Carbon Nanotubes Improved the Germination and Vigor of Plant Species from Peatland Ecosystem Via Remodeling the Membrane Lipidome
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Treatments
- CNT: Imbibition for 24 h with select CNTs before exposure at room temperature.
- CNT + stratification: Imbibition for 24 h with select CNTs and incubated in the fridge at 2—4 °C for 15 days before exposure at room temperature.
2.2. Tetrazolium Test
2.3. Electrical Conductivity
2.4. Evaluation of Abnormal and Normal Seedlings
2.5. Germination Evaluation
2.6. Seedling Vigor Index (SVI)
2.7. Lipid Extraction
2.8. Analysis of Plant Membrane Lipids
2.9. Data Analysis
3. Results
3.1. Tetrazolium Test
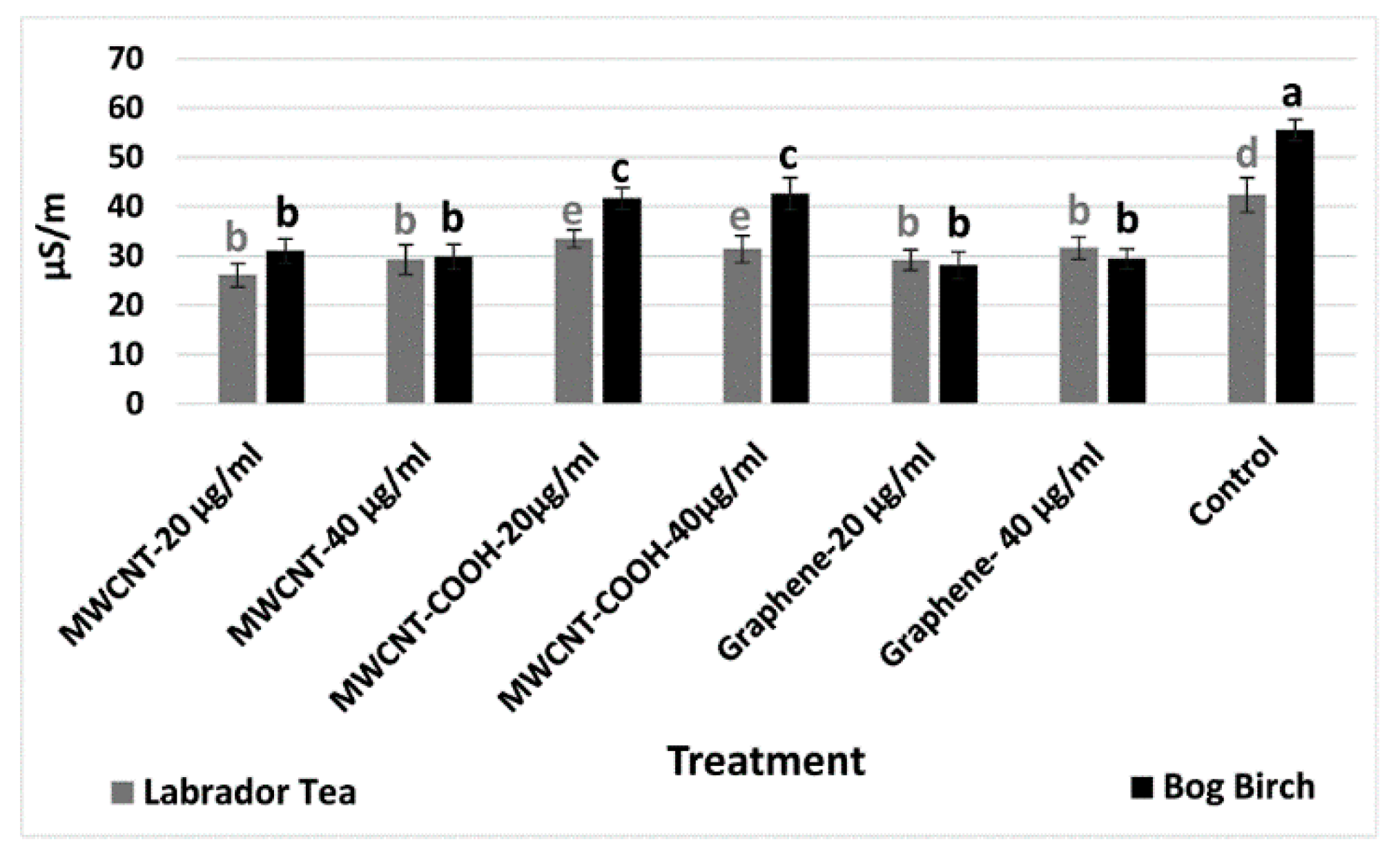
3.2. Electrical Conductivity Test
3.3. Evaluation of Normal and Abnormal Seedlings
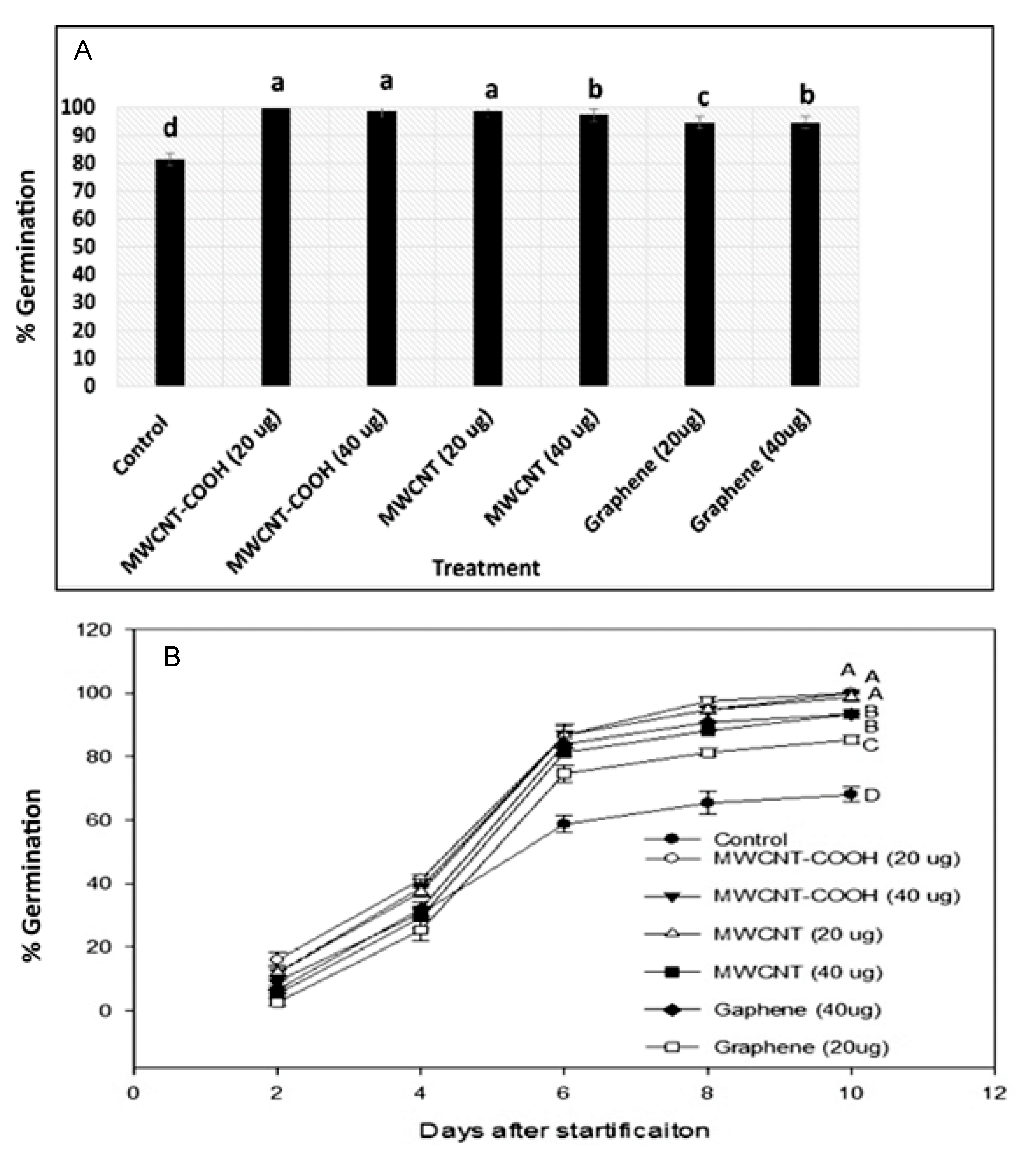
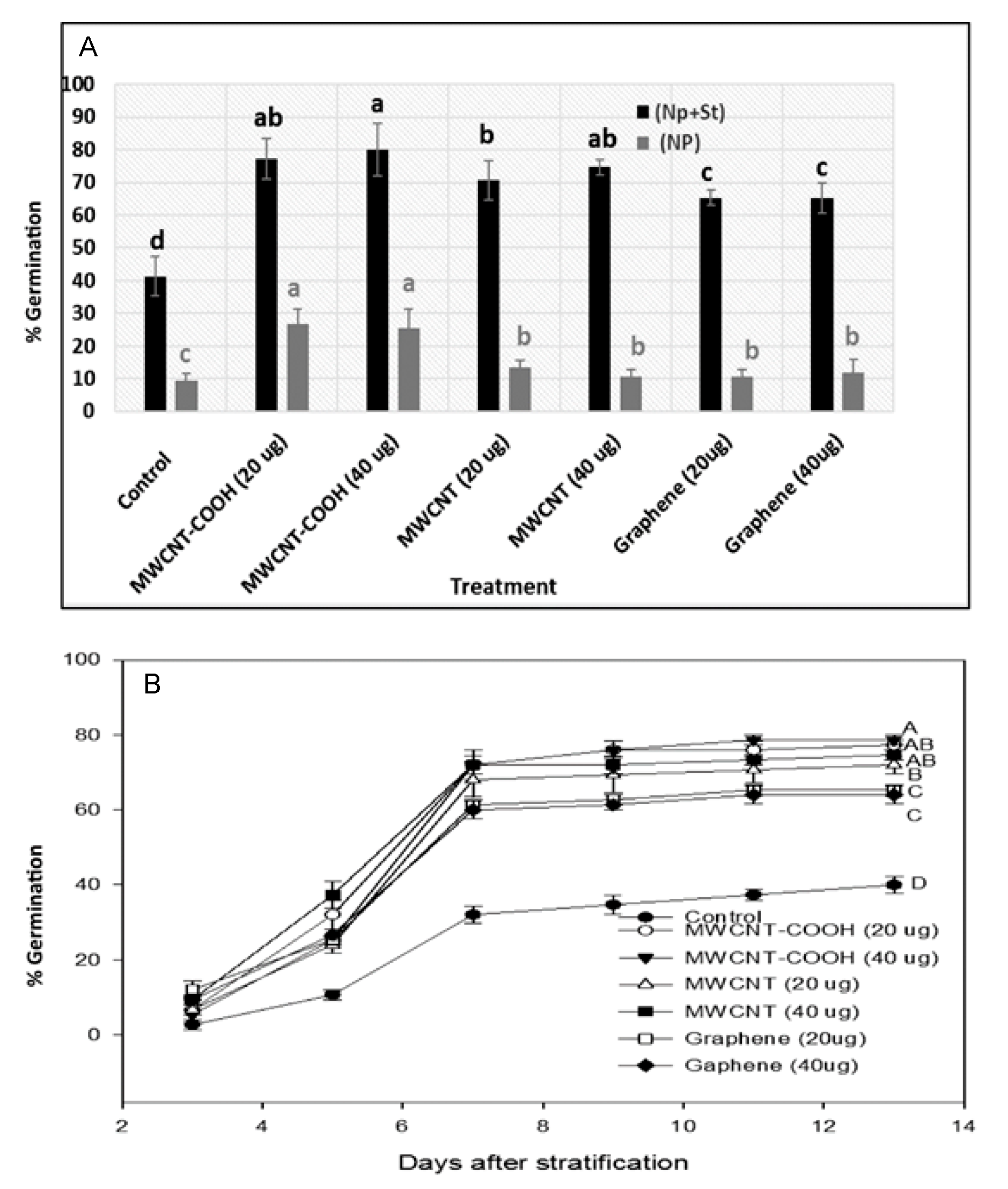
3.4. Germination Percentages
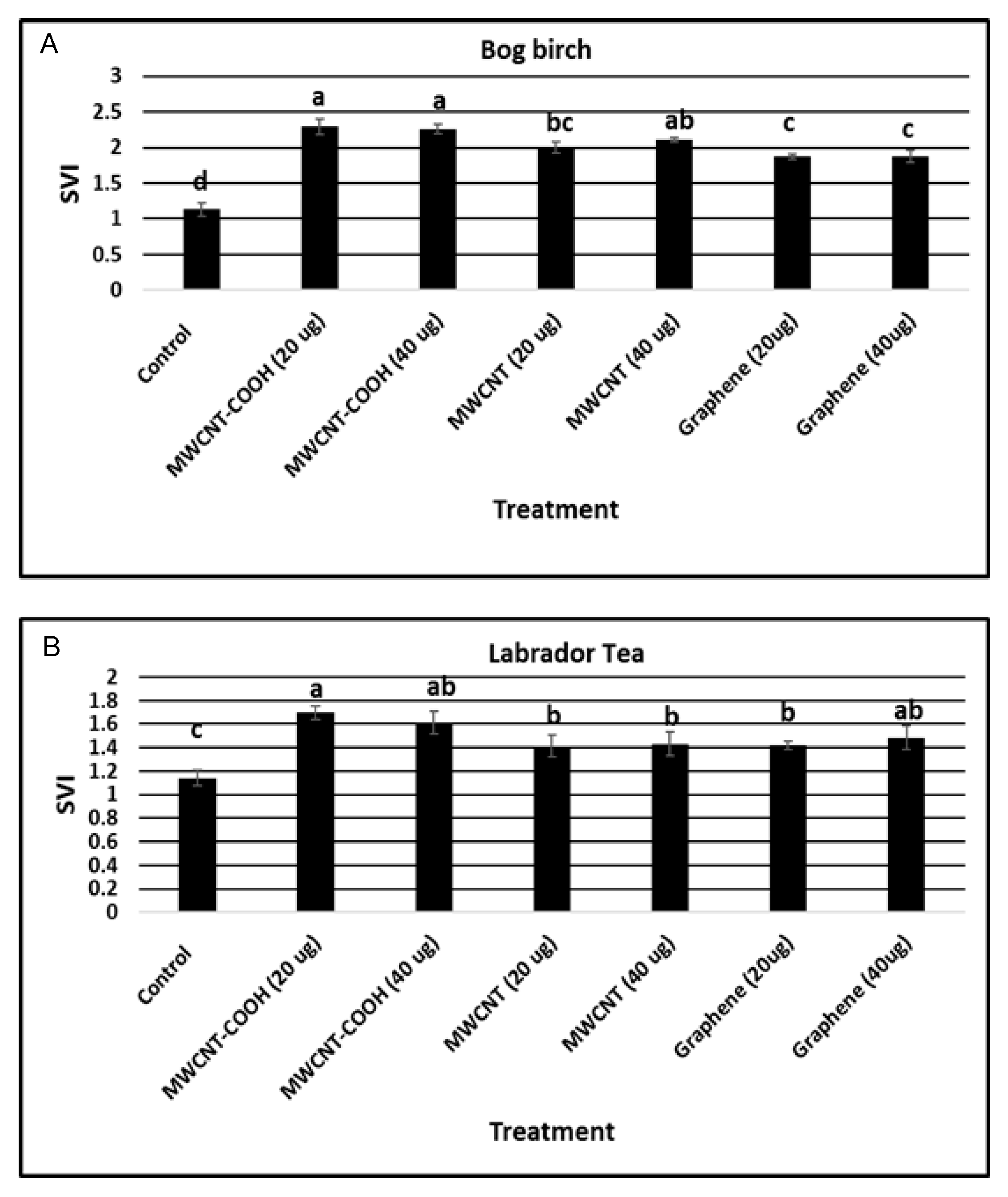
3.5. Seedling Vigor Index (SVI)
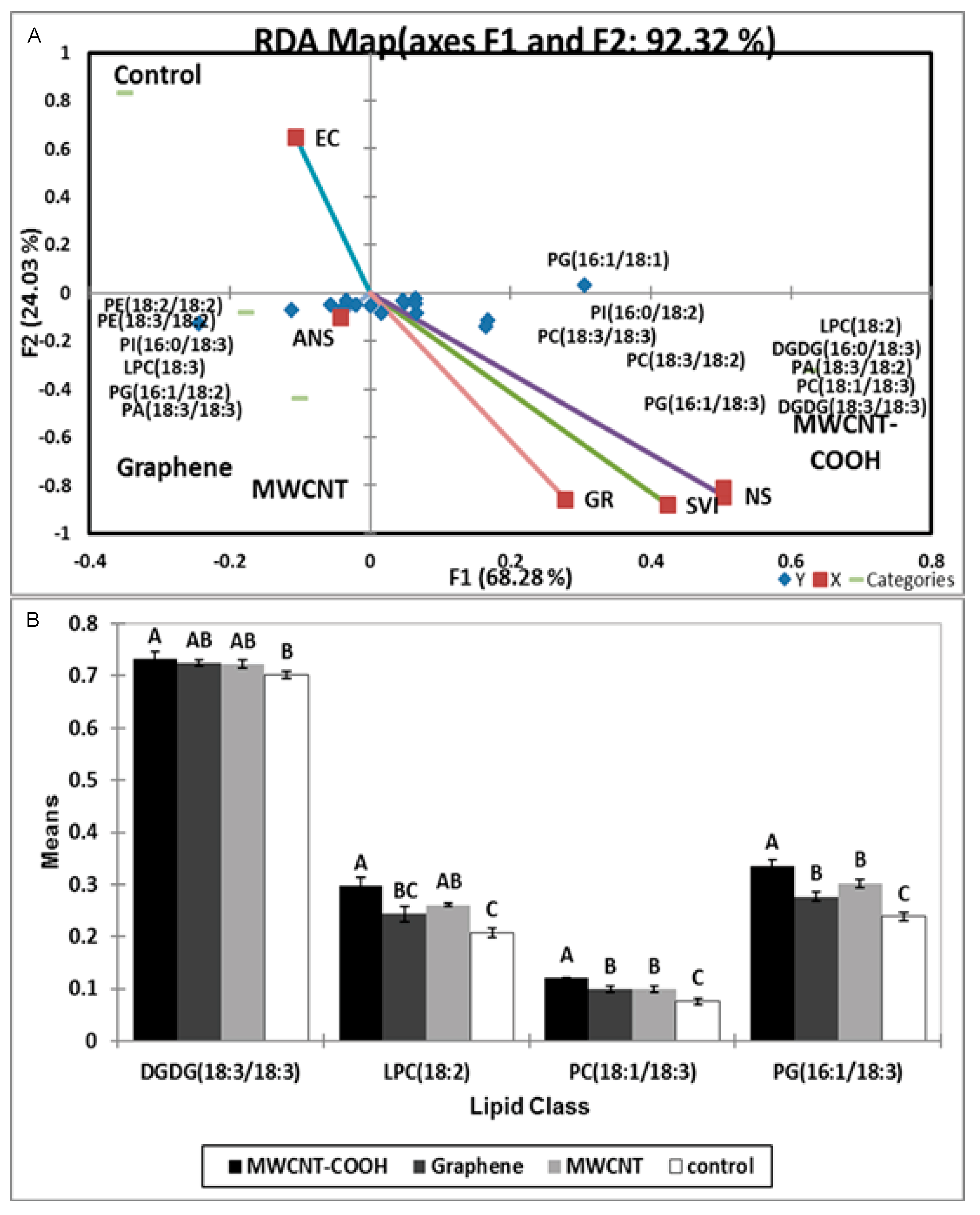
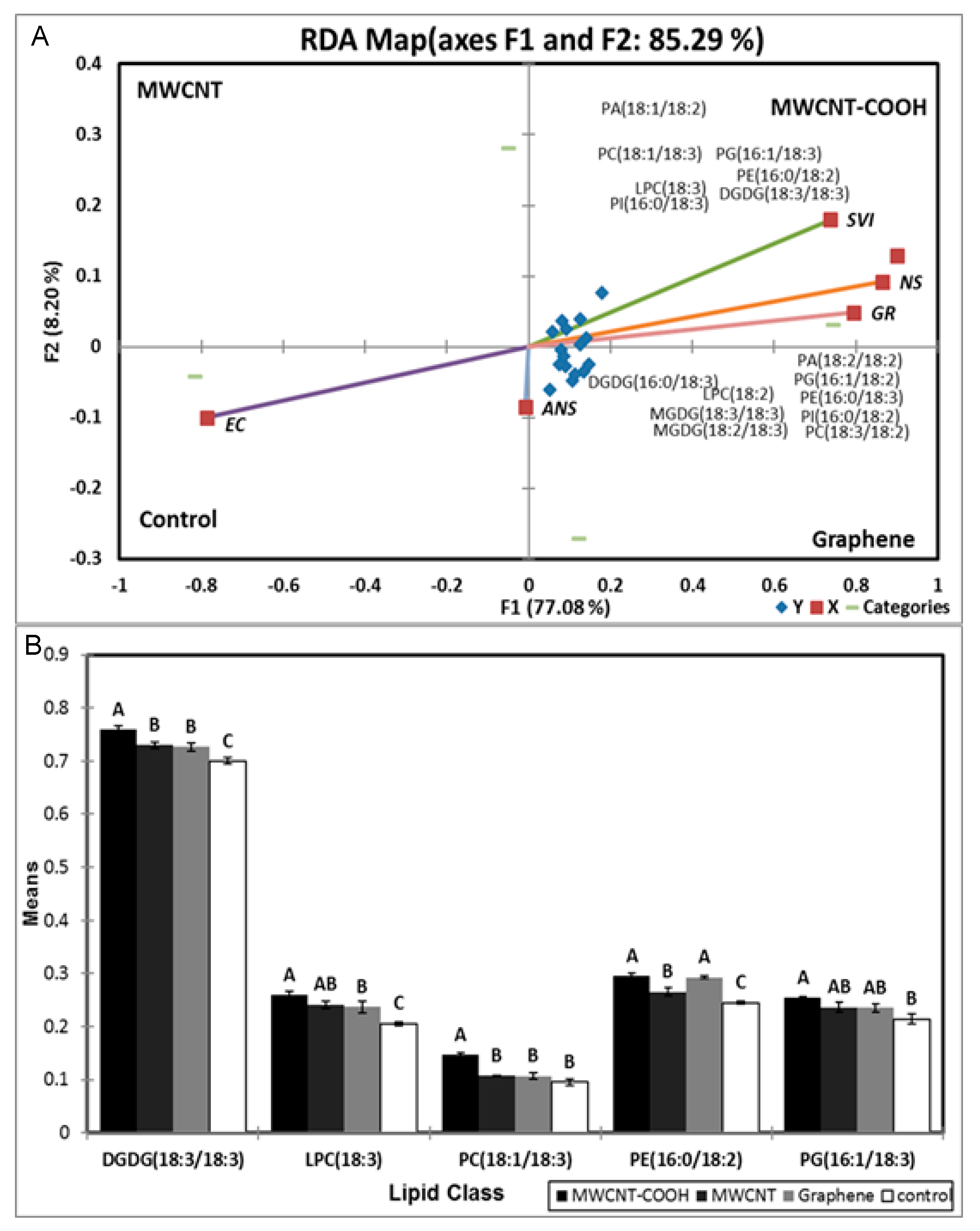
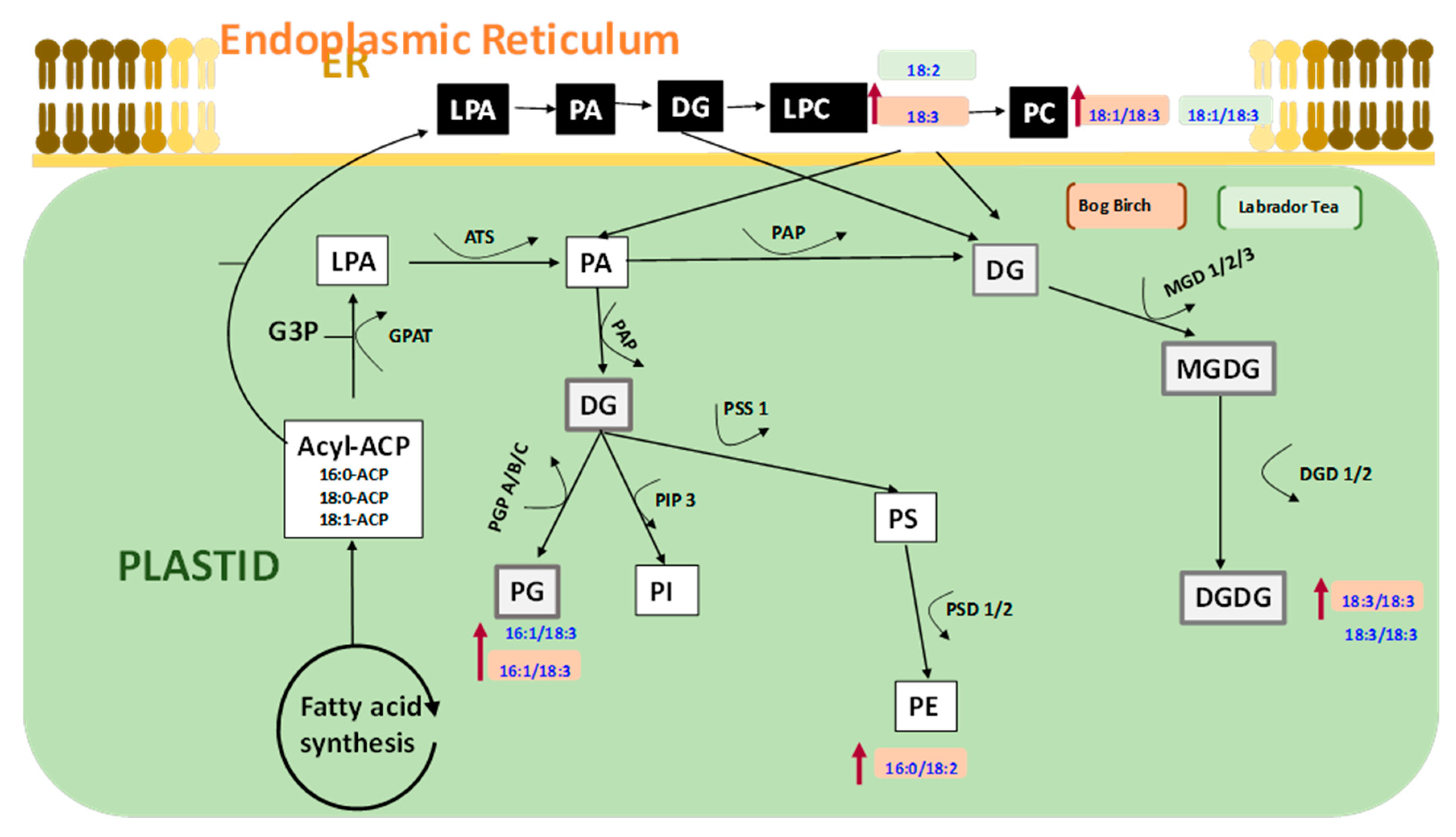
3.6. Possible Role of Lipid Metabolism in Resolving Seed Dormancy in Peatland Boreal Forest Species
4. Discussion
4.1. Effects of Seed Priming with Carbon Nanoparticles on Seedling Quality
4.2. Effect of Carbon Nanoparticles in Enhancing Germination and Overcoming Seed Dormancy
4.3. Effect of Carbon Nanoparticles in Enhancing Seedling Vigor Index
4.4. Possible Role of Lipid Metabolism in Resolving Seed Dormancy in Peatland Boreal Forest Species Following Nanopriming
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bentsink, L.; Koornneef, M. Seed Dormancy and Germination. Arab. Book Am. Soc. Plant Biol. 2008, 6, e0119. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [PubMed]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [PubMed]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Ali, M.H. Use of Nanotechnology to Improve Plant Performance in Boreal Forest Ecosystem. Master’s Thesis, Memorial University of Newfoundland, Grenfell Campus, St. John’s, NL, Canada, 2019. [Google Scholar]
- Ali, M.H.; Sobez, J.-M.; Pham, T.H.; Nadeem, M.; Liu, C.; Galagedara, L.; Cheema, M.; Thomas, R. Carbon nanoparticles functionlized with carboxylic acid improved the germination and seedling vigor in upland boreal forest species. Nanomaterials 2020, 10, 176. [Google Scholar] [CrossRef]
- Kelly, K.; Staden, J.; Bell, W. Seed coat structure and dormancy. Plant Growth Regul. 1992, 11, 201–209. [Google Scholar]
- Smykal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant Sci. 2014, 5, 351. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, B.; Wang, Q.; Shi, X.; Xiao, Z.; Lin, J.; Fang, X. Carbon nanotubes as molecular transporters for walled plant cells. Nano Lett. 2009, 9, 1007–1010. [Google Scholar] [CrossRef]
- Kaur, J.; Schoonmaker, A.; Sobze, J.-M. Length of cold stratification period affects germination in green alder (Alnus viridis (Chaix) DC. subsp. crispa (Aiton) Turrill) seed collected from northwestern Alberta. Native Plants J. 2016, 17, 95–102. [Google Scholar]
- George, Y. Birch Seeds Will Germinate under a Water-Light Treatment without Pre-Chilling; Forest Research Note NE-124; US Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Upper Darby, PA, USA, 1961; p. 124.
- Hallett, B.P.; Bewley, J.D. Membranes and seed dormancy: Beyond the anaesthetic hypothesis. Seed Sci. Res. 2002, 12, 69–82. [Google Scholar] [CrossRef]
- Ramos, K.M.O.; Matos, J.M.M.; Martins, R.C.C.; Martins, I.S. Electrical Conductivity Testing as Applied to the Assessment of Freshly Collected Kielmeyera coriacea Mart. Seeds. ISRN Agron. 2012, 2012, 1–5. [Google Scholar] [CrossRef]
- Vieira, R.D.; Scappa Neto, A.; Bittencourt, S.R.M.D.; Panobianco, M. Electrical conductivity of the seed soaking solution and soybean seedling emergence. Sci. Agric. 2004, 61, 164–168. [Google Scholar] [CrossRef]
- Ahmet Korkmaz, N.O.; Benian, E. Assessment of Vigor Characteristics of Processing Tomato Cultivars by Using Various Vigor Tests. Asian J. Plant Sci. 2004, 3, 181–186. [Google Scholar]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 1–21. [Google Scholar] [CrossRef]
- Sivasubramaniam, K.; Geetha, R.; Sujatha, K.; Raja, K.; Sripunitha, A.; Selvarani, R. Seed Priming: Triumphs and Tribulations. Madras Agric. J. 2011, 98, 197–209. [Google Scholar]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, J.; Anand, A.; Nagarajan, S. Biochemical and biophysical changes associated with magnetopriming in germinating cucumber seeds. Plant Physiol. Biochem. 2012, 57, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Kartoolinejad, D.; Naghdi, R. Effects of priming with multi-walled carbon nanotubes on seed physiological characteristics of Hopbush (Dodonaeaviscosa L.) under drought stress. Int. J. Environ. Stud. 2017, 74, 528–539. [Google Scholar] [CrossRef]
- Li-Shar, H.; Claus, G. Lipid and Fatty Acid Changes During Germination of Alfalfa Seeds. Phytochemistry 1990, 29, 1441–1445. [Google Scholar]
- Yu, X.M.; Li, A.H.; Li, W.Q. How membranes organize during seed germination: Three patterns of dynamic lipid remodelling define chilling resistance and affect plastid biogenesis. Plant Cell Environ. 2015, 38, 1391–1403. [Google Scholar] [CrossRef]
- Ma, Z.H.; Bykova, N.V.; Igamberdiev, A.U. Cell signaling mechanisms and metabolic regulation of germination and dormancy in barley seeds. Crop J. 2017, 5, 459–477. [Google Scholar] [CrossRef]
- De Paiva, E.P.; Torres, S.B.; De Almeida, J.P.N.; Sá, F.V.D.S.; Oliveira, R.R.T. Tetrazolium test for the viability of gherkin seeds. Rev. Cienc. Agron. 2017, 48, 118–124. [Google Scholar] [CrossRef]
- Carvalho, S.M.C.; Torres, S.B.; Sousa, E.C.; Sousa, D.M.M.; Pereira, K.T.O.; Paiva, E.P.D.; Matias, J.R.; Santos, B.R.V.D. Viability of Carica papaya L. Seeds by the Tetrazolium Test. J. Agric. Sci. 2018, 10, 335. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Zeng, Y.J.; Wang, Y.R. Methods of topographical tetrazolium testing for seed viability of Nitraria tangutorum Bobr. and N. sibirica Pall. Seed Sci. Technol. 2009, 37, 691–698. [Google Scholar] [CrossRef]
- Sharma, P.; Mishra, M.; Singh, N.I.; Chauhan, J.S. Determination of Integrity of Seed Membrane Systems and Mineral Ions Leakage in Horse Gram. World J. Agric. Sci. 2011, 7, 476–479. [Google Scholar]
- Mukherjee, A.; Peralta-Videa, J.; Gardea-Torresdey, J.; White, J.C. Effects and Uptake of Nanoparticles in Plants. In Engineered Nanoparticles and the Environment: Biophysicochemical Processes and Toxicity, 1st ed.; John Wiley and Sons: New York, NY, USA, 2016; pp. 386–408. [Google Scholar]
- Ferguson, J.M.; Keys, R.D.; McLaughlin, F.W.; Warren, J.M. Seed and Seed Quality; NC State Extension: Patterson Hall, NC, USA, 1991; p. 448. [Google Scholar]
- Franklin, S.E. Narrow-linear and small-area forest disturbance detection and mapping from high spatial resolution imagery. J. Appl. Remote Sens. 2009, 3, 033570. [Google Scholar] [CrossRef]
- Poff, K.E.; Sharma, J.; Richards, M. Cold-Moist Stratification Improves Germination in a Temperate Terrestrial Orchid. Castanea 2016, 81, 292–301. [Google Scholar] [CrossRef]
- Canas, J.E.; Long, M.; Nations, S.; Vadan, R.; Dai, L.; Luo, M.; Ambikapathi, R.; Lee, E.H.; Olszyk, D. Effects of Functionalized and Nonfunctionalized Single-Walled Carbon Nanotubes on Root Elongation of Select Crop Species. Environ. Toxicol. Chem. 2008, 27, 1922–1931. [Google Scholar] [CrossRef]
- Haghighi, M.; Silva, J.A.T. The effect of carbon nanotubes on the seed germination and seedling growth of four vegetable species. J. Crop Sci. Biotechnol. 2014, 17, 201–208. [Google Scholar] [CrossRef]
- Mondal, A.; Basu, R.; Das, S.; Nandy, P. Beneficial role of carbon nanotubes on mustard plant growth: An agricultural prospect. J. Nanopart. Res. 2011, 13, 4519–4528. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Colman, B.P.; McGill, B.M.; Wright, J.P.; Bernhardt, E.S. Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS ONE 2012, 7, e47674. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Puri, S.; Jamwal, A.; Sharma, S. Studies on seed germination and seedling growth in Kalmegh (Andrographis paniculata Wall. Ex Nees) under abiotic stress conditions. Int. J. Sci. Environ. Technol. 2012, 1, 197–204. [Google Scholar]
- Savithramma, N.A.S.; Bhumi, G. Effect of Nanoparticles on Seed Germination and Seedling Growth of Boswellia Ovalifoliolata—An Endemic and Endangered Medicinal Tree Taxon. Nano Vis. 2012, 2222, 61–68. [Google Scholar]
- Srinivasan, C.; Saraswathi, R. Nano-agriculture—Carbon nanotubes enhance tomato seed germination and plant growth. Curr. Sci. 2010, 99, 274–275. [Google Scholar]
- Doria, E.; Pagano, A.; Ferreri, C.; Larocca, A.V.; Macovei, A.; Araújo, S.D.S.; Balestrazzi, A. How does the seed pre-germinative metabolism fight against imbibition damage? Emerging roles of fatty acid cohort and antioxidant defence. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Roiter, Y.; Ornatska, M.; Rammohan, A.R.; Balakrishnan, J.; Heine, D.R.; Minko, S. Interaction of Nanoparticles with Lipid Membrane. Nano Lett. 2008, 8, 941–944. [Google Scholar] [CrossRef]
- McDonnell, E.M.; Pulford, F.G.; Mirbahar, R.B.; Tomos, A.D.; Laidman, D.L. Membrane Lipids and Phosphatidyl Choline Turnover in Embryos from Germinating Low and High Vigour Wheat (Triticum aestivum). J. Exp. Bot. 1982, 33, 631–642. [Google Scholar]
- Kraszewski, S.; Bianco, A.; Tarek, M.; Ramseyer, C. Insertion of short amino-functionalized single-walled carbon nanotubes into phospholipid bilayer occurs by passive diffusion. PLoS ONE 2012, 7, e40703. [Google Scholar] [CrossRef]
- Maatta, S.; Scheu, B.; Roth, M.R.; Tamura, P.; Li, M.; Williams, T.D.; Wang, X.; Welti, R. Levels of Arabidopsis thaliana Leaf Phosphatidic Acids, Phosphatidylserines, and Most Trienoate-Containing Polar Lipid Molecular Species Increase during the Dark Period of the Diurnal Cycle. Front. Plant Sci. 2012, 3, 49. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.H.; Sobze, J.-M.; Pham, T.H.; Nadeem, M.; Liu, C.; Galagedara, L.; Cheema, M.; Thomas, R. Carbon Nanotubes Improved the Germination and Vigor of Plant Species from Peatland Ecosystem Via Remodeling the Membrane Lipidome. Nanomaterials 2020, 10, 1852. https://doi.org/10.3390/nano10091852
Ali MH, Sobze J-M, Pham TH, Nadeem M, Liu C, Galagedara L, Cheema M, Thomas R. Carbon Nanotubes Improved the Germination and Vigor of Plant Species from Peatland Ecosystem Via Remodeling the Membrane Lipidome. Nanomaterials. 2020; 10(9):1852. https://doi.org/10.3390/nano10091852
Chicago/Turabian StyleAli, Md. Hossen, Jean-Marie Sobze, Thu Huong Pham, Muhammad Nadeem, Chen Liu, Lakshman Galagedara, Mumtaz Cheema, and Raymond Thomas. 2020. "Carbon Nanotubes Improved the Germination and Vigor of Plant Species from Peatland Ecosystem Via Remodeling the Membrane Lipidome" Nanomaterials 10, no. 9: 1852. https://doi.org/10.3390/nano10091852
APA StyleAli, M. H., Sobze, J.-M., Pham, T. H., Nadeem, M., Liu, C., Galagedara, L., Cheema, M., & Thomas, R. (2020). Carbon Nanotubes Improved the Germination and Vigor of Plant Species from Peatland Ecosystem Via Remodeling the Membrane Lipidome. Nanomaterials, 10(9), 1852. https://doi.org/10.3390/nano10091852






