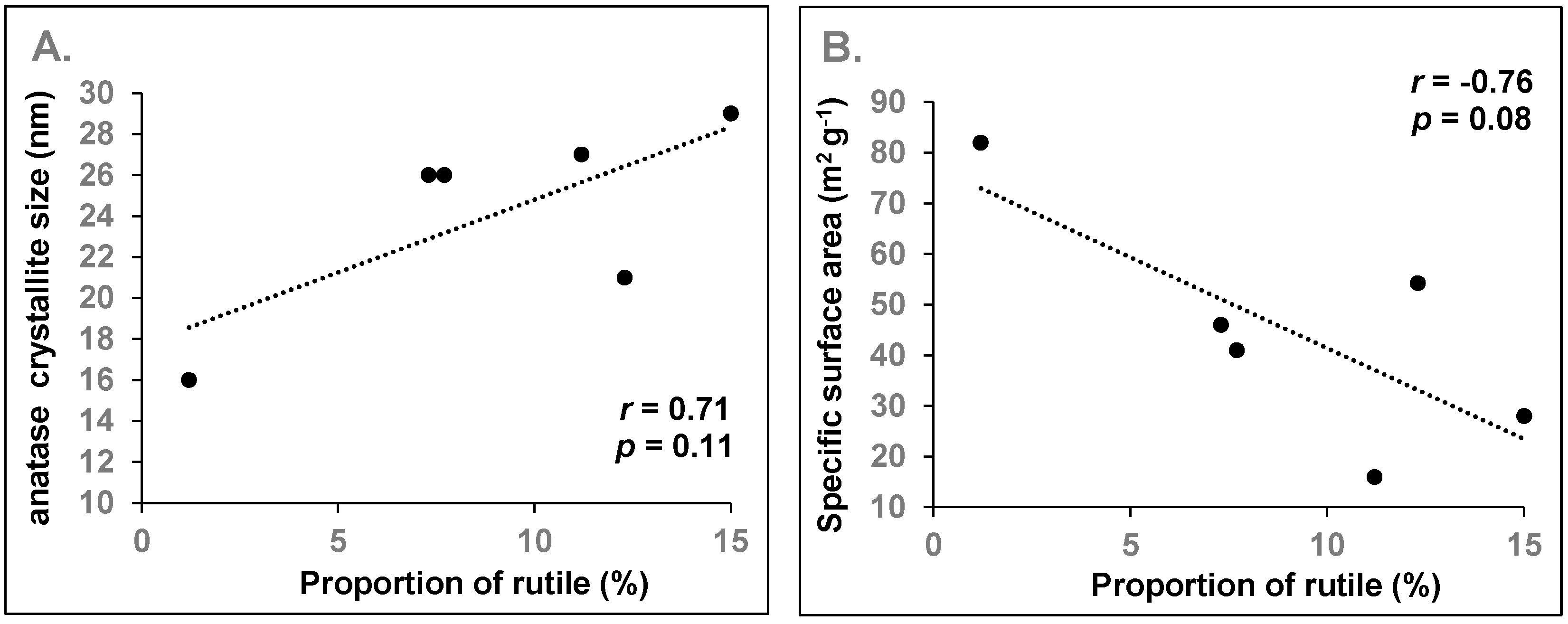
3.1. Crystalline Phase and Crystallite Size
3.1.1. Aluminum Oxides
3.1.2. Copper Oxides
3.1.3. Titanium Oxides
3.1.4. Zinc Oxides
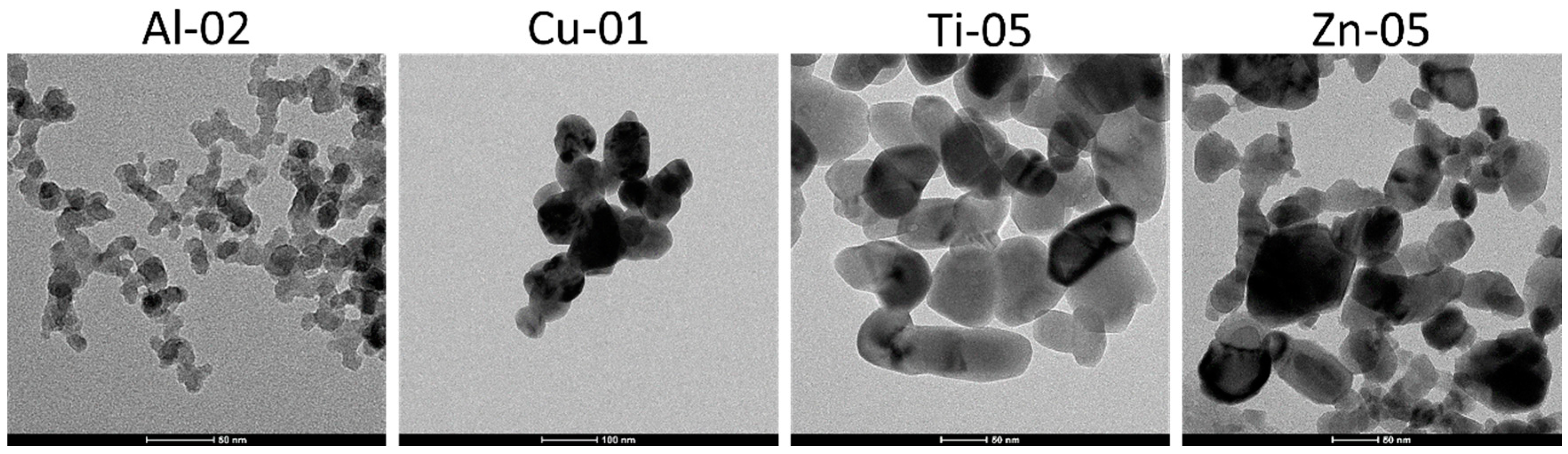
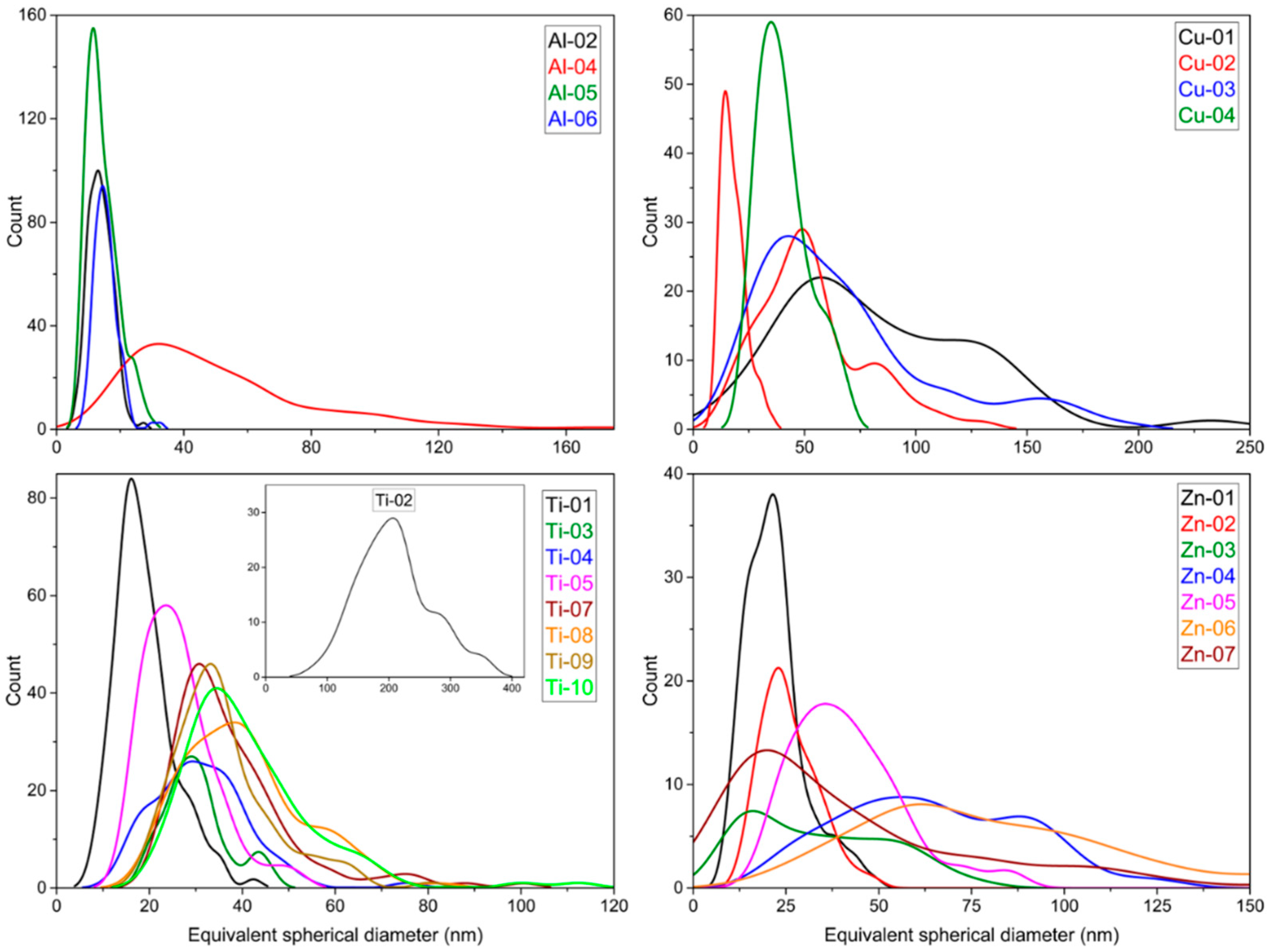
3.2. Particle Shape and Particle Size Distribution
3.3. Comparison of Particle Size Measurements
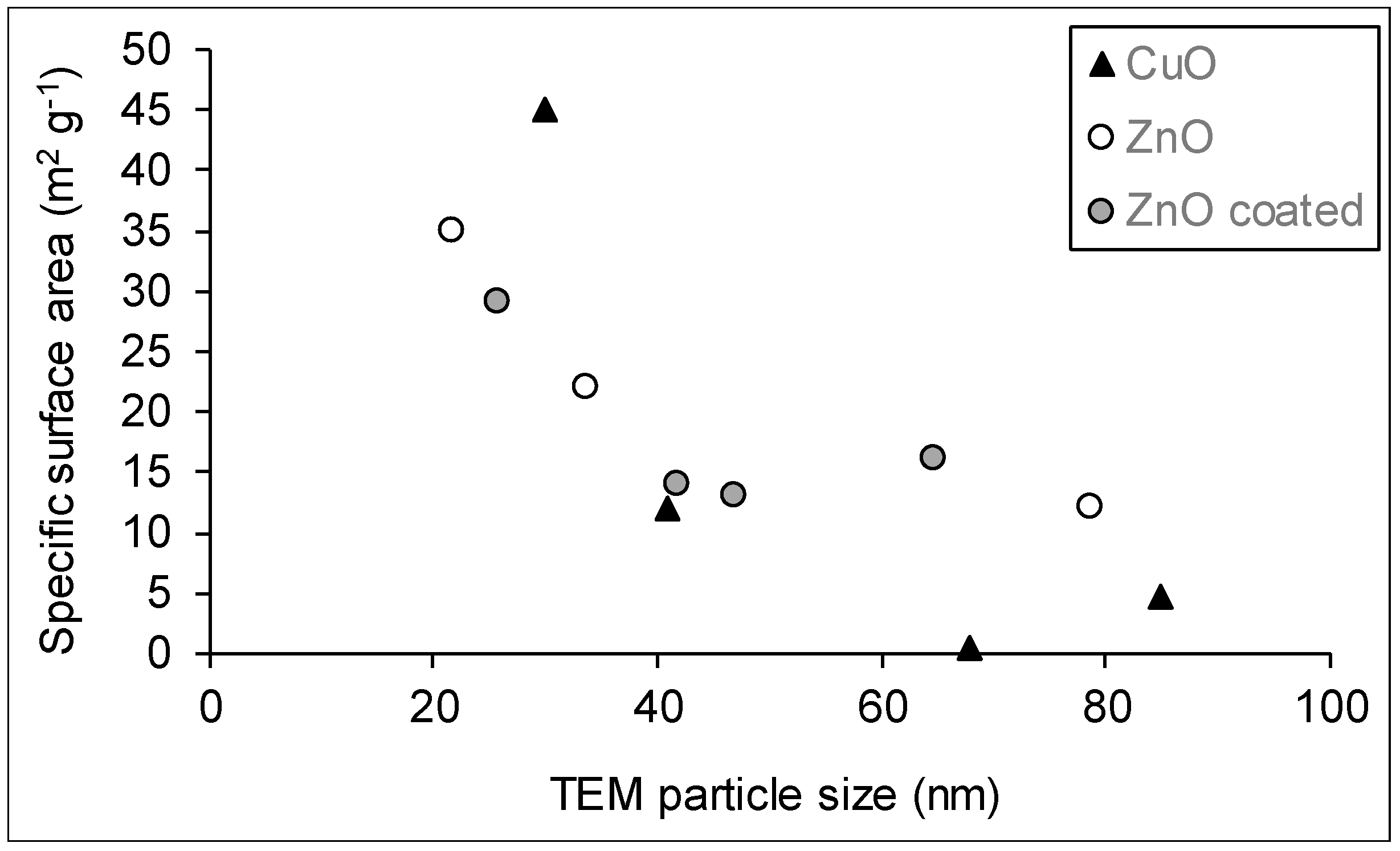
3.4. Specific Surface Area
4. Implications for Risk Assessment
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krug, H.F. Nanosafety research—Are we on the right track? Angew. Chem. Int. Ed. 2014, 53, 12304–12319. [Google Scholar] [CrossRef] [Green Version]
- DeLoid, G.M.; Cohen, J.M.; Pyrgiotakis, G.; Demokritou, P. Preparation, characterization, and in vitro dosimetry of dispersed, engineered nanomaterials. Nat. Protoc. 2017, 12, 355–371. [Google Scholar] [CrossRef]
- Gao, X.; Lowry, G.V. Progress towards standardized and validated characterization for measuring physicochemical properties of manufactured nanomaterials relevant to nano health and safety risks. NanoImpact 2018, 9, 14–30. [Google Scholar] [CrossRef]
- Rasmussen, K.; Rauscher, H.; Mech, A.; Riego Sintes, J.; Gilliland, D.; Gonzalez, M.; Kearns, P.; Moss, K.; Visser, M.; Groenewold, M.; et al. Physico-chemical properties of manufactured nanomaterials—Characterization and relevant methods. An outlook based on the OECD testing programme. Regul. Toxicol. Pharmacol. 2018, 92, 8–28. [Google Scholar] [CrossRef]
- Meli, F.; Klein, T.; Buhr, E.; Frase, C.G.; Gleber, G.; Krumrey, M.; Duta, A.; Duta, S.; Korpelainen, V.; Bellotti, R.; et al. Traceable size determination of nanoparticles, a comparison among European metrology institutes. Meas. Sci. Tech. 2012, 23, 125005. [Google Scholar] [CrossRef]
- Rice, S.B.; Chan, C.; Brown, S.C.; Eschbach, P.; Han, L.; Esnor, D.S.; Stefaniak, A.B.; Bonevich, J.; Vladar, A.E.; Hight Walker, A.R.; et al. Particle size distributions by transmission electron microscopy: An interlaboratory comparison case study. Metrologia 2013, 50, 663–678. [Google Scholar] [CrossRef] [Green Version]
- Grulke, E.A.; Wu, X.; Ji, Y.; Buhr, E.; Yamamoto, K.; Song, N.W.; Stefaniak, A.B.; Schwegler-Berry, D.; Burchett, W.W.; Lambert, J.; et al. Differentiating gold nanorod samples using particle size and shape distributions from transmission electron microscope images. Metrologia 2018, 55, 254–267. [Google Scholar] [CrossRef]
- Mülhopt, S.; Diabaté, S.; Dilger, M.; Adelhelm, C.; Anderlohr, C.; Bergfeldt, T.; Gómez de la Torre, J.; Jiang, Y.; Valsami-Jones, E.; Langevin, D.; et al. Characterization of nanoparticle batch-to-batch variability. Nanomaterials 2018, 8, 311. [Google Scholar] [CrossRef] [Green Version]
- Babick, F.; Mielke, J.; Wohlleben, W.; Weigel, S.; Hodoroaba, V.-D. How reliably can a material be classified as a nanomaterial? Available particle sizing techniques at work. J. Nanopart. Res. 2016, 18, 158. [Google Scholar] [CrossRef] [Green Version]
- Avramescu, M.L.; Rasmussen, P.E.; Chénier, M.; Gardner, H.D. Influence of pH, particle size and crystal form on dissolution behavior of engineered nanomaterials. Environ. Sci. Pollut. Res. Int. 2017, 24, 1553–1564. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, P.; Avramescu, M.-L.; Chénier, M.; Gardner, D. Purchasing metal oxide nanopowders for research: Buyers beware! In Proceedings of the NanoSAFE’18 Conference, Grenoble, France, 5–9 November 2018. [Google Scholar]
- Kunc, F.; Balhara, V.; Brinkmann, A.; Sun, Y.; Leek, D.M.; Johnston, L.J. Quantification and stability of surface amine groups on silica nanoparticles using solution NMR. Anal. Chem. 2018, 90, 13322–13330. [Google Scholar]
- Sun, Y.; Kunc, F.; Balhara, V.; Coleman, B.; Kodra, O.; Reza, M.; Chen, M.; Brinkmann, A.; Lopinski, G.P.; Johnston, L.J. Quantification of amine functional groups on silica nanoparticles: A multi-method approach. Nanoscale Adv. 2019, 1, 1598–1607. [Google Scholar] [CrossRef] [Green Version]
- Kunc, F.; Kodra, O.; Brinkmann, A.; Lopinski, G.P.; Johnston, L.J. A multi-method approach for quantification of surface coatings on commercial zinc oxide nanomaterials. Nanomaterials 2020, 10, 678. [Google Scholar] [CrossRef] [Green Version]
- Wohlleben, W.; Ma-Hock, L.; Boyko, V.; Cox, G.; Egenolf, H.; Freiberger, H.; Hinrichsen, B.; Hirth, S.; Landsiedel, R. Nanospecific guidance in reach: A comparative physical chemical characterization of 15 materials with methodical correlations. J. Ceram. Sci. Tech. 2013, 4, 93–104. [Google Scholar]
- Farcal, L.; Torres Andon, F.; Di Cristo, L.; Rotoli, B.M.; Bussolati, O.; Bergamaschi, E.; Mech, A.; Hartmann, N.B.; Rasmussen, K.; Reigo-Sintes, J.; et al. Comprehensive in vitro toxicity testing of a panel of representative oxide nanomaterials: First steps towards an intelligent testing strategy. PLoS ONE 2015, 10, e0127174. [Google Scholar] [CrossRef]
- Kermanizadeh, A.; Gosens, I.; MacCalman, L.; Johnston, H.; Danielsen, P.H.; Jacobsen, N.R.; Lenz, A.-G.; Fernandes, T.; Schins, R.P.F.; Cassee, F.R.; et al. A multilaboratory toxicological assessment of a panel of 10 engineered nanomaterials to human health—ENPRA project—The highlights, limitations, and current and future challenges. J. Toxic. Environ. Health, Part. B 2016, 19, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Mirshafiee, V.; Sun, B.; Chang, C.H.; Liao, Y.-P.; Jiang, W.; Jiang, J.; Liu, X.; Wang, X.; Xia, T.; Nel, A.E. Toxicological profiling of metal oxide nanoparticles in liver context reveals pyroptosis in Kupffer cells and macrophages versus apoptosis in hepatocytes. ACS Nano 2018, 12, 3836–3852. [Google Scholar] [CrossRef]
- Nath, D.; Singh, F.; Das, R. X-ray diffraction analysis by Williamson-Hall, Halder-Wagner and size-strain plot methods of CdSe nanoparticles- a comparative study. Mater. Chem. Phys. 2020, 239, 122021. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Uvarov, V.; Popov, I. Metrological characterization of X-ray diffraction methods for determination of crystallite size in nano-scale materials. Mater. Charact. 2007, 58, 883–891. [Google Scholar] [CrossRef]
- Uvarov, V.; Popov, I. Metrological characterization of X-ray diffraction methods at different acquisition geometries for determination of crystallite size in nano-scale materials. Mater. Charact. 2013, 85, 111–123. [Google Scholar] [CrossRef]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Chenari, H.M.; Seibel, C.; Hauschild, D.; Reinert, F.; Abdollahian, H. Titanium dioxide nanoparticles: Synthesis, X-ray line analysis and chemical composition study. Mater. Res. 2016, 19, 1319–1323. [Google Scholar] [CrossRef]
- Kumar, S.; Mote, V.; Prakash, R.; Kumar, V. X-ray analysis of α-Al2O3 particles by Williamson–Hall methods. Mater. Focus 2016, 5, 545–549. [Google Scholar] [CrossRef]
- Hu, F.; Wu, X.; Wang, Y.; Lai, X. Ultrathin-Al2O3 nanofibers with large specific surface area and their enhanced thermal stability by si-doping. RSC Adv. 2015, 5, 54053–54058. [Google Scholar] [CrossRef]
- Lodziana, Z.; Parlinski, K. Dynamical stability of the alpha and theta phases of alumina. Phys. Rev. B Condens. Matter 2003, 67, 174106. [Google Scholar] [CrossRef] [Green Version]
- Weibel, A.; Bouchet, R.; Boulc’h, F.; Knauth, P. The big problem of small particles: A comparison of methods for determination of particle size in nanocrystalline anatase powders. Chem. Mater. 2005, 17, 2378–2385. [Google Scholar] [CrossRef]
- Wohlleben, W.; Mielke, J.; Bianchin, A.; Ghanem, A.; Freiberger, H.; Rauscher, H.; Gemeinert, M.; Hodoroaba, V.-D. Reliable nanomaterial classification of powders using the volume-specific surface area method. J. Nanopart. Res. 2017, 19, 61. [Google Scholar] [CrossRef] [Green Version]
- SRM 1898, NIST, Standard Reference Material; NIST: Gaithersburg, MD, USA, 1989.
- Misra, S.K.; Dybowska, A.; Berhanu, D.; Louma, S.N.; Valsami-Jones, E. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci. Total Environ. 2012, 438, 225–232. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Elder, A.; Gelein, R.; Mercer, P.; Biswas, P. Does nanoparticle activity depend upon size and crystal phase? Nanotoxicology 2008, 2, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Tang, Y.; Tang, F.G.; Li, X.L.; Xu, S.; Fan, X.Y.; Yang, Y.J. Cellular toxicity of TiO2 nanoparticles in anatase and rutile crystal phase. Biol. Trace Elem. Res. 2011, 141, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.; Kennedy, A.J.; Detzel, C.; Vikesland, P.; Chappel, M.A. Moving beyond mass: The unmet need to consider dose metrics in environmental nanotoxicology studies. Environ. Sci. Technol. 2012, 46, 10881–10882. [Google Scholar] [CrossRef] [PubMed]
- Arts, J.H.E.; Hadi, M.; Irfan, M.-A.; Keene, A.M.; Kreiling, R.; Lyon, D.; Maier, M.; Michel, K.; Petry, T.; Sauer, U.G.; et al. A decision-making framework for the grouping and testing of nanomaterials (DF4nanogrouping). Reg. Tox. Pharm. 2015, 71, S1–S27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oomen, A.G.; Bleeker, E.A.J.; Bos, P.M.J.; van Broekhuizen, F.; Gottardo, S.; Gronewold, M.; Hristozov, D.; Hund-Rinke, K.; Irfan, M.-A.; Marcomini, A.; et al. Grouping and read-across approaches for risk assessment of nanomaterials. Int. J. Environ. Res. Public Health 2015, 12, 13415–13434. [Google Scholar] [CrossRef] [Green Version]




| Code | Formula 1 | Trade Name 2 | Supplier 3 | Purity | Coating |
|---|---|---|---|---|---|
| Al-01 | Al2O3 | N/A | Sigma Aldrich (cat no. 544833) | uncoated | |
| Al-02 | Al2O3 | Aeroxide Alu C | Sigma Aldrich (cat no. 718475) | 99.8% | uncoated |
| Al-03 | Al2O3 | N/A | Skyspring Nanomaterials, Inc. | 99.99% | hydrophobic coating |
| Al-04 | Al2O3 | Aluminium oxide, alpha phase | Plasmachem GmbH | 99.8% | uncoated |
| Al-05 | Al2O3 | Aluminium oxide, gamma phase | Plasmachem GmbH | >99.9% | uncoated |
| Al-06 | Al2O3 | Aluminium oxide, theta phase | Plasmachem GmbH | 99.8% | uncoated |
| Al-07 | Al2O3 | N/A | mkNano | 99% | unspecified |
| Cu-01 | CuO | N/A | Sigma Aldrich (cat no. 544868) | >95% | uncoated |
| Cu-02 | CuO | N/A | Sigma Aldrich (cat no. 792004) | 100% | uncoated |
| Cu-03 | CuO | N/A | mkNano | 99% | uncoated |
| Cu-04 | CuO | N/A | Plasmachem GmbH | >99% | uncoated |
| Ti-01 | TiO2 | SRM 1898/Aeroxide TiO2 P25 | NIST | 99.4% | uncoated |
| Ti-02 | TiO2 | Kronos 2310 titanium dioxide | Brenntag Canada | >92.5% | surface treated with Al, Si and Zr compounds |
| Ti-03 | TiO2 | TiO2-Anatase, 50 nm | mkNano | 98% | uncoated |
| Ti-04 | TiO2 | TiO2-Anatase, 100 nm | mkNano | 99.7% | uncoated |
| Ti-05 | TiO2 | TiO2-Rutile, 50 nm | mkNano | 99% | uncoated |
| Ti-06 | TiO2 | TiO2-Anatase, 5 nm, water dispersible | mkNano | 99.9% | uncoated |
| Ti-07 | TiO2 | Titanium oxide, silica coated anatase/rutile, 20 nm | Nanostructured & Amorphous Materials, Inc. | >96% | silica coated |
| Ti-08 | TiO2 | Titanium oxide, silica & alumina coated, anatase/rutile, 20 nm | Nanostructured & Amorphous Materials, Inc. | >92% | silica and alumina coated |
| Ti-09 | TiO2 | Titanium oxide, silica & stearic acid coated anatase/rutile, 20 nm | Nanostructured & Amorphous Materials, Inc. | >96% | silica & stearic acid coated |
| Ti-10 | TiO2 | Titanium oxide, silica & silicone oil coated anatase/rutile, 20 nm | Nanostructured & Amorphous Materials, Inc. | >92% | silica & silicone oil coated |
| Ti-11 | TiO2 | N/A | Sigma (cat no. 774510) | unspecified | |
| Zn-01 | ZnO | N/A | US Research Nanomaterials Inc. | >99% | uncoated |
| Zn-02 | ZnO | N/A | Skyspring Nanomaterials, Inc. (Cat no. 8412DL) | 99% | treated w/stearic acid |
| Zn-03 | ZnO | N/A | Nanostructured & Amorphous Materials, Inc. | >99% | uncoated |
| Zn-04 | ZnO | N/A | Nanostructured & Amorphous Materials, Inc. | 98% | 3-amino-propyltri-ethoxysilane |
| Zn-05 | ZnO | N/A | Nanostructured & Amorphous Materials, Inc. | 98% | silicone oil (lipophilic) |
| Zn-06 | ZnO | Z-COTE | BASF | 100% | uncoated, hydrophobic |
| Zn-07 | ZnO | Z-COTE HP1 | BASF | 97.5% | triethoxy-caprylylsilane |
| XRD Results | Suppliers | ||||
|---|---|---|---|---|---|
| Sample | Phases Detected | Crystallite Size, nm | ±SD | Phases Detected | Particle Size, nm |
| Al-01 | Gamma/delta Al2O3 1 | 4 | 0.4 | gamma phase | < 50 |
| Minor: Corundum α-Al2O3) | 53 | 9.6 | N/A 2 | N/A | |
| Al-02 | Delta-Al2O3 | 5 | 2.7 | N/A | 13 3 |
| Traces corundum (α-Al2O3) | N/A | N/A | N/A | N/A | |
| Al-03 | Corundum (α-Al2O3) | 76 | 8.0 | alpha phase | <100 |
| Minor: Theta-Al2O3 | 11 | 2.4 | N/A | N/A | |
| Al-04 | Corundum (α-Al2O3) | 77 | 5.4 | alpha phase | 40 |
| Minor: Theta-Al2O3 | 13 | 3.5 | N/A | N/A | |
| Al-05 | Gamma-Al2O3 | 3 | 0.8 | gamma phase | 40 |
| Minor: Corundum (α-Al2O3) | 49 | 38.6 | N/A | N/A | |
| Al-06 | Theta-Al2O3 | 10 | 9.1 | theta phase | 15 |
| Al-07 | Dialuminum trioxide | 2 | 1.2 | N/A (nanowires) | 5 × 1000 |
| Minor: Aluminum oxide | 10 | 2.5 | |||
| Cu-01 | 100% CuO | 49 | 28.1 | CuO | <50 |
| Cu-02 | 100% CuO | 14 | 5.4 | CuO (nanotubes) | (10−12) × (75−100) |
| Cu-03 | 100% CuO | 46 | 15.3 | CuO | 40 |
| Cu-04 | 100% CuO | 19 | 6.5 | CuO | 40 |
| Ti-01 | 87.7% Anatase (β-TiO2) | 21 | 5.0 | Anatase, 76% | 19 ± 2 |
| 12.3% Rutile (α-TiO2) | 26 | 7.7 | Rutile, 24% | 37 ± 6 | |
| Ti-02 | 100% Rutile (α-TiO2) | 146 | 23.7 | Rutile | |
| Ti-03 | 98.8% Anatase (β-TiO2) | 16 | 2.2 | Anatase | 50 |
| 1.2% Rutile (α-TiO2) | N/A | N/A | |||
| Ti-04 | 100% Anatase (β-TiO2) | 19 | 6.4 | Anatase | 100 |
| Ti-05 | 98.8% Rutile (α-TiO2) | 49 | 9.0 | Rutile | 50 |
| 1.2% Anatase (β-TiO2) | 27 | 10.9 | |||
| Ti-06 | 100% Anatase (β-TiO2) | 6 | 1.8 | Anatase | 5 |
| Ti-07 | 92.7% Anatase (β-TiO2) | 26 | 9.5 | Anatase, 80–90% | 20 |
| 7.3% Rutile (α-TiO2) | N/A | N/A | Rutile, 10–20% | ||
| Ti-08 | 85% Anatase (β-TiO2) | 29 | 4.9 | Anatase, 80–90% | 20 |
| 15% Rutile (α-TiO2) | 49 | 11.0 | Rutile, 10–20% | ||
| Ti-09 | 92.3% Anatase (β-TiO2) | 26 | 9.1 | Anatase, 80–90% | 20 |
| 7.7% Rutile (α-TiO2) | N/A | N/A | Rutile, 10–20% | ||
| Ti-10 | 88.8% Anatase (β-TiO2) | 27 | 4.9 | Anatase, 80–90% | 20 |
| 11.2% Rutile (α-TiO2) | 46 | 6.4 | Rutile, 10–20% | ||
| Ti-11 | Beta-TiO2Other phases: unidentified | 25 | 22.9 | N/A (nanowire) | 100 × 10,000 |
| Zn-01 | 100% Zincite (ZnO) | 18 | 3.4 | ZnO | 35–45 |
| Zn-02 | 100% Zincite (ZnO) | 22 | 6.1 | ZnO | 10–30 |
| Zn-03 | 100% Zincite (ZnO) | 32 | 4.7 | ZnO | 30 |
| Zn-04 | 100% Zincite (ZnO) | 30 | 5.3 | ZnO | 30 |
| Zn-05 | 100% Zincite (ZnO) | 41 | 7.7 | ZnO | 30 |
| Zn-06 | 100% Zincite (ZnO) | 50 | 3.8 | ZnO | 53 ± 23 |
| Zn-07 | 100% Zincite (ZnO) | 42 | 2.9 | ZnO | 54 ± 26 |
| Sample | TEM | Supplier 1 | ||||||
|---|---|---|---|---|---|---|---|---|
| Shape 2 | n 3 | deq, (std Error), nm 4 | SD, nm 4 | Aspect Ratio | SD | Shape | Size, nm (Method) | |
| Al-01 | nanorods, IR | N/A | <50 (TEM) | |||||
| Al-02 | SP, EL, IR | 128 | 13.8 (0.3) | 4 | 1.4 | 0.2 | 13 5 (TEM) | |
| Al-03 | EL, IR | N/A | <100 | |||||
| Al-04 | ~SP, HX | 158 | 58 (3) | 44 | 1.1 | 0.2 | spherical | ~40 (TEM) |
| Al-05 | nanorods, plates | 232 | 14.2 (0.3) 21, 10 | 5 | 2.3 | 1.2 | spherical, elongated | ~40 (TEM) |
| Al-06 | EL, IR | 111 | 15.5 (0.4) | 4 | 1.3 | 0.3 | ~15 | |
| Al-07 | nanowires | 119 | 6.0 (0.1) 6 | 1.2 | nanowire | 5 × 1000 6 | ||
| Cu-01 | SP, EL | 78 | 85 (5) | 44 | 1.3 | 0.2 | < 50 (TEM) | |
| Cu-02 | nanorods, IR | 71 | 30 (1) 54, 18 | 9 | 3.1 | 1.4 | nanotube | (10−12) × (75−100) |
| Cu-03 | SP, IR | 108 | 68 (4) | 40 | 1.3 | 0.2 | 40 | |
| Cu-04 | SP, EL, IR | 105 | 41 (1) | 12 | 1.4 | 0.2 | spherical | ~40 |
| Ti-01 | SP, EL, EL, RC | 137 | 18.7 (0.5) | 6 | 1.3 | 0.2 | 19, anatase; 37, rutile (XRD) | |
| Ti-02 | SP, EL, IR | 129 | 209 (5) | 60 | 1.3 | 0.2 | ||
| Ti-03 | IR | 52 | 30 (1) | 7 | 1.4 | 0.3 | 50 | |
| Ti-04 | EL, IR | 75 | 31 (1) | 10 | 1.5 | 0.4 | 100 | |
| Ti-05 | EL, RC, IR | 114 | 26.4 (0.7) | 8 | 1.3 | 0.2 | 50 | |
| Ti-06 | single particles not detected | N/A | <5 | <5 | ||||
| Ti-07 | EL, RC, IR | 121 | 38 (1) | 14 | 1.6 | 0.4 | spherical, ellipsoidal | 20 (TEM) |
| Ti-08 | EL, RC, IR | 100 | 40 (1) | 13 | 1.4 | 0.3 | spherical, ellipsoidal | 20 (TEM) |
| Ti-09 | SP, EL, RC | 106 | 36 (1) | 11 | 1.7 | 0.4 | spherical | 20 |
| Ti-10 | SP, EL, IR | 101 | 42 (1) | 15 | 1.5 | 0.5 | spherical | 20 |
| Ti-11 | Nanorods | 90 | 790 (40), 65 (3) | 377, 32 | 13 | 7 | nanowires | 100 × 10,000 |
| Zn-01 | SP, EL | 132 | 22 (1) | 8 | 1.4 | 0.2 | nearly spherical | 35–45 (TEM) |
| Zn-02 | SP, EL, HX | 77 | 26 (1) | 8 | 1.3 | 0.2 | 10–30 | |
| Zn-03 | SP, EL, RC | 71 | 34 (2) | 19 | 1.3 | 0.2 | spherical | 30 (TEM) |
| Zn-04 | SP, EL, RC | 124 | 65 (2) | 25 | 1.3 | 0.2 | spherical | 30 |
| Zn-05 | SP, EL, IR | 129 | 42 (1) | 15 | 1.3 | 0.2 | spherical | 30 |
| Zn-06 | EL, IR | 132 | 79 (3) | 34 | 1.4 | 0.3 | 53 ± 23 | |
| Zn-07 | rods, EL, RC | 154 | 47 (3) | 44 | 2.3 | 1.5 | 54 ± 26 | |
| Sample | Specific Surface Area, m2/g | ||
|---|---|---|---|
| BET | Supplier 1 | Calculated (TEM) 2 | |
| Al-01 | 129 | >40 | |
| Al-02 | 97 | 85–115 | |
| Al-03 | 16, 35 3 | ||
| Al-04 | 9.8 | >10 | 25.9 |
| Al-05 | 124 | >40 | |
| Al-06 | 100 | 90–110 | |
| Al-07 | 466 | ||
| Cu-01 | 4.6 | 29 | 11.2 |
| Cu-02 | 45 | 60–100 | 31.7 |
| Cu-03 | 0.5 | 80 | 14.0 |
| Cu-04 | 12 | >10 | 23.2 |
| Ti-01 4 | 54 | 55.55 | 83.5 |
| Ti-02 | 17 | 6.8 | |
| Ti-03 | 82 | 52.9 | |
| Ti-04 | 91 | 51.2 | |
| Ti-05 | 24 | 54.6 | |
| Ti-06 | 152 | 356 | |
| Ti-07 | 46 | 40 | 41.8 |
| Ti-08 | 28 | 40 | 39.7 |
| Ti-09 | 41 | 10 | 44.1 |
| Ti-10 | 16 | 10 | 37.8 |
| Ti-11 | 24 | ||
| Zn-01 | 35 | ~65 | 48.6 |
| Zn-02 | 29 | >60 | 41.2 |
| Zn-03 | 22 | 15 | 31.5 |
| Zn-04 | 16 | 8 | 16.5 |
| Zn-05 | 14 | 15 | 25.5 |
| Zn-06 | 12 | 13.5 | |
| Zn-07 | 13 | 22.8 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bushell, M.; Beauchemin, S.; Kunc, F.; Gardner, D.; Ovens, J.; Toll, F.; Kennedy, D.; Nguyen, K.; Vladisavljevic, D.; Rasmussen, P.E.; et al. Characterization of Commercial Metal Oxide Nanomaterials: Crystalline Phase, Particle Size and Specific Surface Area. Nanomaterials 2020, 10, 1812. https://doi.org/10.3390/nano10091812
Bushell M, Beauchemin S, Kunc F, Gardner D, Ovens J, Toll F, Kennedy D, Nguyen K, Vladisavljevic D, Rasmussen PE, et al. Characterization of Commercial Metal Oxide Nanomaterials: Crystalline Phase, Particle Size and Specific Surface Area. Nanomaterials. 2020; 10(9):1812. https://doi.org/10.3390/nano10091812
Chicago/Turabian StyleBushell, Michael, Suzanne Beauchemin, Filip Kunc, David Gardner, Jeffrey Ovens, Floyd Toll, David Kennedy, Kathy Nguyen, Djordje Vladisavljevic, Pat E. Rasmussen, and et al. 2020. "Characterization of Commercial Metal Oxide Nanomaterials: Crystalline Phase, Particle Size and Specific Surface Area" Nanomaterials 10, no. 9: 1812. https://doi.org/10.3390/nano10091812





