Deuterium Adsorption on Free-Standing Graphene
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. NPG Sample Preparation Prior to D-Exposure
3.2. Raman Evidence of Low-Defected Graphane
3.3. Photoelectron Spectroscopy Fingerprints of D-NPG
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| XPS | X-ray photoelectron spectroscopy |
| UPS | Ultraviolet photoelectron spectroscopy |
| NPG | Nanoporous graphene |
References
- Sofo, J.O.; Chaudhari, A.S.; Barber, G.D. Graphane: A two-dimensional hydrocarbon. Phys. Rev. B 2007, 75, 153401. [Google Scholar] [CrossRef] [Green Version]
- Samarakoon, D.K.; Wang, X.Q. Tunable Band Gap in Hydrogenated Bilayer Graphene. ACS Nano 2010, 4, 4126–4130. [Google Scholar] [CrossRef] [PubMed]
- Shkrebtii, A.I.; Heritage, E.; McNelles, P.; Cabellos, J.L.; Mendoza, B.S. Graphene and graphane functionalization with hydrogen: Electronic and optical signatures. Phys. Status Solidi C 2012, 9, 1378–1383. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385. [Google Scholar] [CrossRef]
- Falin, A.; Cai, Q.; Santos, E.J.; Scullion, D.; Qian, D.; Zhang, R.; Yang, Z.; Huang, S.; Watanabe, K.; Taniguchi, T.; et al. Mechanical properties of atomically thin boron nitride and the role of interlayer interactions. Nat. Comm. 2017, 8, 15815. [Google Scholar] [CrossRef] [Green Version]
- Giorgio, C.D.; Blundo, E.; Pettinari, G.; Felici, M.; Lu, Y.; Cucolo, A.M.; Polimeni, A.; Bobba, F. Nanoscale Measurements of Elastic Properties and Hydrostatic Pressure in H2-Bulged MoS2 Membranes. Adv. Mater. Interfaces 2020, 7, 2001024. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacon, M.; Bradley, S.J.; Nann, T. Graphene Quantum Dots. Part. Part. Syst. Charact. 2014, 31, 415–428. [Google Scholar] [CrossRef]
- Hassoun, J.; Bonaccorso, F.; Agostini, M.; Angelucci, M.; Betti, M.G.; Cingolani, R.; Gemmi, M.; Mariani, C.; Panero, S.; Pellegrini, V.; et al. An Advanced Lithium-Ion Battery Based on a Graphene Anode and a Lithium Iron Phosphate Cathode. Nano Lett. 2014, 14, 4901–4906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massimi, L.; Ourdjini, O.; Lafferentz, L.; Koch, M.; Grill, L.; Cavaliere, E.; Gavioli, L.; Cardoso, C.; Prezzi, D.; Molinari, E.; et al. Surface-Assisted Reactions toward Formation of Graphene Nanoribbons on Au(110) Surface. J. Phys. Chem. C 2015, 119, 2427–2437. [Google Scholar] [CrossRef]
- Betti, M.G.; Biasotti, M.; Boscá, A.; Calle, F.; Carabe-Lopez, J.; Cavoto, G.; Chang, C.; Chung, W.; Cocco, A.; Colijn, A.; et al. A design for an electromagnetic filter for precision energy measurements at the tritium endpoint. Prog. Part. Nucl. Phys. 2019, 106, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Betti, M.G.; Biasotti, M.; Boscá, A.; Calle, F.; Canci, N.; Cavoto, G.; Chang, C.; Cocco, A.; Colijn, A.; Conrad, J.; et al. Neutrino physics with the PTOLEMY project: Active neutrino properties and the light sterile case. J. Cosmol. Astropart. Phys. 2019, 2019, 047. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Shang, J.; Lim, S.; Li, D.; Xiong, Q.; Shen, Z.; Lin, J.; Yu, T. Modulating the electronic structures of graphene by controllable hydrogenation. Appl. Phys. Lett. 2010, 97, 233111. [Google Scholar] [CrossRef]
- Burgess, J.S.; Matis, B.R.; Robinson, J.T.; Bulat, F.A.; Perkins, F.K.; Houston, B.H.; Baldwin, J.W. Tuning the electronic properties of graphene by hydrogenation in a plasma enhanced chemical vapor deposition reactor. Carbon 2011, 49, 4420–4426. [Google Scholar] [CrossRef]
- Felten, A.; McManus, D.; Rice, C.; Nittler, L.; Pireaux, J.J.; Casiraghi, C. Insight into hydrogenation of graphene: Effect of hydrogen plasma chemistry. Appl. Phys. Lett. 2014, 105, 183104. [Google Scholar] [CrossRef]
- Elias, D.C.; Nair, R.R.; Mohiuddin, T.M.G.; Morozov, S.V.; Blake, P.; Halsall, M.P.; Ferrari, A.C.; Boukhvalov, D.W.; Katsnelson, M.I.; Geim, A.K.; et al. Control of Graphene’s Properties by Reversible Hydrogenation: Evidence for Graphane. Science 2009, 323, 610–613. [Google Scholar] [CrossRef] [Green Version]
- Haberer, D.; Vyalikh, D.V.; Taioli, S.; Dora, B.; Farjam, M.; Fink, J.; Marchenko, D.; Pichler, T.; Ziegler, K.; Simonucci, S.; et al. Tunable Band Gap in Hydrogenated Quasi-Free-Standing Graphene. Nano Lett. 2010, 10, 3360–3366. [Google Scholar] [CrossRef]
- Paris, A.; Verbitskiy, N.; Nefedov, A.; Wang, Y.; Fedorov, A.; Haberer, D.; Oehzelt, M.; Petaccia, L.; Usachov, D.; Vyalikh, D.; et al. Kinetic Isotope Effect in the Hydrogenation and Deuteration of Graphene. Adv. Funct. Mater. 2013, 23, 1628–1635. [Google Scholar] [CrossRef] [Green Version]
- Ryu, S.; Han, M.Y.; Maultzsch, J.; Heinz, T.F.; Kim, P.; Steigerwald, M.L.; Brus, L.E. Reversible Basal Plane Hydrogenation of Graphene. Nano Lett. 2008, 8, 4597–4602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitener, K.E.; Lee, W.K.; Campbell, P.M.; Robinson, J.T.; Sheehan, P.E. Chemical hydrogenation of single-layer graphene enables completely reversible removal of electrical conductivity. Carbon 2014, 72, 348–353. [Google Scholar] [CrossRef]
- Son, J.; Lee, S.; Kim, S.J.; Park, B.C.; Lee, H.K.; Kim, S.; Kim, J.H.; Hong, B.H.; Hong, J. Hydrogenated monolayer graphene with reversible and tunable wide band gap and its field-effect transistor. Nat. Commun. 2016, 7, 13261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haberer, D.; Petaccia, L.; Wang, Y.; Quian, H.; Farjam, M.; Jafari, S.A.; Sachdev, H.; Federov, A.V.; Usachov, D.; Vyalikh, D.V.; et al. Electronic properties of hydrogenated quasi-free-standing graphene. Phys. Status Solidi (b) 2011, 248, 2639–2643. [Google Scholar] [CrossRef]
- Balog, R.; Andersen, M.; Jørgensen, B.; Sljivancanin, Z.; Hammer, B.; Baraldi, A.; Larciprete, R.; Hofmann, P.; Hornekær, L.; Lizzit, S. Controlling Hydrogenation of Graphene on Ir(111). ACS Nano 2013, 7, 3823–3832. [Google Scholar] [CrossRef] [PubMed]
- Panahi, M.; Solati, N.; Kaya, S. Modifying hydrogen binding strength of graphene. Surf. Sci. 2019, 679, 24–30. [Google Scholar] [CrossRef]
- Ito, Y.; Tanabe, Y.; Qiu, H.J.; Sugawara, K.; Heguri, S.; Tu, N.H.; Huynh, K.K.; Fujita, T.; Takahashi, T.; Tanigaki, K.; et al. High-Quality Three-Dimensional Nanoporous Graphene. Angew. Chem. Int. Ed. 2014, 53, 4822–4826. [Google Scholar] [CrossRef]
- Tanabe, Y.; Ito, Y.; Sugawara, K.; Hojo, D.; Koshino, M.; Fujita, T.; Aida, T.; Xu, X.; Huynh, K.K.; Shimotani, H.; et al. Electric Properties of Dirac Fermions Captured into 3D Nanoporous Graphene Networks. Adv. Mater. 2016, 28, 10304–10310. [Google Scholar] [CrossRef]
- Di Bernardo, I.; Avvisati, G.; Mariani, C.; Motta, N.; Chen, C.; Avila, J.; Asensio, M.C.; Lupi, S.; Ito, Y.; Chen, M.; et al. Two-Dimensional Hallmark of Highly Interconnected Three-Dimensional Nanoporous Graphene. ACS Omega 2017, 2, 3691–3697. [Google Scholar] [CrossRef]
- Di Bernardo, I.; Avvisati, G.; Chen, C.; Avila, J.; Asensio, M.C.; Hu, K.; Ito, Y.; Hines, P.; Lipton-Duffin, J.; Rintoul, L.; et al. Topology and doping effects in three-dimensional nanoporous graphene. Carbon 2018, 131, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Sha, X.; Jackson, B. First-principles study of the structural and energetic properties of H atoms on a graphite (0001) surface. Surf. Sci. 2002, 496, 318–330. [Google Scholar] [CrossRef]
- Ruffieux, P.; Gröning, O.; Bielmann, M.; Mauron, P.; Schlapbach, L.; Gröning, P. Hydrogen adsorption on sp2-bonded carbon: Influence of the local curvature. Phys. Rev. B 2002, 66, 245416. [Google Scholar] [CrossRef] [Green Version]
- Abdelnabi, M.M.S.; Blundo, E.; Betti, M.G.; Cavoto, G.; Placidi, E.; Polimeni, A.; Ruocco, A.; Hu, K.; Ito, Y.; Mariani, C. Towards free-standing graphane: Atomic hydrogen and deuterium bonding to nano-porous graphene. Nanotechnology 2020, 32, 035707. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Qiu, H.J.; Fujita, T.; Tanabe, Y.; Tanigaki, K.; Chen, M. Bicontinuous Nanoporous N-doped Graphene for the Oxygen Reduction Reaction. Adv. Mater. 2014, 26, 4145–4150. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Cong, W.; Fujita, T.; Tang, Z.; Chen, M. High Catalytic Activity of Nitrogen and Sulfur Co-Doped Nanoporous Graphene in the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2015, 54, 2131–2136. [Google Scholar] [CrossRef]
- Ito, Y.; Tanabe, Y.; Han, J.; Fujita, T.; Tanigaki, K.; Chen, M. Multifunctional Porous Graphene for High-Efficiency Steam Generation by Heat Localization. Adv. Mater. 2015, 27, 4302–4307. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Qin, L.; Zhang, S.; Zheng, J.; Sun, J.; Ito, Y.; Wu, Y. Building a Reactive Armor Using S-Doped Graphene for Protecting Potassium Metal Anodes from Oxygen Crossover in K–O2 Batteries. ACS Energy Lett. 2020, 5, 1788–1793. [Google Scholar] [CrossRef]
- Hodgson, E.; Moroño, A.; Gonzalez, S. Insulator degradation due to radiation induced ion and dark currents in vacuum. J. Nucl. Mater. 2009, 386–388, 999–1001. [Google Scholar] [CrossRef]
- Muñoz, P.; Hernández, T.; García-Cortés, I.; Sánchez, F.; Maira, A.; Iadicicco, D.; Vanazzi, M.; Utili, M.; Di Fonzo, F.; Moroño, A. Radiation effects on deuterium permeation for PLD alumina coated Eurofer steel measured during 1.8 MeV electron irradiation. J. Nucl. Mater. 2018, 512, 118–125. [Google Scholar] [CrossRef]
- Muñoz, P.; Moroño, A.; Sánchez, F.; Maira, A.; García-Cortés, I. Ionizing radiation effects on the thermal stability of deuterium trapping in reaction bonded SiC. J. Nucl. Mater. 2018, 508, 219–225. [Google Scholar] [CrossRef]
- Iadicicco, D.; Bassini, S.; Vanazzi, M.; Muñoz, P.; Moroño, A.; Hernandez, T.; García-Cortés, I.; Sánchez, F.; Utili, M.; Ferré, F.G.; et al. Efficient hydrogen and deuterium permeation reduction in Al2O3 coatings with enhanced radiation tolerance and corrosion resistance. Nucl. Fusion 2018, 58, 126007. [Google Scholar] [CrossRef]
- Massimi, L.; Angelucci, M.; Gargiani, P.; Betti, M.G.; Montoro, S.; Mariani, C. Metal-Phthalocyanine Ordered Layers on Au (110): Metal-Dependent Adsorption Energy. J. Chem. Phys. 2014, 140, 244704. [Google Scholar] [CrossRef] [Green Version]
- Massimi, L.; Betti, M.G.; Caramazza, S.; Postorino, P.; Mariani, C.; Latini, A.; Leardini, F. In-vacuum thermolysis of ethane 1,2-diamineborane for the synthesis of ternary borocarbonitrides. Nanotechnology 2016, 27, 435601. [Google Scholar] [CrossRef] [PubMed]
- Leardini, F.; Flores, E.; Galvis, A.R.; Ferrer, I.J.; Ares, J.R.; Sánchez, C.; Molina, P.; van der Meulen, H.P.; Gómez Navarro, C.; López Polin, G.; et al. Chemical vapor deposition growth of boron–carbon–nitrogen layers from methylamine borane thermolysis products. Nanotechnology 2018, 29, 025603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leardini, F.; Jiménez-Arévalo, N.; Ferrer, I.J.; Ares, J.R.; Molina, P.; Navarro, C.G.; Manzanares, Y.; Granados, D.; Urbanos, F.J.; García-García, F.J.; et al. A fast synthesis route of boron–carbon–nitrogen ultrathin layers towards highly mixed ternary B–C–N phases. 2D Mater. 2019, 6, 035015. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Shin, Y.E.; Sa, Y.J.; Park, S.; Lee, J.; Shin, K.H.; Joo, S.H.; Ko, H. An ice-templated, pH-tunable self-assembly route to hierarchically porous graphene nanoscroll networks. Nanoscale 2014, 6, 9734–9741. [Google Scholar] [CrossRef]
- Jiménez-Arévalo, N.; Leardini, F.; Ferrer, I.J.; Ares, J.R.; Sánchez, C.; Saad Abdelnabi, M.M.; Betti, M.G.; Mariani, C. Ultrathin Transparent B–C–N Layers Grown on Titanium Substrates with Excellent Electrocatalytic Activity for the Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2020, 3, 1922–1932. [Google Scholar] [CrossRef]
- Yeh, J.; Lindau, I. Atomic subshell photoionization cross sections and asymmetry parameters: 1≤ Z≤ 103. At. Data Nucl. Data Tables 1985, 32, 1–155. [Google Scholar] [CrossRef]
- Lesiak, B.; Stobinski, L.; Malolepszy, A.; Mazurkiewicz, M.; Kövér, L.; Tóth, J. Preparation of graphene oxide and characterisation using electron spectroscopy. J. Electron Spectrosc. Relat. Phenom. 2014, 193, 92–99. [Google Scholar] [CrossRef]
- Rojas, J.; Toro-Gonzalez, M.; Molina-Higgins, M.; Castano, C. Facile radiolytic synthesis of ruthenium nanoparticles on graphene oxide and carbon nanotubes. Mater. Sci. Eng. B 2016, 205, 28–35. [Google Scholar] [CrossRef]
- Malard, L.; Pimenta, M.; Dresselhaus, G.; Dresselhaus, M. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Mohiuddin, T.M.G.; Lombardo, A.; Nair, R.R.; Bonetti, A.; Savini, G.; Jalil, R.; Bonini, N.; Basko, D.M.; Galiotis, C.; Marzari, N.; et al. Uniaxial strain in graphene by Raman spectroscopy: G peak splitting, Grüneisen parameters, and sample orientation. Phys. Rev. B 2009, 79, 205433. [Google Scholar] [CrossRef]
- Ding, F.; Ji, H.; Chen, Y.; Herklotz, A.; Dörr, K.; Mei, Y.; Rastelli, A.; Schmidt, O.G. Stretchable Graphene: A Close Look at Fundamental Parameters through Biaxial Straining. Nano Lett. 2010, 10, 3453. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, X.F.; Shi, W.; Wu, J.B.; Jiang, D.S.; Tan, P.H. Phonon and Raman scattering of two-dimensional transition metal dicalchogenides from monolayer, multilayer to bulk material. Chem. Soc. Rev. 2015, 44, 2757–2785. [Google Scholar] [CrossRef] [Green Version]
- Felton, J.; Blundo, E.; Ling, S.; Glover, J.; Kudrynskyi, Z.R.; Makarovsky, O.; Kovalyuk, Z.D.; Besley, E.; Walker, G.; Polimeni, A.; et al. The Interaction of Hydrogen with the van der Waals Crystal γ-InSe. Molecules 2020, 25, 2526. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.; Liu, X.; Christopher, J.W.; Cantley, L.; Wadehra, A.; Kim, B.L.; Goldberg, B.B.; Swan, A.K.; Bunch, J.S. Band gap engineering with ultralarge biaxial strains in suspended monolayer MoS2. Nano Lett. 2016, 16, 5836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blundo, E.; Di Giorgio, C.; Pettinari, G.; Yildirim, T.; Felici, M.; Lu, Y.; Bobba, F.; Polimeni, A. Engineered Creation of Periodic Giant, Nonuniform Strains in MoS2 Monolayers. Adv. Mater. Interfaces 2020, 7, 2000621. [Google Scholar] [CrossRef]
- Mathew, S.; Gopinadhan, K.; Chan, T.K.; Yu, X.J.; Zhan, D.; Cao, L.; Rusydi, A.; Breese, M.B.H.; Dhar, S.; Shen, Z.X.; et al. Magnetism in MoS2 induced by proton irradiation. Appl. Phys. Lett. 2012, 101, 102103. [Google Scholar] [CrossRef] [Green Version]
- Tedeschi, D.; Blundo, E.; Felici, M.; Pettinari, G.; Liu, B.; Yildrim, T.; Petroni, E.; Zhang, C.; Zhu, Y.; Sennato, S.; et al. Controlled Micro/Nanodome Formation in Proton-Irradiated Bulk Transition-Metal Dichalcogenides. Adv. Mater. 2019, 31, 1903795. [Google Scholar] [CrossRef]
- Blundo, E.; Felici, M.; Yildirim, T.; Pettinari, G.; Tedeschi, D.; Miriametro, A.; Liu, B.; Ma, W.; Lu, Y.; Polimeni, A. Evidence of the direct-to-indirect band gap transition in strained two-dimensional WS2, MoS2, and WSe2. Phys. Rev. Res. 2020, 2, 012024. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Yu, T.; Kim, K.J.; Ni, Z.; You, Y.; Lim, S.; Shen, Z.; Wang, S.; Lin, J. Thickness-Dependent Reversible Hydrogenation of Graphene Layers. ACS Nano 2009, 3, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Wojtaszek, M.; Tombros, N.; Caretta, A.; van Loosdrecht, P.H.M.; van Wees, B.J. A road to hydrogenating graphene by a reactive ion etching plasma. J. Appl. Phys. 2011, 110, 063715. [Google Scholar] [CrossRef] [Green Version]
- Lacovig, P.; Pozzo, M.; Alfe, D.; Vilmercati, P.; Baraldi, A.; Lizzit, S. Growth of dome-shaped carbon nanoislands on Ir (111): The intermediate between carbidic clusters and quasi-free-standing graphene. Phys. Rev. Lett. 2009, 103, 166101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scardamaglia, M.; Forte, G.; Lizzit, S.; Baraldi, A.; Lacovig, P.; Larciprete, R.; Mariani, C.; Betti, M.G. Metal-phthalocyanine array on the moiré pattern of a graphene sheet. J. Nanoparticle Res. 2011, 13, 6013–6020. [Google Scholar] [CrossRef]
- Scardamaglia, M.; Lisi, S.; Lizzit, S.; Baraldi, A.; Larciprete, R.; Mariani, C.; Betti, M.G. Graphene-Induced Substrate Decoupling and Ideal Doping of a Self-Assembled Iron-phthalocyanine Single Layer. J. Phys. Chem. C 2013, 117, 3019–3027. [Google Scholar] [CrossRef]
- Eberlein, T.; Bangert, U.; Nair, R.R.; Jones, R.; Gass, M.; Bleloch, A.L.; Novoselov, K.S.; Geim, A.; Briddon, P.R. Plasmon spectroscopy of free-standing graphene films. Phys. Rev. B 2008, 77, 233406. [Google Scholar] [CrossRef] [Green Version]
- Politano, A.; Campi, D.; Formoso, V.; Chiarello, G. Evidence of confinement of the π plasmon in periodically rippled graphene on Ru(0001). Phys. Chem. Chem. Phys. 2013, 15, 11356–11361. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, G.; Liscio, A.; Ruocco, A. The evolution of hydrogen induced defects and the restoration of π-plasmon as a monitor of the thermal reduction of graphene oxide. Appl. Surf. Sci. 2020, 512, 145605. [Google Scholar] [CrossRef]
- Barinov, A.; Gregoratti, L.; Dudin, P.; La Rosa, S.; Kiskinova, M. Imaging and Spectroscopy of Multiwalled Carbon Nanotubes during Oxidation: Defects and Oxygen Bonding. Adv. Mater. 2009, 21, 1916–1920. [Google Scholar] [CrossRef]
- Scardamaglia, M.; Amati, M.; Llorente, B.; Mudimela, P.; Colomer, J.F.; Ghijsen, J.; Ewels, C.; Snyders, R.; Gregoratti, L.; Bittencourt, C. Nitrogen ion casting on vertically aligned carbon nanotubes: Tip and sidewall chemical modification. Carbon 2014, 77, 319–328. [Google Scholar] [CrossRef]
- Susi, T.; Kaukonen, M.; Havu, P.; Ljungberg, M.P.; Ayala, P.; Kauppinen, E.I. Core level binding energies of functionalized and defective graphene. Beilstein J. Nanotechnol. 2014, 5, 121–132. [Google Scholar] [CrossRef] [Green Version]
- D’Acunto, G.; Ripanti, F.; Postorino, P.; Betti, M.G.; Scardamaglia, M.; Bittencourt, C.; Mariani, C. Channelling and induced defects at ion-bombarded aligned multiwall carbon nanotubes. Carbon 2018, 139, 768–775. [Google Scholar] [CrossRef]
- Balog, R.; Jørgensen, B.; Nilsson, L.; Andersen, M.; Rienks, E.; Bianchi, M.; Fanetti, M.; Lægsgaard, E.; Baraldi, A.; Lizzit, S.; et al. Bandgap opening in graphene induced by patterned hydrogen adsorption. Nat. Mater. 2010, 9, 315–319. [Google Scholar] [CrossRef] [PubMed]


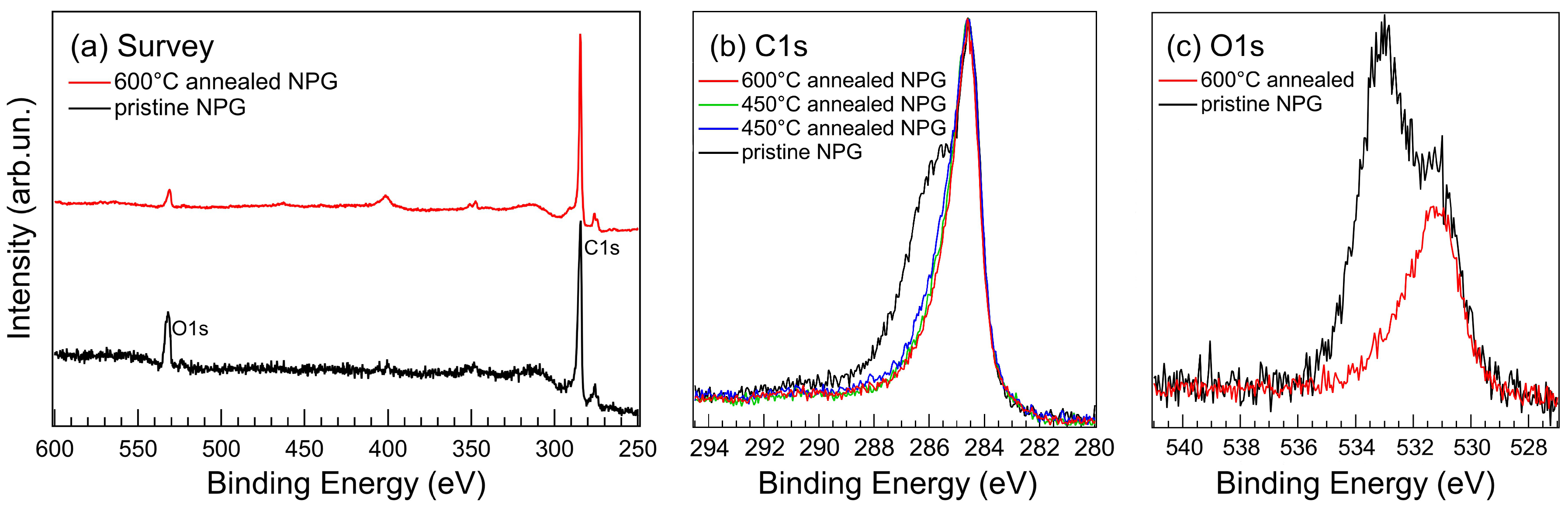


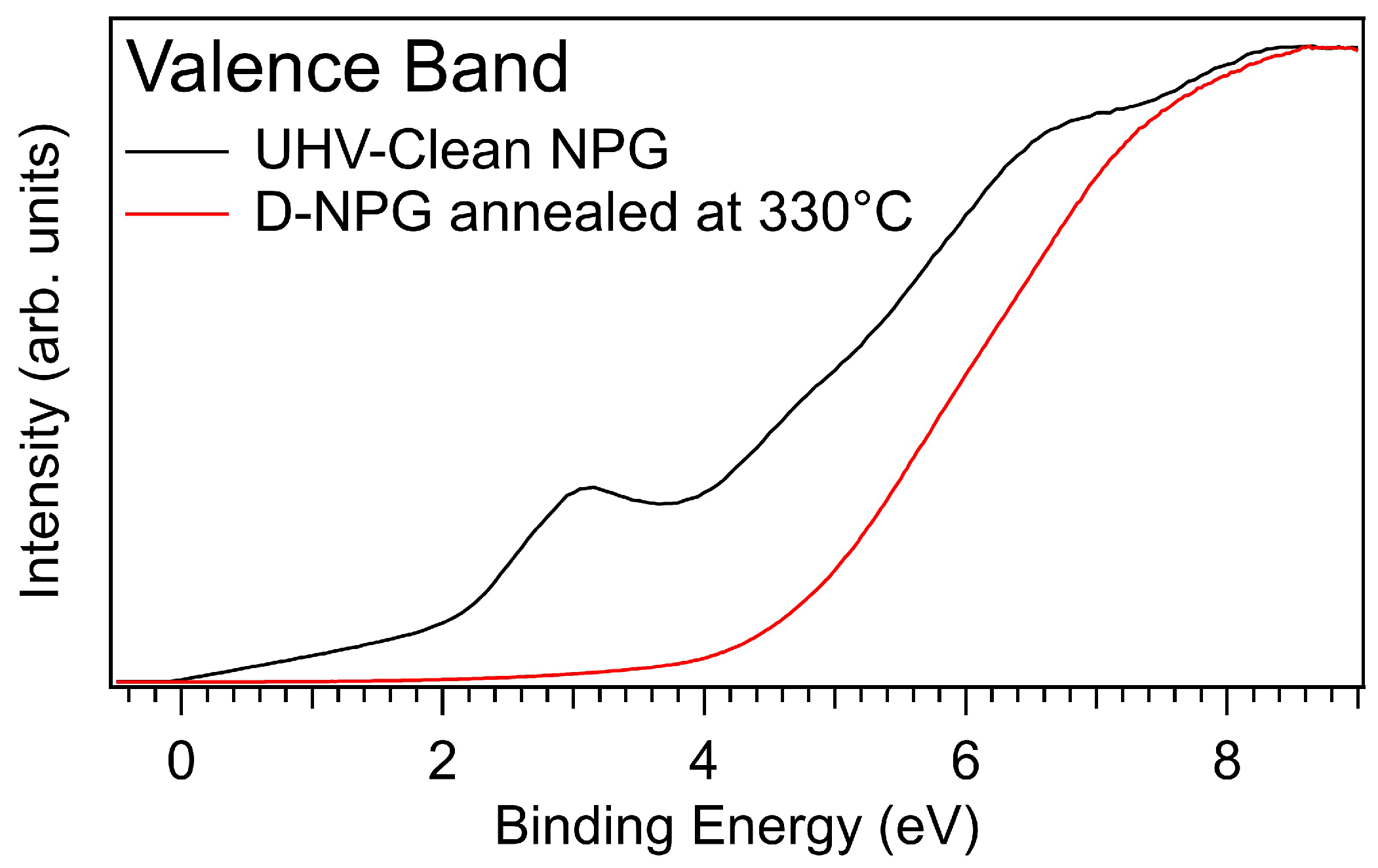
| UHV-clean NPG | ||||
| C 1s component | BE (eV) | Asymm. Param. | FWHM (eV) | Area/Area (%) |
| sp | 284.6 | 0.1 | 1.1 | 71.3 |
| sp | 285.3 | 0 | 1.3 | 14.8 |
| -plasmon | 291.3 | 0 | 4.0 | 5.3 |
| CO | 286.3 | 0 | 1.7 | 8.6 |
| as-deposited D-NPG | ||||
| C 1s component | BE (eV) | asymm. param. | FWHM (eV) | Area/Area (%) |
| sp | 284.6 | 0.1 | 1.1 | 41.3 |
| sp | 285.6 | 0 | 1.9 | 42.8 |
| -plasmon | 291.0 | 0 | 4.0 | 0.3 |
| CO | 287.0 | 0 | 2.5 | 15.6 |
| D-NPG annealed at 150 C | ||||
| C 1s component | BE (eV) | asymm. param. | FWHM (eV) | Area/Area (%) |
| sp | 284.6 | 0.1 | 1.1 | 42.3 |
| sp | 285.6 | 0 | 1.7 | 43.6 |
| -plasmon | 291.1 | 0 | 4.0 | 0.3 |
| CO | 287.0 | 0 | 2.2 | 13.8 |
| D-NPG annealed at 250 C | ||||
| C 1s component | BE (eV) | asymm. param. | FWHM (eV) | Area/Area (%) |
| sp | 284.5 | 0.1 | 1.1 | 43.1 |
| sp | 285.4 | 0 | 1.7 | 43.7 |
| -plasmon | 291.0 | 0 | 4.0 | 0.7 |
| CO | 286.7 | 0 | 2.2 | 12.5 |
| D-NPG annealed at 330 C | ||||
| C 1s component | BE (eV) | asymm. param. | FWHM (eV) | Area/Area (%) |
| sp | 284.5 | 0.1 | 1.1 | 44.3 |
| sp | 285.3 | 0 | 1.6 | 43.6 |
| -plasmon | 291.0 | 0 | 4.0 | 1.9 |
| CO | 286.3 | 0 | 2.1 | 10.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelnabi, M.M.S.; Izzo, C.; Blundo, E.; Betti, M.G.; Sbroscia, M.; Di Bella, G.; Cavoto, G.; Polimeni, A.; García-Cortés, I.; Rucandio, I.; et al. Deuterium Adsorption on Free-Standing Graphene. Nanomaterials 2021, 11, 130. https://doi.org/10.3390/nano11010130
Abdelnabi MMS, Izzo C, Blundo E, Betti MG, Sbroscia M, Di Bella G, Cavoto G, Polimeni A, García-Cortés I, Rucandio I, et al. Deuterium Adsorption on Free-Standing Graphene. Nanomaterials. 2021; 11(1):130. https://doi.org/10.3390/nano11010130
Chicago/Turabian StyleAbdelnabi, Mahmoud Mohamed Saad, Chiara Izzo, Elena Blundo, Maria Grazia Betti, Marco Sbroscia, Giulia Di Bella, Gianluca Cavoto, Antonio Polimeni, Isabel García-Cortés, Isabel Rucandio, and et al. 2021. "Deuterium Adsorption on Free-Standing Graphene" Nanomaterials 11, no. 1: 130. https://doi.org/10.3390/nano11010130
APA StyleAbdelnabi, M. M. S., Izzo, C., Blundo, E., Betti, M. G., Sbroscia, M., Di Bella, G., Cavoto, G., Polimeni, A., García-Cortés, I., Rucandio, I., Moroño, A., Hu, K., Ito, Y., & Mariani, C. (2021). Deuterium Adsorption on Free-Standing Graphene. Nanomaterials, 11(1), 130. https://doi.org/10.3390/nano11010130







