Design of PEGylated Three Ligands Silica Nanoparticles for Multi-Receptor Targeting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Hybrid Triethoxysilyl Sulfo Cyanine 5.5
2.2. Synthesis of SiNPs (Example for Fluorescent SiNPs Type B)
2.3. Cyanine Encapsulation Quantification (Example of Fluorescent SiNPs Type B)
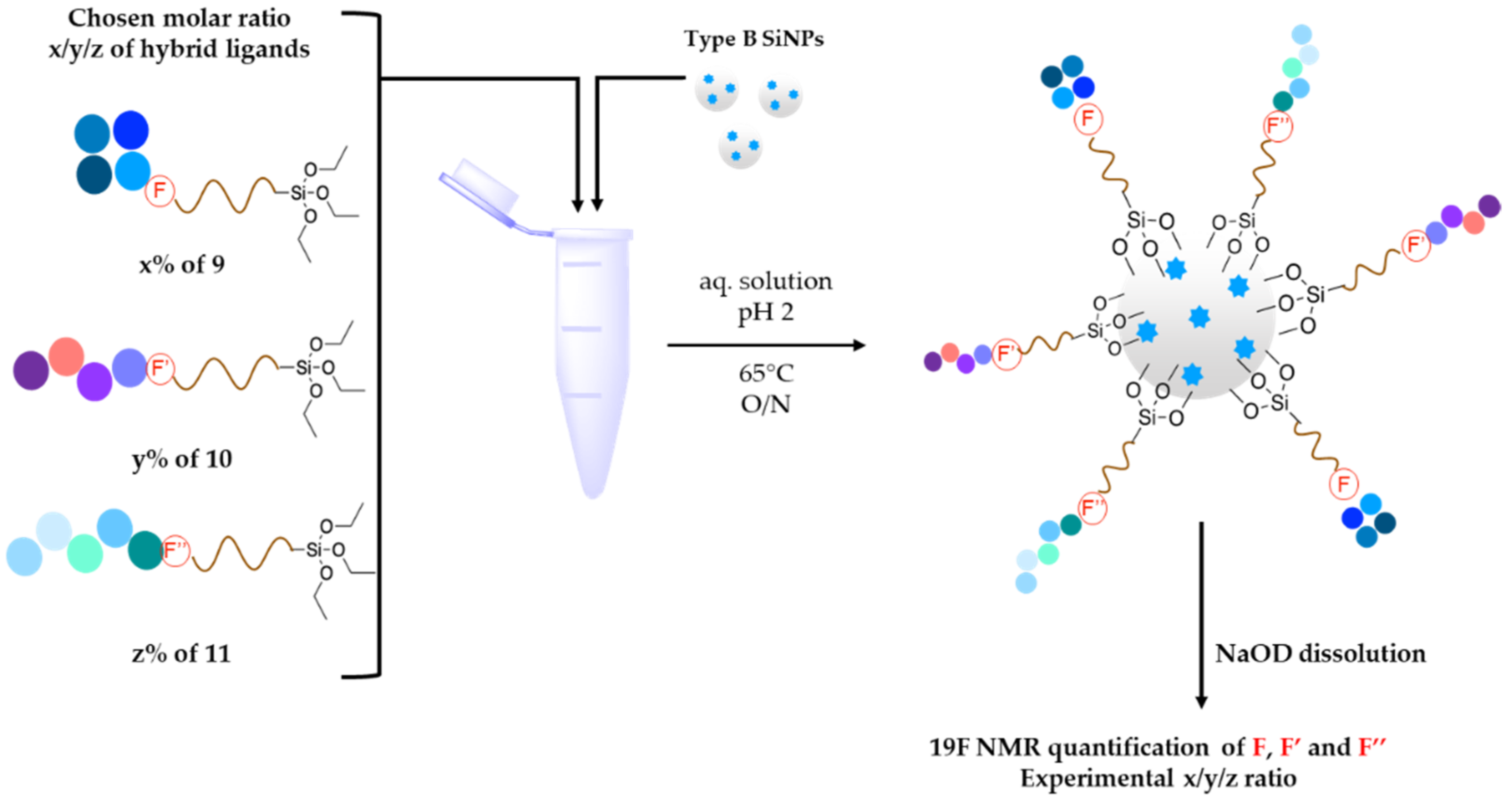
2.4. Grafting of Hybrid Peptides on Fluorescent SiNPs (Example for 9, 10 and 11 Hybrid PEG–Peptides, with a 1/1/1 Ratio)
2.5. Grafting Quantification by 19F NMR (Example of SiNP 7)
2.6. Anchoring Stability Study (Example for Type B2)
2.7. Cell Culture
2.8. Flow Cytometry (FACS)
2.9. Spheroids Binding Assay
2.10. Microscopy
3. Results
3.1. Synthesis and Characterization of Cyanine-Containing Fluorescent Nanoparticles
3.1.1. What Is the Best NP Synthesis Procedure to Avoid Fluorophore Degradation? Stöber or Micro-Emulsion?
3.1.2. How Many Fluorophores Are Trapped in SiNPs?
3.1.3. How to Avoid Aggregation? Conservation of SiNPs
3.2. Influence of the Surface of the SiNPs for Grafting a Silylated PEG
3.2.1. Synthesis and Characterization of SiNPs with Different Surfaces
3.2.2. Is an Extra Shell of Silica Useful for Grafting? PEG Grafting on Type A, B, C and D SiNPs
3.2.3. What Is the Conformation of the PEG Chain on the Surface of the Particle?
3.3. Is the Siloxane Bond between SiNP Surfaces and PEG Stable? Does the Nature of the Silane Moiety Impact on the Anchoring Stability?
3.4. Design and Synthesis of Hybrid Ligands
3.5. Hybrid Ligand Grafting
3.6. Binding Efficiency
3.7. Cell Labeling and Interaction with Melanoma/Endothelial Mixed Spheroids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Kumar, R.; Mishra, G.; Sharma, U.; Singh, G.; Ahalawat, S. Sol-Gel processing of silica nanoparticles and their applications. Adv. Colloid Interface Sci. 2014, 214, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Fontecave, T.; Sanchez, C.; Azaïs, T.; Boissière, C. Chemical Modification as a Versatile Tool for Tuning Stability of Silica Based Mesoporous Carriers in Biologically Relevant Conditions. Chem. Mater. 2012, 24, 4326–4336. [Google Scholar] [CrossRef]
- Hong, X.; Wang, Z.; Yang, J.; Zheng, Q.; Zong, S.; Sheng, Y.; Zhu, D.; Tang, C.; Cui, Y. Silylated BODIPY dyes and their use in dye-encapsulated silica nanoparticles with switchable emitting wavelengths for cellular imaging. Analyst 2012, 137, 4140–4149. [Google Scholar] [CrossRef]
- Hudson, S.P.; Padera, R.F.; Langer, R.; Kohane, D.S. The biocompatibility of mesoporous silicates. Biomaterials 2008, 29, 4045–4055. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Miller, M.L.; Di Pasqua, A.J. Biocompatibility of Mesoporous Silica Nanoparticles? Comments Inorg. Chem. 2015, 36, 61–80. [Google Scholar] [CrossRef]
- Vera, M.L.; Cánneva, A.; Huck-Iriart, C.; Requejo, F.G.; Gonzalez, M.; Dell’Arciprete, M.L.; Calvo, A. Fluorescent silica nanoparticles with chemically reactive surface: Controlling spatial distribution in one-step synthesis. J. Colloid Interface Sci. 2017, 496, 456–464. [Google Scholar] [CrossRef]
- Li, M.; Cheng, F.; Xue, C.; Wang, H.; Chen, C.; Du, Q.; Ge, D.; Sun, B. Surface Modification of Stöber Silica Nanoparticles with Controlled Moiety Densities Determines Their Cytotoxicity Profiles in Macrophages. Langmuir 2019, 35, 14688–14695. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Hu, X.-X.; Zhanga, X.-B. Dye-Doped Fluorescent Silica Nanoparticles for Live Cell and In Vivo Bioimaging. Nanomaterials 2016, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Miletto, I.; Gilardino, A.; Zamburlin, P.; Dalmazzo, S.; Lovisolo, D.; Caputo, G.; Viscardi, G.; Martra, G. Highly bright and photostable cyanine dye-doped silica nanoparticles for optical imaging: Photophysical characterization and cell tests. Dye. Pigment. 2010, 84, 121–127. [Google Scholar] [CrossRef]
- Bagwe, R.P.; Yang, C.; Hilliard, L.R.; Tan, W. Optimization of Dye-Doped Silica Nanoparticles Prepared Using a Reverse Microemulsion Method. Langmuir 2004, 20, 8336–8342. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Ding, L.-J.; Zhang, W.; Zhang, X.-A.; Zhang, Y.-L.; Lin, Z.-Z.; Wang, X.-D. Synthesis of highly stable cyanine-dye-doped silica nanoparticle for biological applications. Methods Appl. Fluoresc. 2018, 6, 034002. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Sciortino, M.; Alonzo, G.; De Schrijver, A.; Pagliaro, M. From Molecules to Systems: Sol−Gel Microencapsulation in Silica-Based Materials. Chem. Rev. 2011, 111, 765–789. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Li, Y.; Zhang, W.; Liu, S.; Xie, Z.; Jing, X. Near-Infrared Polymeric Nanoparticles with High Content of Cyanine for Bimodal Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2016, 8, 24426–24432. [Google Scholar] [CrossRef] [PubMed]
- Ciccione, J.; Jia, T.; Coll, J.-L.; Parra, K.; Amblard, M.; Jebors, S.; Martinez, J.; Mehdi, A.; Subra, G. Unambiguous and Controlled One-Pot Synthesis of Multifunctional Silica Nanoparticles. Chem. Mater. 2016, 28, 885–889. [Google Scholar] [CrossRef]
- Jia, T.; Choi, J.; Ciccione, J.; Henry, M.; Mehdi, A.; Martinez, J.; Eymin, B.; Subra, G.; Coll, J.-L. Heteromultivalent targeting of integrin αvβ3 and neuropilin 1 promotes cell survival via the activation of the IGF-1/insulin receptors. Biomaterials 2018, 155, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Vaganay, E.; Carpentier, G.; Coudert, P.; Guzman-Gonzales, V.; Manuel, R.; Eymin, B.; Coll, J.-L.; Ruggiero, F. A collagen Vα1-derived fragment inhibits FGF-2 induced-angiogenesis by modulating endothelial cells plasticity through its heparin-binding site. Matrix Biol. 2020, 94, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Iovino, G.; Malvindi, M.; Agnello, S.; Buscarino, G.; Alessi, A.; Pompa, P.; Gelardi, F. Optical and morphological properties of infrared emitting functionalized silica nanoparticles. Mater. Chem. Phys. 2013, 142, 763–769. [Google Scholar] [CrossRef]
- Arriagada, F.; Osseo-Asare, K. Synthesis of Nanosize Silica in a Nonionic Water-in-Oil Microemulsion: Effects of the Water/Surfactant Molar Ratio and Ammonia Concentration. J. Colloid Interface Sci. 1999, 211, 210–220. [Google Scholar] [CrossRef]
- Wang, M.; Liu, W.; Zhang, Y.; Dang, M.; Zhang, Y.; Tao, J.; Chen, K.; Peng, X.; Tengb, Z. Intercellular adhesion molecule 1 antibody-mediated mesoporous drug delivery system for targeted treatment of triple-negative breast cancer. J. Colloid Interface Sci. 2019, 538, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Van Blaaderen, A.; Vrij, A. Synthesis and characterization of colloidal dispersions of fluorescent, monodisperse silica spheres. Langmuir 1992, 8, 2921–2931. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clayton, K.N.; Salameh, J.W.; Wereley, S.T.; Kinzer-Ursem, T.L. Physical characterization of nanoparticle size and surface modification using particle scattering diffusometry. Biomicrofluidics 2016, 10, 054107. [Google Scholar] [CrossRef] [Green Version]
- Bagwe, R.P.; Hilliard, L.R.; Tan, W. Surface Modification of Silica Nanoparticles to Reduce Aggregation and Nonspecific Binding. Langmuir 2006, 22, 4357–4362. [Google Scholar] [CrossRef] [Green Version]
- Valot, L.; Maumus, M.; Montheil, T.; Martinez, J.; Noël, D.; Mehdi, A.; Subra, G. Biocompatible Glycine-Assisted Catalysis of the Sol-Gel Process: Development of Cell-Embedded Hydrogels. ChemPlusChem 2019, 84, 1720–1729. [Google Scholar] [CrossRef] [Green Version]
- Alauzun, J.; Mehdi, A.; Reyé, C.; Corriu, R.J.P. Direct synthesis of bifunctional mesoporous organosilicas containing chelating groups in the framework and reactive functional groups in the channel pores. J. Mater. Chem. 2006, 17, 349–356. [Google Scholar] [CrossRef]
- Salon, M.-C.B.; Belgacem, N. Competition between hydrolysis and condensation reactions of trialkoxysilanes, as a function of the amount of water and the nature of the organic group. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 366, 147–154. [Google Scholar] [CrossRef]
- Meyer, M.; Vechambre, C.; Viau, L.; Mehdi, A.; Fontaine, O.; Mourad, E.; Monge, S.; Chenal, J.-M.; Chazeau, L.; Vioux, A. Single-ion conductor nanocomposite organic–inorganic hybrid membranes for lithium batteries. J. Mater. Chem. A 2014, 2, 12162–12165. [Google Scholar] [CrossRef]
- Keefe, A.J.; Jiang, S. Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity. Nat. Chem. 2012, 4, 59–63. [Google Scholar] [CrossRef]
- Pocock, K.; Delon, L.C.; Khatri, A.; Prestidge, C.A.; Gibson, R.J.; Barbe, C.; Thierry, B. Uptake of silica particulate drug carriers in an intestine-on-a-chip: Towards a better in vitro model of nanoparticulate carrier and mucus interactions. Biomater. Sci. 2019, 7, 2410–2420. [Google Scholar] [CrossRef] [PubMed]
- Zara, G.P.; Cavalli, R.; Bargoni, A.; Fundarò, A.; Vighetto, D.; Gasco, M.R. Intravenous Administration to Rabbits of Non-stealth and Stealth Doxorubicin-loaded Solid Lipid Nanoparticles at Increasing Concentrations of Stealth Agent: Pharmacokinetics and Distribution of Doxorubicin in Brain and Other Tissues. J. Drug Target. 2002, 10, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Adumeau, L.; Genevois, C.; Roudier, L.; Schatz, C.; Couillaud, F.; Mornet, S. Impact of surface grafting density of PEG macromolecules on dually fluorescent silica nanoparticles used for the in vivo imaging of subcutaneous tumors. Biochim. et Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 1587–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rixman, M.A.; Dean, D.; Ortiz, C. Nanoscale Intermolecular Interactions between Human Serum Albumin and Low Grafting Density Surfaces of Poly(ethylene oxide). Langmuir 2003, 19, 9357–9372. [Google Scholar] [CrossRef]
- Yang, Q.; Jones, S.; Parker, C.L.; Zamboni, W.C.; Bear, J.E.; Lai, S.K. Evading Immune Cell Uptake and Clearance Requires PEG Grafting at Densities Substantially Exceeding the Minimum for Brush Conformation. Mol. Pharm. 2014, 11, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Moghaddam, S.P.H.; Mohammadpour, R.; Ghandehari, H. In vitro and in vivo evaluation of degradation, toxicity, biodistribution, and clearance of silica nanoparticles as a function of size, porosity, density, and composition. J. Control. Release 2019, 311–312, 1–15. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef] [Green Version]
- Thomas, N.; Tirand, L.; Chatelut, Étienne; Plénat, F.; Frochot, C.; Dodeller, M.; Guillemin, F.; Barberi-Heyob, M. Tissue distribution and pharmacokinetics of an ATWLPPR-conjugated chlorin-type photosensitizer targeting neuropilin-1 in glioma-bearing nude mice. Photochem. Photobiol. Sci. 2008, 7, 433–441. [Google Scholar] [CrossRef]
- Dyab, A.K. Destabilisation of Pickering emulsions using pH. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 402, 2–12. [Google Scholar] [CrossRef]









| Type of SiNPs | Size (TEM) (nm) | Size (DLS) (nm) | PdI | Zeta Potential (ζ) at pH 7.6 (mV) | Hybrid PEG Loading (μmol/g) | N PEG/nm2 | RF/D | Conformational Regime | Cyanine 5.5 Incorporation (yield%) |
|---|---|---|---|---|---|---|---|---|---|
| A | 90 | 130 | 0.12 | −47 | 170 | 3.37 | 5.4 | Brush | n.a. |
| B | 110 | 145 | 0.09 | −50 | 149 | 3.62 | 5.6 | Brush | 23 |
| C | 160 | 210 | 0.02 | −60 | 170 | 6.03 | 7.2 | Brush | 17 |
| D | 135 | 510 a | 0.50 | +10 | 110 | 3.28 | 5.3 | Brush | 19 |
| Type of SiNPs | Total Area/g (nm2/g) | Max Theoretical μmol of PEG/nm2 | Experimental μmol of PEG/nm2 | Yield of Grafting a (%) |
|---|---|---|---|---|
| A | 3 × 1019 | 1.8 × 10−17 | 5.5 × 10−18 | 31.8 |
| B | 2.5 × 1019 | 2.2 × 10−17 | 6 × 10−18 | 28 |
| C | 1.7 × 1019 | 3.4 × 10−17 | 1 × 10−17 | 29.5 |
| D | 2 × 1019 | 2.5 × 10−17 | 5.5 × 10−18 | 21.5 |
| Compound # Ligand/Hybrid Ligand | Ligand MW | Ligand m/z Found | Ligands R = H Hybrid Ligands R =  | 19 F δ (ppm) | Peptide Isoelectric Point (IP) |
|---|---|---|---|---|---|
| 5/9 | 955.4 | 956.9 [M+H]+ |  | −64 | 7.2 |
| 6/10 | 1228.7 | 645.35 [M+2H]2+ |  | −117 | 11.1 |
| 7/11 | 2879.4 | 961.6 [M+3H]3+ |  | −62 | 12.5 |
| 8/12 | 969.5 | 970.8 [M+H]+ |  | −64 | 7.4 |
| SiNP | Theo. Ratio 9/10/11 | Exp. Ratio a 9/10/11 | Overall Theoretical Peptide Loading (µmol/g) | Overall NMR Peptide Loading (µmol/g) | N PEG–Peptide/nm2 | Zeta Potential (ζ) at pH 7.6 (mV) | RF/D b |
|---|---|---|---|---|---|---|---|
| SiNP 1 | 100/0/0 | 100/0/0 | 207 | 26 | 0.6/0/0 | −20 | 2.3 |
| SiNP 2 | 0/100/0 | 0/100/0 | 196 | 20.2 | 0/0.5/0 | −12 | 2.0 |
| SiNP 3 | 0/0/100 | 0/0/100 | 128 | 8.2 | 0/0/0.2 | −19 | 1.3 |
| SiNP 4 | 49/51/0 | 55/45/0 | 198 | 7.4 | 0.9/0.08/0 | / | 0.9/0.8/0 |
| SiNP 5 | 0/63/37 | 0/8/92 | 161 | 7.8 | 0/0.01/0.1 | / | 0/0.3/1.2 |
| SiNP 6 | 62/0/38 | 37/0/63 | 165 | 13.2 | 0.1/0/0.2 | −2 | 1.0/0/1.3 |
| SiNP 7 | 38/39/23 | 7/70/23 | 178 | 11.2 | 0.01/0.2/0.06 | / | 0.4/1.3/0.7 |
| SiNP 8 | / | / | 242 | 149 | 3.62 | −48 | 5.6 |
| SiNP 9 | / | / | 219 | 18 | 0.4 | / | 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurel, M.; Montheil, T.; Martin, J.; Chaar, L.; Guzman-Gonzalez, V.; Couvet, M.; Jacquet, T.; Jia, T.; Eymin, B.; Parra, K.; et al. Design of PEGylated Three Ligands Silica Nanoparticles for Multi-Receptor Targeting. Nanomaterials 2021, 11, 177. https://doi.org/10.3390/nano11010177
Maurel M, Montheil T, Martin J, Chaar L, Guzman-Gonzalez V, Couvet M, Jacquet T, Jia T, Eymin B, Parra K, et al. Design of PEGylated Three Ligands Silica Nanoparticles for Multi-Receptor Targeting. Nanomaterials. 2021; 11(1):177. https://doi.org/10.3390/nano11010177
Chicago/Turabian StyleMaurel, Manon, Titouan Montheil, Julie Martin, Line Chaar, Veronica Guzman-Gonzalez, Morgane Couvet, Thibault Jacquet, Tao Jia, Beatrice Eymin, Karine Parra, and et al. 2021. "Design of PEGylated Three Ligands Silica Nanoparticles for Multi-Receptor Targeting" Nanomaterials 11, no. 1: 177. https://doi.org/10.3390/nano11010177
APA StyleMaurel, M., Montheil, T., Martin, J., Chaar, L., Guzman-Gonzalez, V., Couvet, M., Jacquet, T., Jia, T., Eymin, B., Parra, K., Dumy, P., Martinez, J., Ruggiero, F., Vaganay, E., Mehdi, A., Coll, J. -L., & Subra, G. (2021). Design of PEGylated Three Ligands Silica Nanoparticles for Multi-Receptor Targeting. Nanomaterials, 11(1), 177. https://doi.org/10.3390/nano11010177







