Synthesis, Characterization and Process Optimization of Bone Whitlockite
Abstract
:1. Introduction
2. Materials and Method
2.1. Synthesis of WH
2.1.1. Synthesis of WH at pH 4-Effect of Heating Conditions
2.1.2. Synthesis of WH at pH 5-Effect of Heating Conditions
2.1.3. Synthesis of WH-Effect of Heating Time, Aging and Annealing
2.2. Characterization of Materials
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scotchford, C.A.; Vickers, M.; Yousuf Ali, S. The isolation and characterization of magnesium whitlockite crystals from human articular cartilage. Osteoarthr. Cartil. 1995, 3, 79–94. [Google Scholar] [CrossRef] [Green Version]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Halloran, D.; Vrathasha, V.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 Conjugated to Quantum Dot®s is Biologically Functional. Nanomaterials 2020, 10, 1208. [Google Scholar] [CrossRef] [PubMed]
- Lagier, R.; Baud, C.A. Magnesium whitlockite, a calcium phosphate crystal of special interest in pathology. Pathol. Res. Pract. 2003, 199, 329–335. [Google Scholar] [CrossRef]
- Hughes, J.M.; Jolliff, B.L.; Rakovan, J. The crystal chemistry of whitlockite and merrillite and the dehydrogenation of whitlockite to merrillite. Am. Mineral. 2008, 93, 1300–1305. [Google Scholar] [CrossRef]
- Adcock, C.T.; Tschauner, O.; Hausrath, E.M.; Udry, A.; Luo, S.N.; Cai, Y.; Ren, M.; Lanzirotti, A.; Newville, M.; Kunz, M.; et al. Shock-transformation of whitlockite to merrillite and the implications for meteoritic phosphate. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Saleh, A.T.; Ling, L.S.; Hussain, R. Injectable magnesium-doped brushite cement for controlled drug release application. J. Mater. Sci. 2016, 51, 7427–7439. [Google Scholar] [CrossRef]
- Magalhães, M.C.F.; Costa, M.O.G. On the solubility of whitlockite, Ca9Mg(HPO4)(PO4)6, in aqueous solution at 298.15 K. Monatshefte fur Chemie 2018, 149, 253–260. [Google Scholar] [CrossRef]
- Grünewald, T.A.; Rennhofer, H.; Hesse, B.; Burghammer, M.; Stanzl-tschegg, S.E. Biomaterials Magnesium from bioresorbable implants: Distribution and impact on the nano- and mineral structure of bone. Biomaterials 2016, 76, 250–260. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Mohandas, A.; Hwang, N.S.; Jayakumar, R. Injectable angiogenic and osteogenic carrageenan nanocomposite hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2019, 122, 320–328. [Google Scholar] [CrossRef]
- Cheng, H.; Chabok, R.; Guan, X.; Chawla, A.; Li, Y.; Khademhosseini, A.; Jang, H.L. Synergistic interplay between the two major bone minerals, hydroxyapatite and whitlockite nanoparticles, for osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018, 69, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craciunescu, O.; Tardei, C.; Moldovan, L.; Zarnescu, O. Magnesium substitution effect on porous scaffolds for bone repair. Cent. Eur. J. Biol. 2011, 6, 301–311. [Google Scholar] [CrossRef]
- Sabine, W.K. Crystal Structure of Synthetic Mg-Whitlockite, Ca18Mg2H2(PO4)14. Can. J. Chem. 1973, 52, 1155–1165. [Google Scholar] [CrossRef] [Green Version]
- Lazoryak, B.I.; Strunenkova, T.V.; Golubev, V.N.; Vovk, E.A.; Ivanov, L.N. Triple phosphates of calcium, sodium and trivalent elements with whitlockite-like structure. Mater. Res. Bull. 1996, 31, 207–216. [Google Scholar] [CrossRef]
- Salma-Ancane, K.; Stipniece, L.; Putnins, A.; Berzina-Cimdina, L. Development of Mg-containing porous β-tricalcium phosphate scaffolds for bone repair. Ceram. Int. 2015, 41, 4996–5004. [Google Scholar] [CrossRef]
- O’Neill, E.; Awale, G.; Daneshmandi, L.; Umerah, O.; Lo, K.W.H. The roles of ions on bone regeneration. Drug Discov. Today 2018, 23, 879–890. [Google Scholar] [CrossRef]
- Muthiah Pillai, N.S.; Eswar, K.; Amirthalingam, S.; Mony, U.; Kerala Varma, P.; Jayakumar, R. Injectable Nano Whitlockite Incorporated Chitosan Hydrogel for Effective Hemostasis. ACS Appl. Bio Mater. 2019, 2, 865–873. [Google Scholar] [CrossRef]
- Kannan, S.; Lemos, I.A.F.; Rocha, J.H.G.; Ferreira, J.M.F. Synthesis and characterization of magnesium substituted biphasic mixtures of controlled hydroxyapatite/β-tricalcium phosphate ratios. J. Solid State Chem. 2005, 178, 3190–3196. [Google Scholar] [CrossRef]
- Jang, H.L.; Jin, K.; Lee, J.; Kim, Y.; Nahm, S.H.; Hong, K.S.; Nam, K.T. Revisiting whitlockite, the second most abundant biomineral in bone: Nanocrystal synthesis in physiologically relevant conditions and biocompatibility evaluation. ACS Nano 2014, 8, 634–641. [Google Scholar] [CrossRef]
- Araújo, J.C.; Sader, M.S.; Moreira, E.L.; Moraes, V.C.A.; LeGeros, R.Z.; Soares, G.A. Maximum substitution of magnesium for calcium sites in Mg-β-TCP structure determined by X-ray powder diffraction with the Rietveld refinement. Mater. Chem. Phys. 2009, 118, 337–340. [Google Scholar] [CrossRef]
- Cacciotti, I.; Bianco, A.; Lombardi, M.; Montanaro, L. Mg-substituted hydroxyapatite nanopowders: Synthesis, thermal stability and sintering behaviour. J. Eur. Ceram. Soc. 2009, 29, 2969–2978. [Google Scholar] [CrossRef]
- Stipniece, L.; Salma-Ancane, K.; Jakovlevs, D.; Borodajenko, N.; Berzina-Cimdina, L. The Study of Magnesium Substitution Effect on Physicochemical Properties of Hydroxyapatite. Mater. Sci. Appl. Chem. 2013, 28, 51. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.R.; Lee, J.H.; Kim, Y.T.; Riu, D.H.; Jung, S.J.; Lee, Y.J.; Chung, S.C.; Kim, Y.H. Synthesis of Si, Mg substituted hydroxyapatites and their sintering behaviors. Biomaterials 2003, 24, 1389–1398. [Google Scholar] [CrossRef]
- Frasnelli, M.; Sglavo, V.M. Effect of Mg2+ doping on beta-alpha phase transition in tricalcium phosphate (TCP) bioceramics. Acta Biomater. 2016, 33, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ito, A.; Sogo, Y.; Wang, X.; LeGeros, R.Z. Solubility of Mg-containing β-tricalcium phosphate at 25 °C. Acta Biomater. 2009, 5, 508–517. [Google Scholar] [CrossRef] [Green Version]
- Gomes, S.; Renaudin, G.; Jallot, E.; Nedelec, J.M. Structural characterization and biological fluid interaction of sol-gel-derived Mg-substituted biphasic calcium phosphate ceramics. ACS Appl. Mater. Interfaces 2009, 1, 505–513. [Google Scholar] [CrossRef]
- Kannan, S.; Lemos, A.F.; Rocha, J.H.G.; Ferreira, J.M.F. Characterization, and mechanical performance of the Mg-stabilized β-Ca3(PO4)2 prepared from Mg-substituted Ca-deficient apatite. J. Am. Ceram. Soc. 2006, 89, 2757–2761. [Google Scholar] [CrossRef]
- Diallo-Garcia, S.; Laurencin, D.; Krafft, J.M.; Casale, S.; Smith, M.E.; Lauron-Pernot, H.; Costentin, G. Influence of magnesium substitution on the basic properties of hydroxyapatites. J. Phys. Chem. C 2011, 115, 24317–24327. [Google Scholar] [CrossRef]
- Tas, A.C. Transformation of Brushite (CaHPO4·2H2O) to Whitlockite (Ca9Mg(HPO4)(PO4)6) or Other CaPs in Physiologically Relevant Solutions. J. Am. Ceram. Soc. 2016, 99, 1200–1206. [Google Scholar] [CrossRef]
- Stähli, C.; Thüring, J.; Galea, L.; Tadier, S.; Bohner, M.; Döbelin, N. Hydrogen-substituted $β$-tricalcium phosphate synthesized in organic media. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdelkader, S.; Khattech, I.; Rey, C.; Jemal, M. Synthése, caractérisation et thermochimie d’apatites calco-magnésiennes hydroxylées et fluorées. Thermochim. Acta 2001, 376, 25–36. [Google Scholar] [CrossRef]
- Li, G.C.; Wang, P.; Liu, C.B. Hydrothermal Synthesis of Whitlockite. Wuji Cailiao Xuebao/Journal Inorg. Mater. 2017, 32, 1128–1132. [Google Scholar] [CrossRef]
- Jin, Y.Z.; Zheng, G.B.; Jang, H.L.; Lee, K.M.; Lee, J.H. Whitlockite Promotes Bone Healing in Rabbit Ilium Defect Model. J. Med. Biol. Eng. 2019, 39, 944–951. [Google Scholar] [CrossRef]
- Lin, C.C.; Wang, Y.; Zhou, Y.; Zeng, Y. A rapid way to synthesize magnesium whitlockite microspheres for high efficiency removing heavy metals. Desalin. Water Treat. 2019, 162, 220–227. [Google Scholar] [CrossRef]
- Shah, F.A.; Lee, B.E.J.; Tedesco, J.; Larsson Wexell, C.; Persson, C.; Thomsen, P.; Grandfield, K.; Palmquist, A. Micrometer-Sized Magnesium Whitlockite Crystals in Micropetrosis of Bisphosphonate-Exposed Human Alveolar Bone. Nano Lett. 2017, 17, 6210–6216. [Google Scholar] [CrossRef]
- Wang, X.; Shi, J.; Li, Z.; Zhang, S.; Wu, H.; Jiang, Z.; Yang, C.; Tian, C. Facile one-pot preparation of chitosan/calcium pyrophosphate hybrid microflowers. ACS Appl. Mater. Interfaces 2014, 6, 14522–14532. [Google Scholar] [CrossRef]
- Hussin, R.; Hamdan, S.; Halim, D.N.F.A.; Husin, M.S. The origin of emission in strontium magnesium pyrophosphate doped with Dy2O3. Mater. Chem. Phys. 2010, 121, 37–41. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Smirnov, V.V.; Antonova, O.S.; Smirnov, S.V.; Shvorneva, L.I.; Kutsev, S.V.; Barinov, S.M. Magnesium-substituted calcium phosphate cements with (Ca + Mg)/P = 2. Dokl. Chem. 2016, 467, 100–104. [Google Scholar] [CrossRef]
- Prado Da Silva, M.H.; Lima, J.H.C.; Soares, G.A.; Elias, C.N.; De Andrade, M.C.; Best, S.M.; Gibson, I.R. Transformation of monetite to hydroxyapatite in bioactive coatings on titanium. Surf. Coatings Technol. 2001, 137, 270–276. [Google Scholar] [CrossRef]
- Kostov-Kytin, V.V.; Dyulgerova, E.; Ilieva, R.; Petkova, V. Powder X-ray diffraction studies of hydroxyapatite and β-TCP mixtures processed by high energy dry milling. Ceram. Int. 2018, 44, 8664–8671. [Google Scholar] [CrossRef]
- Kim, H.D.; Jang, H.L.; Ahn, H.Y.; Lee, H.K.; Park, J.; Lee, E.-S.; Lee, E.A.; Jeong, Y.H.; Kim, D.G.; Nam, K.T.; et al. Biomimetic whitlockite inorganic nanoparticles-mediated in situ remodeling and rapid bone regeneration. Biomaterials 2017, 112, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Chen, F.; Wu, J.; Zhu, Y.J.; Hao, C.N.; Duan, J.L. Magnesium whitlockite hollow microspheres: A comparison of microwave-hydrothermal and conventional hydrothermal syntheses using fructose 1,6-bisphosphate, and application in protein adsorption. RSC Adv. 2016, 6, 33393–33402. [Google Scholar] [CrossRef]
- Marahat, M.H.; Zahari, M.A.A.; Mohamad, H.; Kasim, S.R. Effect of magnesium ion (Mg2+) substitution and calcination to the properties of biphasic calcium phosphate (BCP). AIP Conf. Proc. 2019, 2068, 1–6. [Google Scholar] [CrossRef]
- Kannan, S.; Ventura, J.M.; Ferreira, J.M.F. Aqueous precipitation method for the formation of Mg-stabilized β-tricalcium phosphate: An X-ray diffraction study. Ceram. Int. 2007, 33, 637–641. [Google Scholar] [CrossRef]
- Mayer, I.; Featherstone, J.D.B. The Thermal Decomposition of Mg-Containing Carbonate Apatites. J. Solid State Chem. 1985, 56, 230–235. [Google Scholar] [CrossRef]
- Suchanek, W.L.; Byrappa, K.; Shuk, P.; Riman, R.E.; Janas, V.F.; Tenhuisen, K.S. Preparation of magnesium-substituted hydroxyapatite powders by the mechanochemical-hydrothermal method. Biomaterials 2004, 25, 4647–4657. [Google Scholar] [CrossRef]
- Sader, M.S.; Legeros, R.Z.; Soares, G.A. Human osteoblasts adhesion and proliferation on magnesium-substituted tricalcium phosphate dense tablets. J. Mater. Sci. Mater. Med. 2009, 20, 521–527. [Google Scholar] [CrossRef]
- Sikder, P.; Bhaduri, S.B. Microwave assisted synthesis and characterization of single-phase tabular hexagonal newberyite, an important bioceramic. J. Am. Ceram. Soc. 2018, 101, 2537–2544. [Google Scholar] [CrossRef]
- Hu, M.; Xiao, F.; Ke, Q.F.; Li, Y.; Chen, X.D.; Guo, Y.P. Cerium-doped whitlockite nanohybrid scaffolds promote new bone regeneration via SMAD signaling pathway. Chem. Eng. J. 2019, 359, 1–12. [Google Scholar] [CrossRef]
- Nouri-Felekori, M.; Khakbiz, M.; Nezafati, N. Synthesis and characterization of Mg, Zn and Sr-incorporated hydroxyapatite whiskers by hydrothermal method. Mater. Lett. 2019, 243, 120–124. [Google Scholar] [CrossRef]
- Khan, A.F.; Awais, M.; Khan, A.S.; Tabassum, S.; Chaudhry, A.A.; Rehman, I.U. Raman spectroscopy of natural bone and synthetic apatites. Appl. Spectrosc. Rev. 2013, 48, 329–355. [Google Scholar] [CrossRef]
- Liao, J.; Hamada, K.; Senna, M. Synthesis of Ca–Mg Apatite via a Mechanochemical Hydrothermal Process. J. Mater. Synth. Process. 2000, 8, 305–306. [Google Scholar] [CrossRef]
- Ren, F.; Leng, Y.; Xin, R.; Ge, X. Synthesis, characterization, and ab initio simulation of magnesium-substituted hydroxyapatite. Acta Biomater. 2010, 6, 2787–2796. [Google Scholar] [CrossRef]

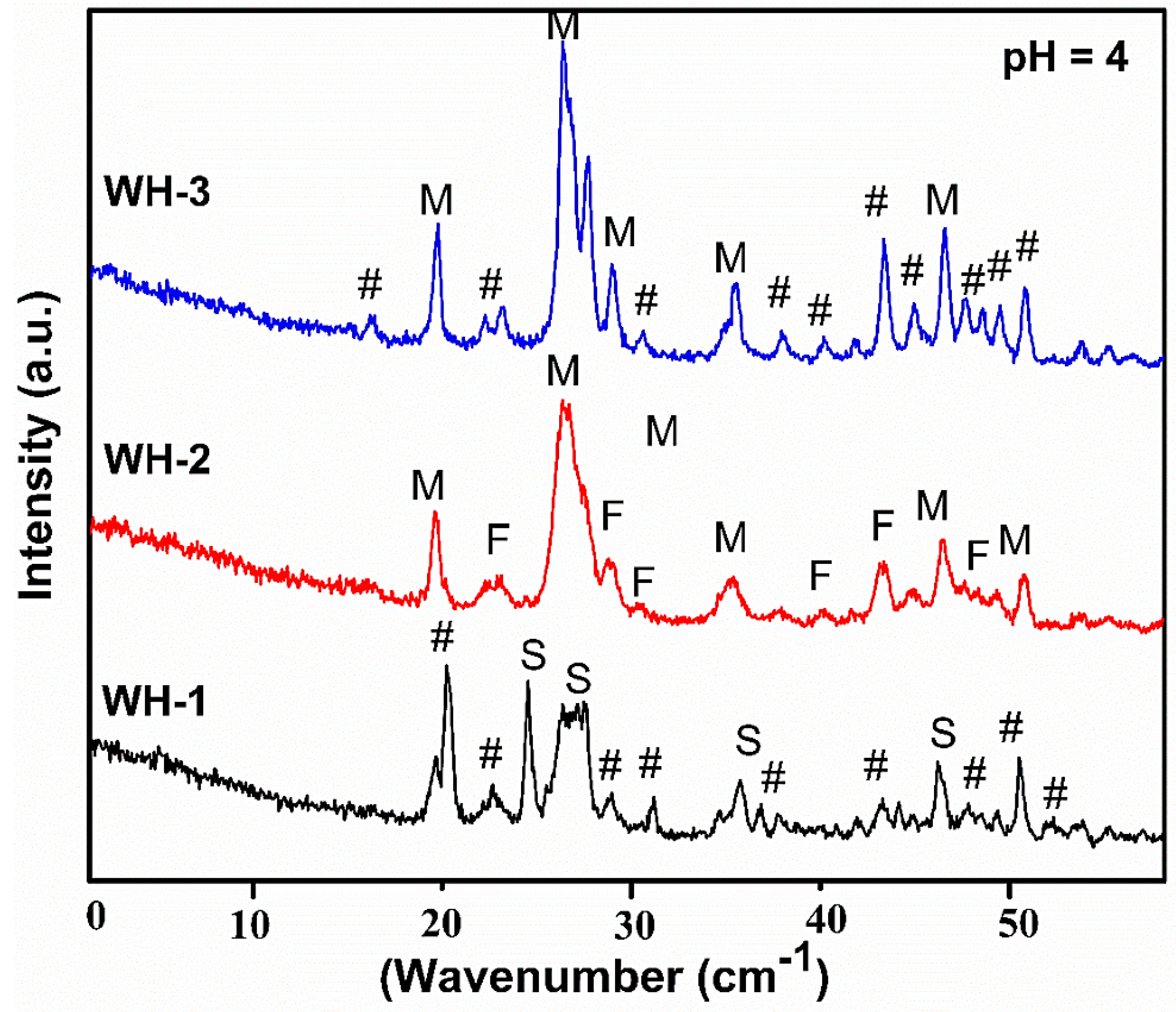
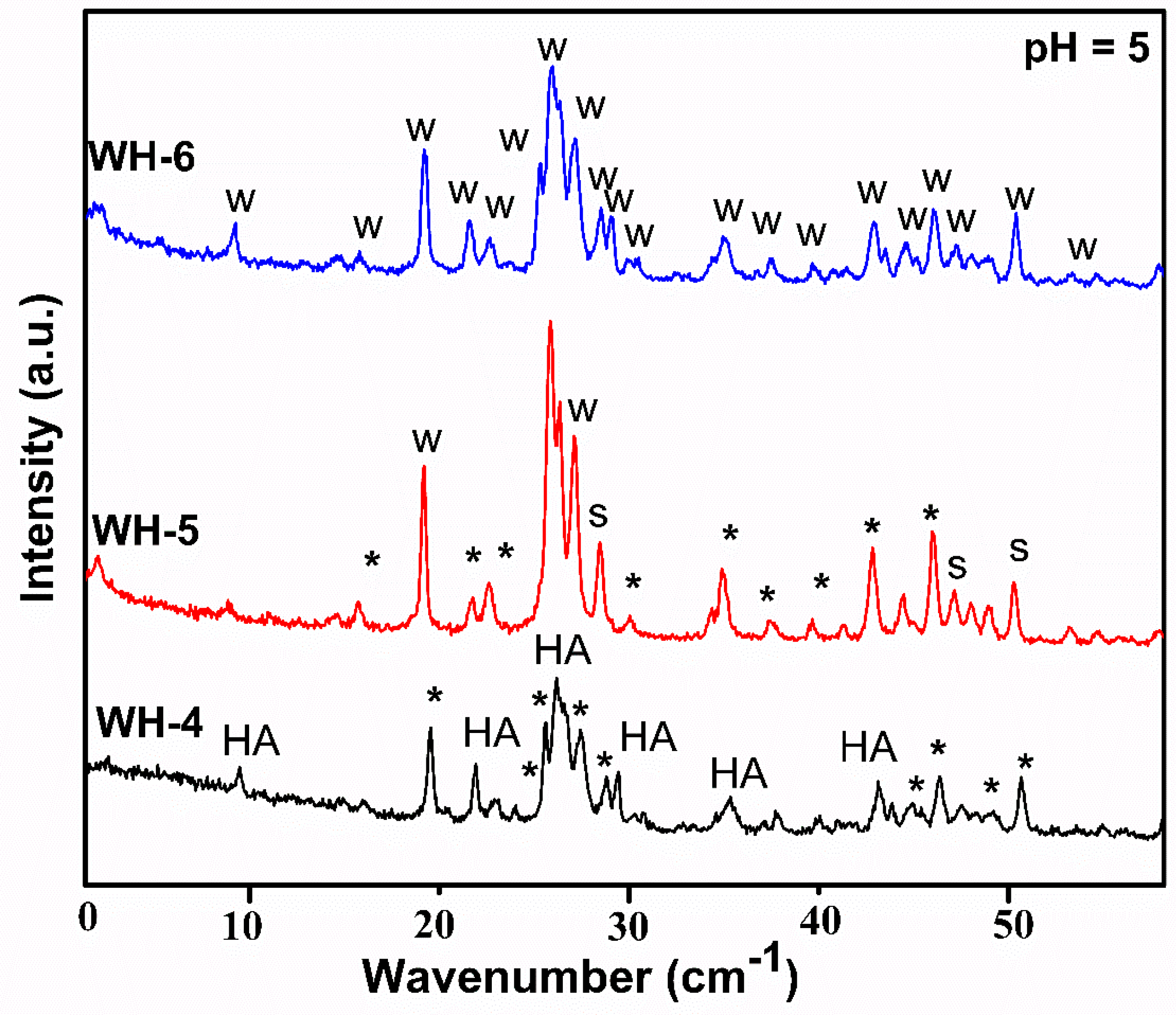
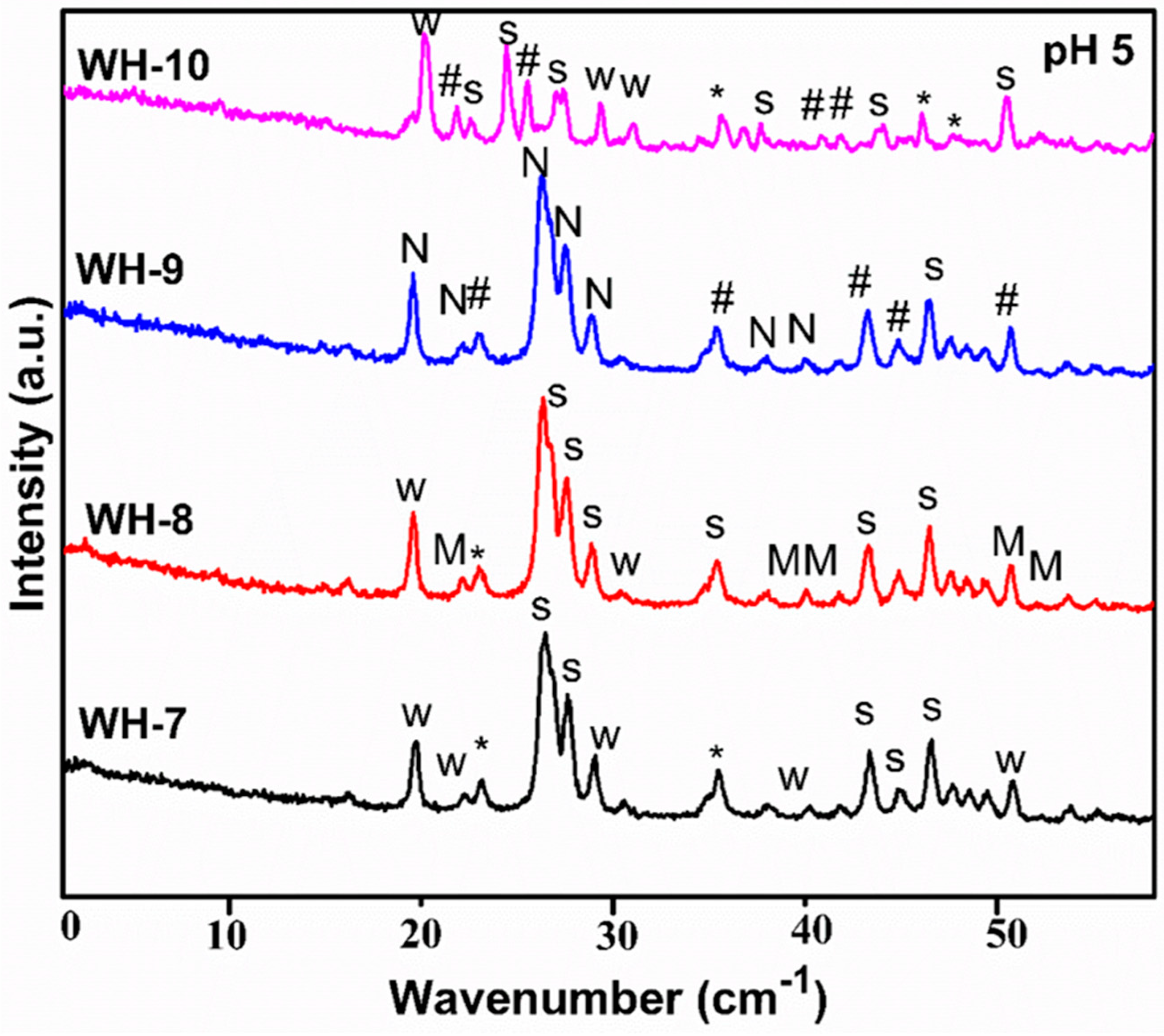

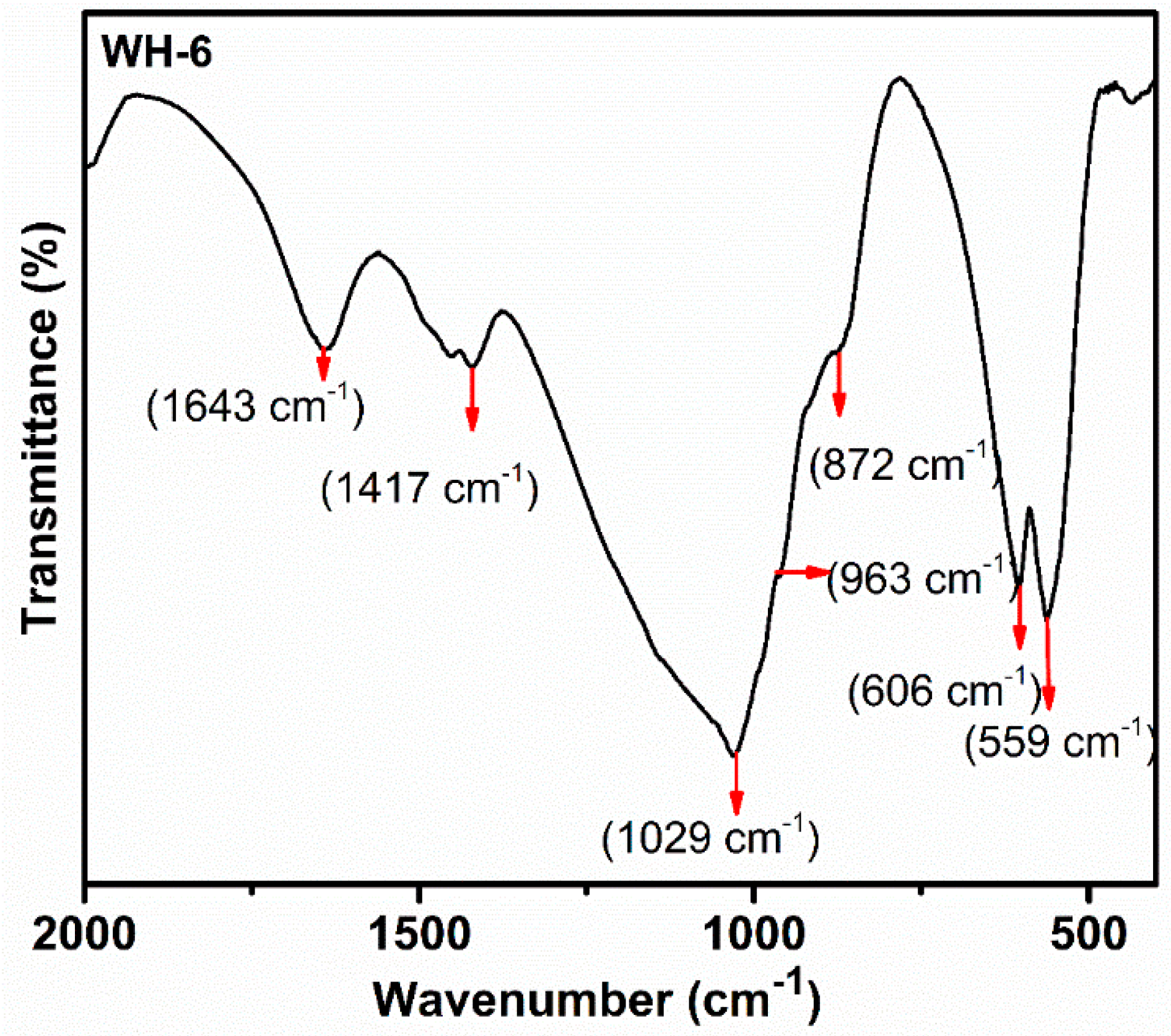
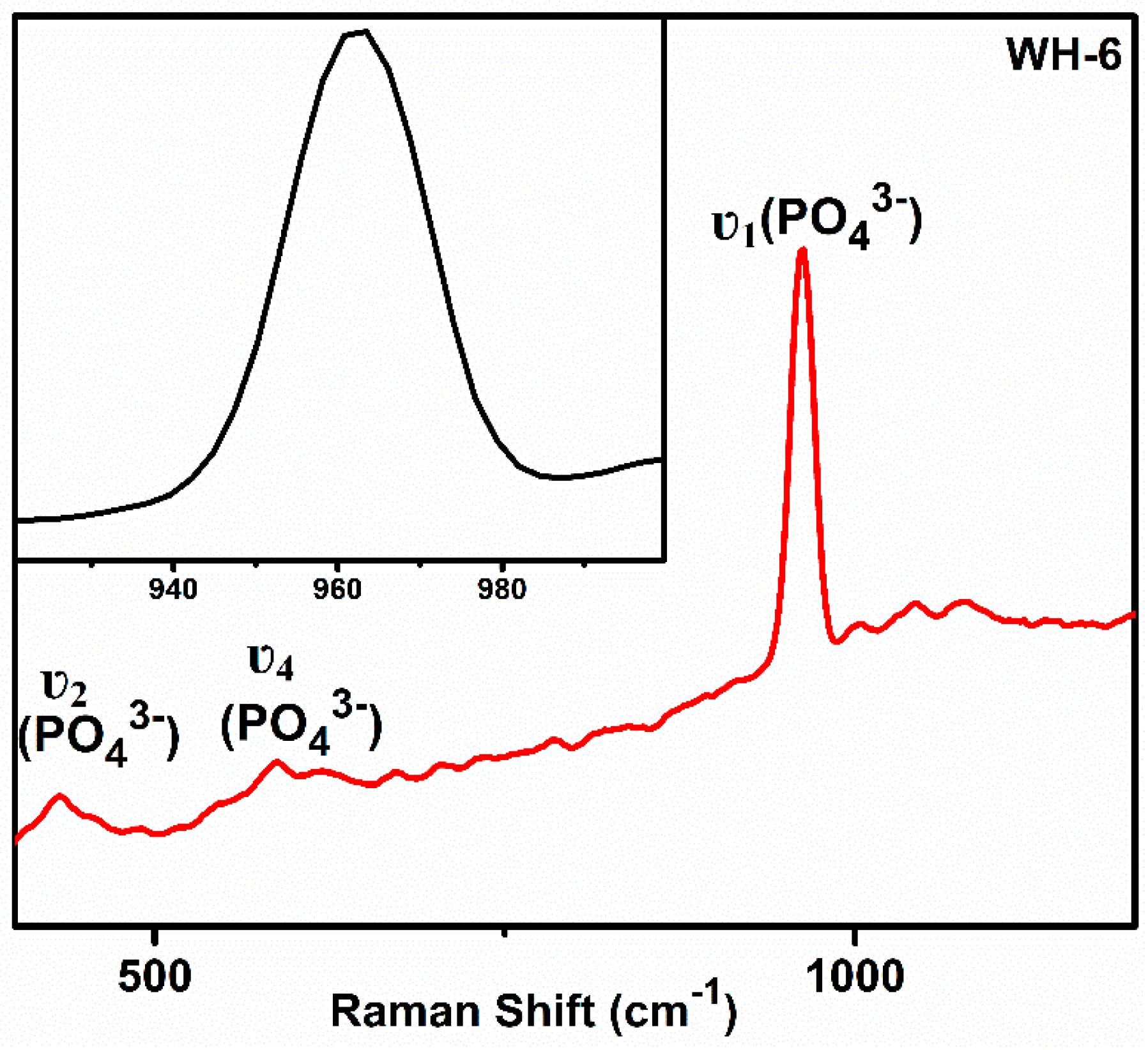

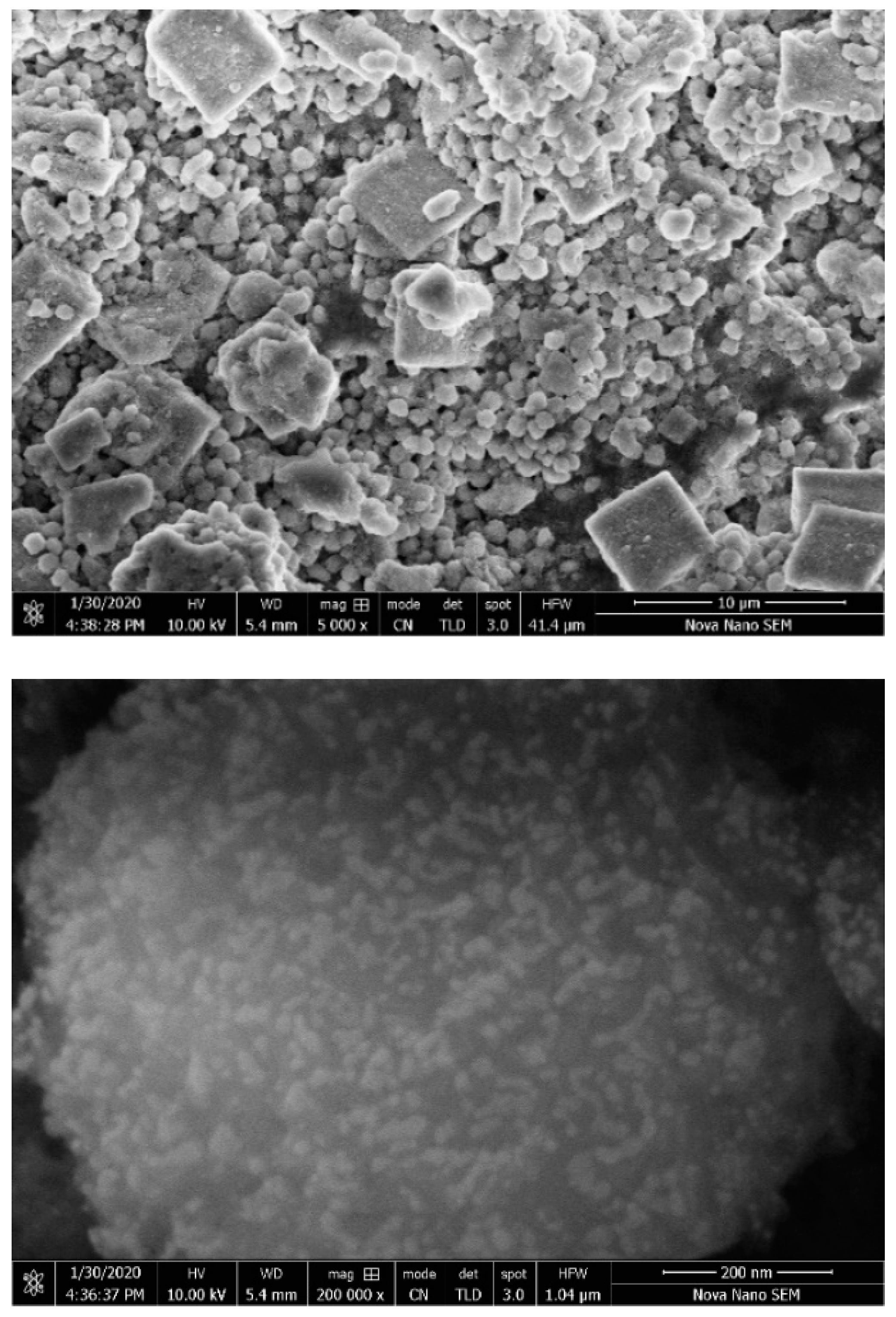
| Sr. # | Sample Name | pH | Reaction Conditions | Aging Time | Product |
|---|---|---|---|---|---|
| 1. | WH-1 | 4 | 80 °C/5 h/stirring | 14 h | Ca pyrophosphate, Mg phosphate hydroxide, Stanfieldite (Mg WH) |
| 2. | WH-2 | 4 | Autoclaved/120 °C/5 h | 14 h | Monetite, farringtonite (WH Phase), Ca phosphate oxide |
| 3. | WH-3 | 4 | Refluxed/100 °C/5 h | 14 h | Mg phosphate, Monetite, Ca-Mg mixed phosphates |
| 4. | WH-4 | 5 | 80 °C/10 h/stirring | 14 h | HA, β-TCP (WH Phase), β-TCMP (WH Phase) |
| 5. | WH-5 | 5 | Autoclaved/120 °C/10 h | 14 h | Bone WH, Stanfieldite (WH Phase), β-TCMP (WH Phase) |
| 6. | WH-6 | 5 | Refluxed/100 °C/10 h | 14 h | Bone WH |
| 7. | WH-7 | 5 | Refluxed/100 °C/12 h | 14 h | Stanfieldite, β-TCMP (WH-Phase) |
| 8. | WH-8 | 5 | Refluxed/100 °C/10 h | 18 h | Bone WH, Stanfieldite (WH-Phase), Monetite |
| 9. | WH-9 | 5 | Refluxed/100 °C/10 h | No aging | Newberyite, Ca Phosphate |
| 10. | WH-10 | 5 | Orthophosphoric acid feed rate changed/refluxed/100 °C | 14 h | Bone WH, Stanfieldite, β-TCP and Ca-Mg phosphate |
| 11. | WH-11 | 5 | WH-6/annealed/750 °C/6 h | Bone WH |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batool, S.; Liaqat, U.; Hussain, Z.; Sohail, M. Synthesis, Characterization and Process Optimization of Bone Whitlockite. Nanomaterials 2020, 10, 1856. https://doi.org/10.3390/nano10091856
Batool S, Liaqat U, Hussain Z, Sohail M. Synthesis, Characterization and Process Optimization of Bone Whitlockite. Nanomaterials. 2020; 10(9):1856. https://doi.org/10.3390/nano10091856
Chicago/Turabian StyleBatool, Sadaf, Usman Liaqat, Zakir Hussain, and Manzar Sohail. 2020. "Synthesis, Characterization and Process Optimization of Bone Whitlockite" Nanomaterials 10, no. 9: 1856. https://doi.org/10.3390/nano10091856
APA StyleBatool, S., Liaqat, U., Hussain, Z., & Sohail, M. (2020). Synthesis, Characterization and Process Optimization of Bone Whitlockite. Nanomaterials, 10(9), 1856. https://doi.org/10.3390/nano10091856






