Non-Isothermal Decomposition as Efficient and Simple Synthesis Method of NiO/C Nanoparticles for Asymmetric Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
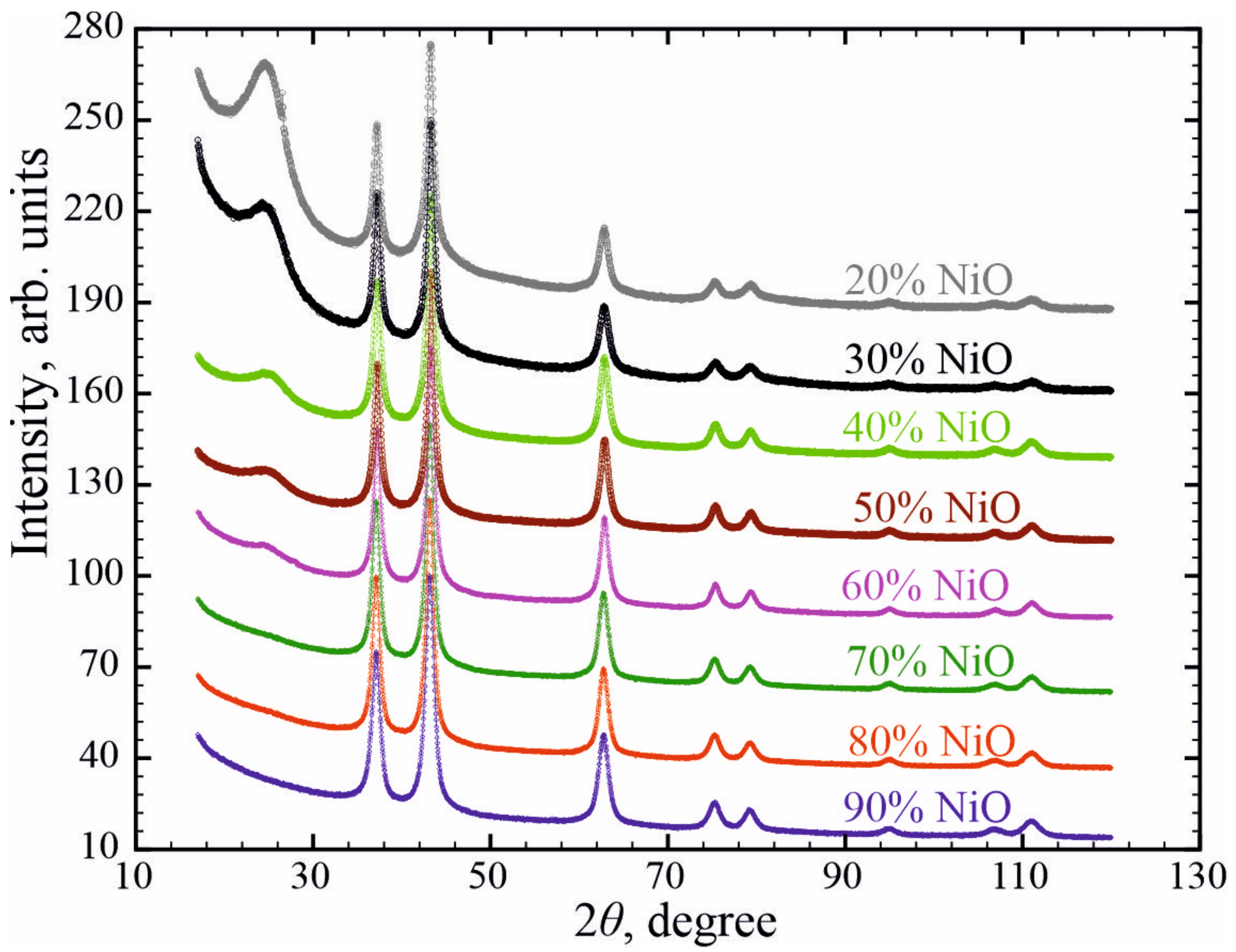
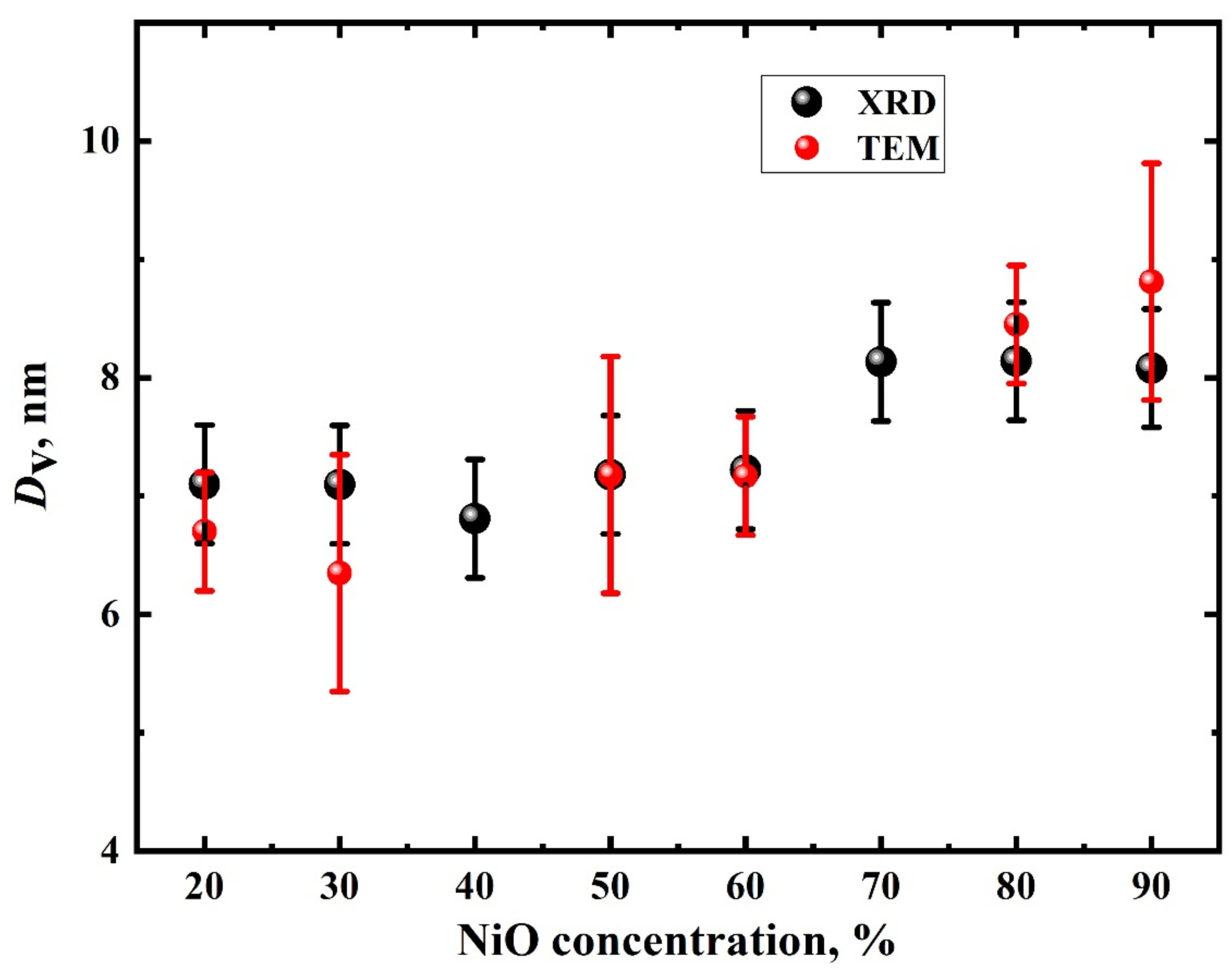
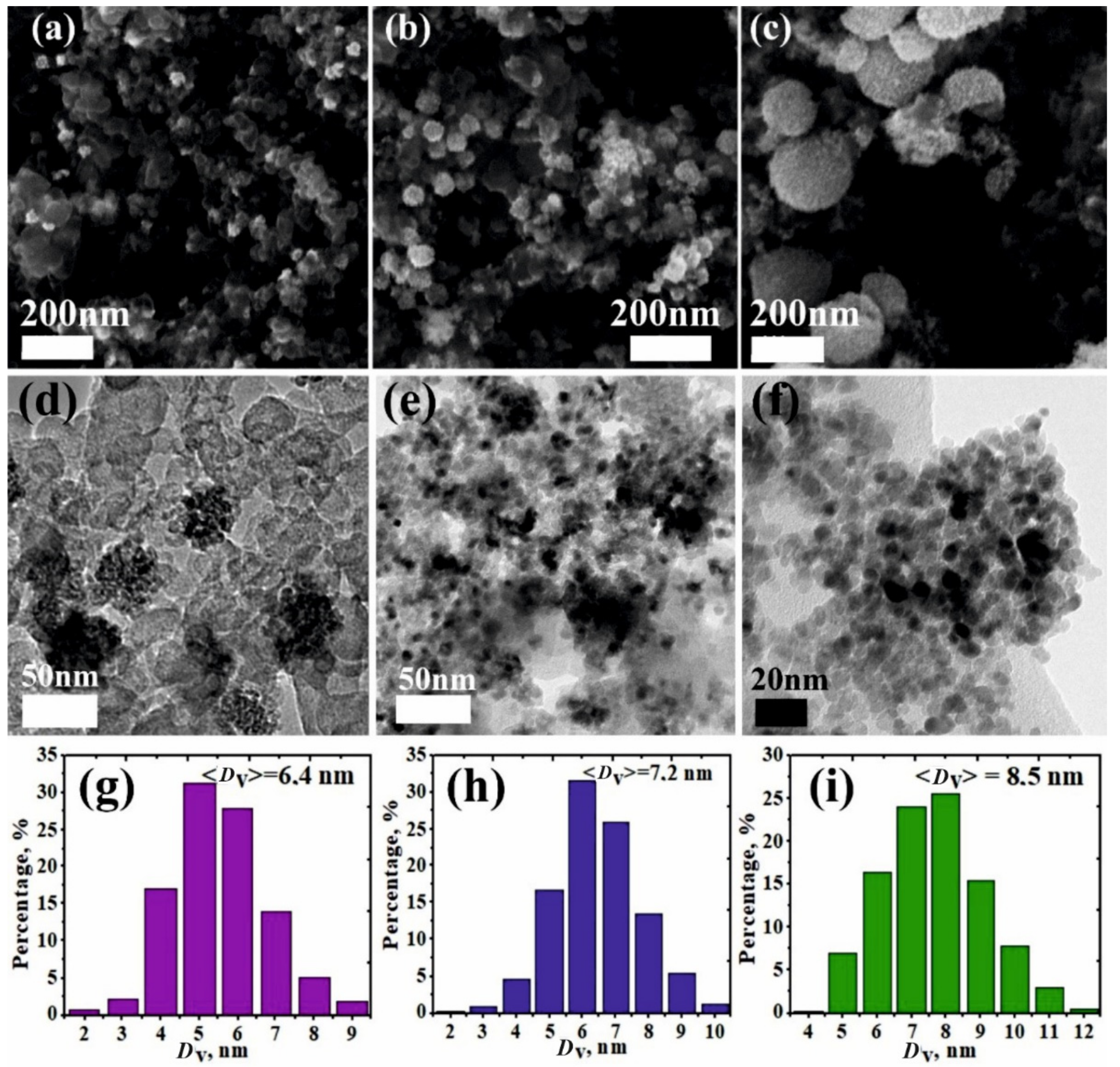
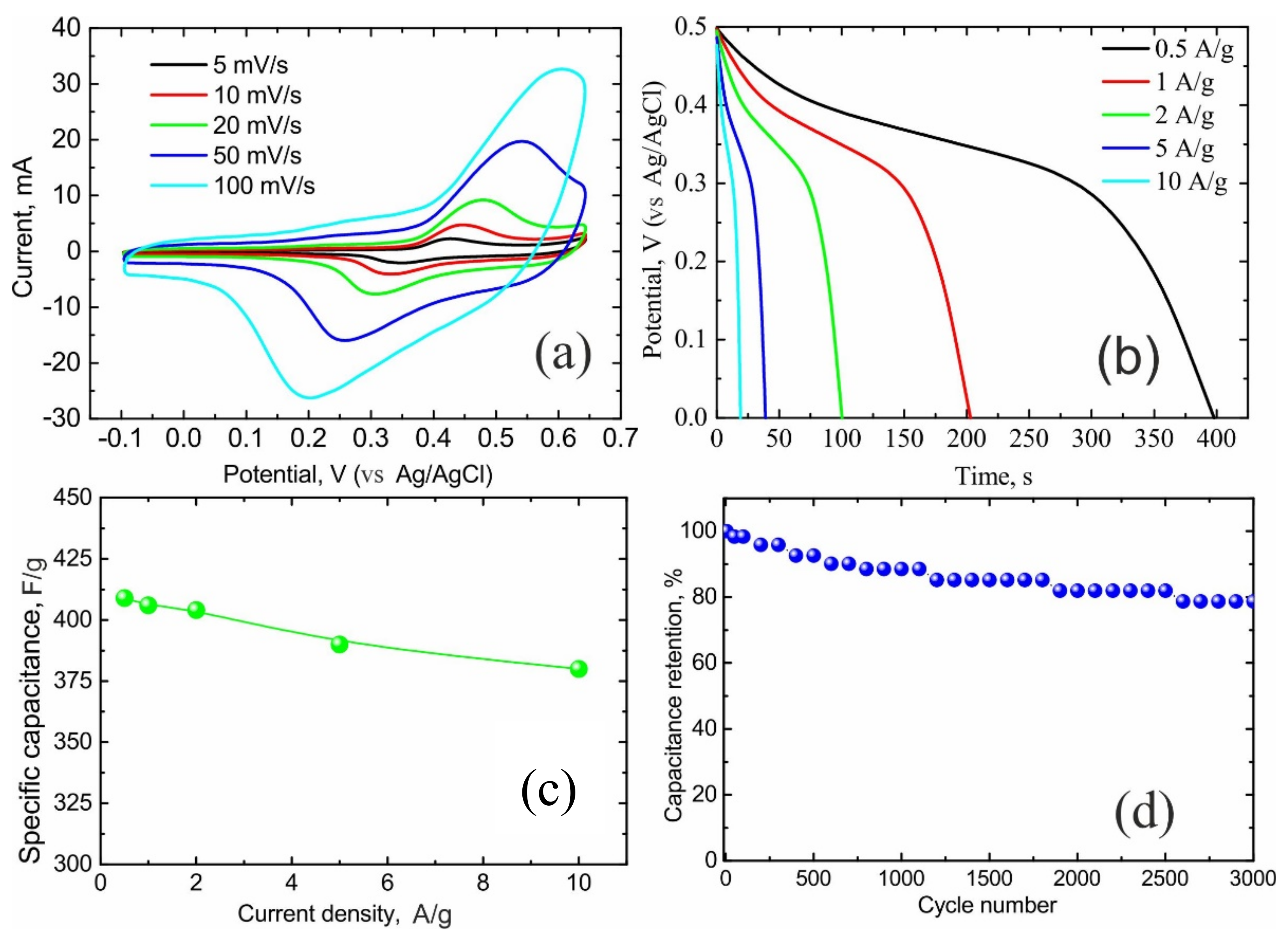
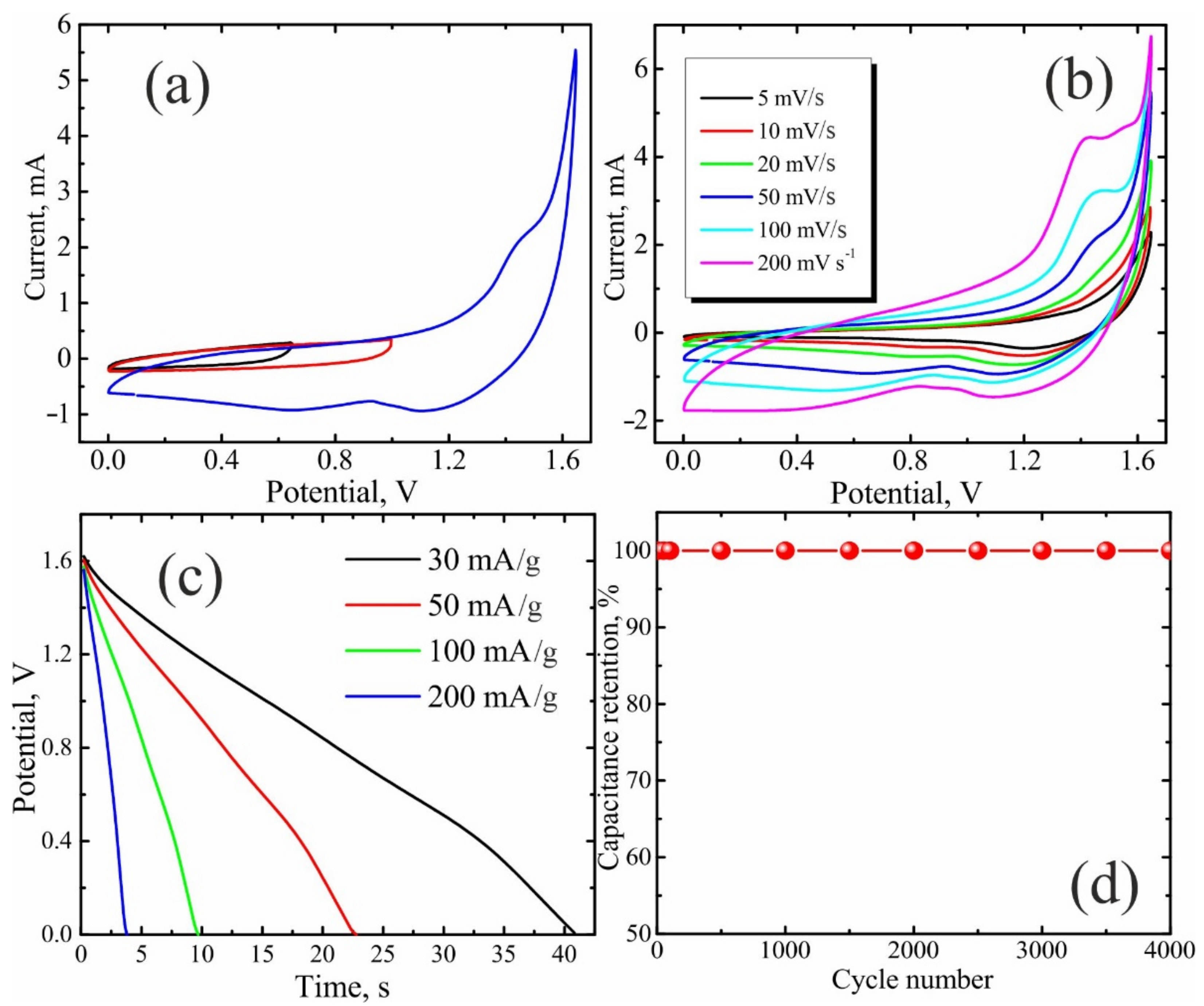
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, X.; Yu, M.; Wang, G.; Tong, Y.; Li, Y. Flexible Solid-State Supercapacitors: Design, Fabrication and Applications. Energy Environ. Sci. 2014, 7, 2160. [Google Scholar] [CrossRef]
- Sk, M.M.; Yue, C.Y.; Ghosh, K.; Jena, R.K. Review on Advances in Porous Nanostructured Nickel Oxides and Their Composite Electrodes for High-Performance Supercapacitors. J. Power Sources 2016, 308, 121–140. [Google Scholar] [CrossRef]
- Paulose, R.; Mohan, R.; Parihar, V. Nanostructured Nickel Oxide and Its Electrochemical Behaviour—A Brief Review. Nano Struct. Nano Objects 2017, 11, 102–111. [Google Scholar] [CrossRef]
- Liu, K.-C.; Anderson, M.A. Porous Nickel Oxide/Nickel Films for Electrochemical Capacitors. J. Electrochem. Soc. 1996, 143, 124. [Google Scholar] [CrossRef]
- Sasi, B.; Gopchandran, K.; Manoj, P.; Koshy, P.; Prabhakara Rao, P.; Vaidyan, V. Preparation of Transparent and Semiconducting NiO Films. Vacuum 2002, 68, 149–154. [Google Scholar] [CrossRef]
- Nandy, S.; Maiti, U.N.; Ghosh, C.K.; Chattopadhyay, K.K. Enhanced P-Type Conductivity and Band Gap Narrowing in Heavily Al Doped NiO Thin Films Deposited by RF Magnetron Sputtering. J. Phys. Condens. Matter 2009, 21, 115804. [Google Scholar] [CrossRef]
- Li, Q.; Li, C.-L.; Li, Y.-L.; Zhou, J.-J.; Chen, C.; Liu, R.; Han, L. Fabrication of Hollow N-Doped Carbon Supported Ultrathin NiO Nanosheets for High-Performance Supercapacitor. Inorg. Chem. Commun. 2017, 86, 140–144. [Google Scholar] [CrossRef]
- Lokhande, V.C.; Lokhande, A.C.; Lokhande, C.D.; Kim, J.H.; Ji, T. Supercapacitive Composite Metal Oxide Electrodes Formed with Carbon, Metal Oxides and Conducting Polymers. J. Alloys Compd. 2016, 682, 381–403. [Google Scholar] [CrossRef]
- Makhlouf, S.A.; Kassem, M.A.; Abdel-Rahim, M.A. Particle Size-Dependent Electrical Properties of Nanocrystalline NiO. J. Mater. Sci. 2009, 44, 3438–3444. [Google Scholar] [CrossRef]
- Yazdani, A.; Zafarkish, H.; Rahimi, K. The Variation of Eg-Shape Dependence of NiO Nanoparticles by the Variation of Annealing Temperature. Mater. Sci. Semicond. Process. 2018, 74, 225–231. [Google Scholar] [CrossRef]
- Bose, P.; Ghosh, S.; Basak, S.; Naskar, M.K. A Facile Synthesis of Mesoporous NiO Nanosheets and Their Application in CO Oxidation. J. Asian Ceram. Soc. 2016, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Ukoba, K.O.; Eloka-Eboka, A.C.; Inambao, F.L. Review of Nanostructured NiO Thin Film Deposition Using the Spray Pyrolysis Technique. Renew. Sustain. Energy Rev. 2018, 82, 2900–2915. [Google Scholar] [CrossRef]
- Aghazadeh, M. Synthesis, Characterization, and Study of the Supercapacitive Performance of NiO Nanoplates Prepared by the Cathodic Electrochemical Deposition-Heat Treatment (CED-HT) Method. J. Mater. Sci. Mater. Electron. 2017, 28, 3108–3117. [Google Scholar] [CrossRef]
- Kumar, A.; Sanger, A.; Kumar, A.; Chandra, R. Single-Step Growth of Pyramidally Textured NiO Nanostructures with Improved Supercapacitive Properties. Int. J. Hydrogen. Energy 2017, 42, 6080–6087. [Google Scholar] [CrossRef]
- Lontio Fomekong, R.; Ngolui Lambi, J.; Ebede, G.R.; Kenfack Tsobnang, P.; Tedjieukeng Kamta, H.M.; Ngnintedem Yonti, C.; Delcorte, A. Effective Reduction in the Nanoparticle Sizes of NiO Obtained via the Pyrolysis of Nickel Malonate Precursor Modified Using Oleylamine Surfactant. J. Solid State Chem. 2016, 241, 137–142. [Google Scholar] [CrossRef]
- Leontyeva, D.V.; Leontyev, I.N.; Avramenko, M.V.; Yuzyuk, Y.I.; Kukushkina, Y.A.; Smirnova, N.V. Electrochemical Dispergation as a Simple and Effective Technique toward Preparation of NiO Based Nanocomposite for Supercapacitor Application. Electrochim. Acta 2013, 114, 356–362. [Google Scholar] [CrossRef]
- Fazlali, F.; Mahjoub, A.; Abazari, R. A New Route for Synthesis of Spherical NiO Nanoparticles via Emulsion Nano-Reactors with Enhanced Photocatalytic Activity. Solid State Sci. 2015, 48, 263–269. [Google Scholar] [CrossRef]
- El-Kemary, M.; Nagy, N.; El-Mehasseb, I. Nickel Oxide Nanoparticles: Synthesis and Spectral Studies of Interactions with Glucose. Mater. Sci. Semicond. Process. 2013, 16, 1747–1752. [Google Scholar] [CrossRef]
- Motahari, F.; Mozdianfard, M.R.; Soofivand, F.; Salavati-Niasari, M. NiO Nanostructures: Synthesis, Characterization and Photocatalyst Application in Dye Wastewater Treatment. RSC Adv. 2014, 4, 27654. [Google Scholar] [CrossRef]
- Danial, A.S.; Saleh, M.M.; Salih, S.A.; Awad, M.I. On the Synthesis of Nickel Oxide Nanoparticles by Sol–Gel Technique and Its Electrocatalytic Oxidation of Glucose. J. Power Sources 2015, 293, 101–108. [Google Scholar] [CrossRef]
- Purushothaman, K.K.; Manohara Babu, I.; Sethuraman, B.; Muralidharan, G. Nanosheet-Assembled NiO Microstructures for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 10767–10773. [Google Scholar] [CrossRef] [PubMed]
- Kulbakov, A.A.; Allix, M.; Rakhmatullin, A.; Mikheykin, A.S.; Popov, Y.V.; Smirnova, N.V.; Maslova, O.; Leontyev, I.N. In Situ Investigation of Non-Isothermal Decomposition of Pt Acetylacetonate as One-Step Size-Controlled Synthesis of Pt Nanoparticles. Phys. Status Solidi 2018, 1800488. [Google Scholar] [CrossRef]
- Kulbakov, A.A.; Kuriganova, A.B.; Allix, M.; Rakhmatullin, A.; Smirnova, N.; Maslova, O.A.; Leontyev, I.N. Non-Isothermal Decomposition of Platinum Acetylacetonate as a Cost-Efficient and Size-Controlled Synthesis of Pt/C Nanoparticles. Catal. Commun. 2018, 117, 14–18. [Google Scholar] [CrossRef]
- Leontyev, I.N.; Kuriganova, A.B.; Allix, M.; Rakhmatullin, A.; Timoshenko, P.E.; Maslova, O.A.; Mikheykin, A.S.; Smirnova, N.V. On the Evaluation of the Average Crystalline Size and Surface Area of Platinum Catalyst Nanoparticles. Phys. Status Solidi Basic Res. 2018, 255. [Google Scholar] [CrossRef]
- Leontyev, I.N.; Kulbakov, A.A.; Allix, M.; Rakhmatullin, A.; Kuriganova, A.B.; Maslova, O.A.; Smirnova, N.V. Thermal Expansion Coefficient of Carbon-Supported Pt Nanoparticles: In-Situ X-Ray Diffraction Study. Phys. Status Solidi Basic Res. 2017, 254. [Google Scholar] [CrossRef]
- Chernysheva, D.; Vlaic, C.; Leontyev, I.; Pudova, L.; Ivanov, S.; Avramenko, M.; Allix, M.; Rakhmatullin, A.; Maslova, O.; Bund, A.; et al. Synthesis of Co3O4/CoOOH via electrochemical dispersion using a pulse alternating current method for lithium-ion batteries and supercapacitors. Solid State Sci. 2018, 86, 53–59. [Google Scholar] [CrossRef]
- Liu, S.; Lee, S.C.; Patil, U.M.; Ray, C.; Sankar, K.V.; Zhang, K.; Kundu, A.; Kang, S.; Park, J.H.; Jun, S.C. Controllable Sulfuration Engineered NiO Nanosheets with Enhanced Capacitance for High Rate Supercapacitors. J. Mater. Chem. A 2017, 5, 4543–4549. [Google Scholar] [CrossRef]
- Shklovskii, B.I.; Éfros, A.L. Percolation Theory and Conductivity of Strongly Inhomogeneous Media. Uspekhi Fiz. Nauk 1975, 117, 401. [Google Scholar] [CrossRef]
- Kuriganova, A.B.; Vlaic, C.A.; Ivanov, S.; Leontyeva, D.V.; Bund, A.; Smirnova, N.V. Electrochemical Dispersion Method for the Synthesis of SnO2 as Anode Material for Lithium Ion Batteries. J. Appl. Electrochem. 2016, 46, 527–538. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, S.; Zhang, E.; Wei, J.; Suo, H.; Zhao, C.; Zhao, X. One-pot synthesis of nickel oxide–carbon composite microspheres on nickel foam for supercapacitors. J. Mater. Sci. 2012, 47, 2182–2187. [Google Scholar] [CrossRef]
- Liu, J.; Wickramaratne, N.P.; Qiao, S.Z.; Jaroniec, M. Molecular-based design and emerging applications of nanoporous carbon spheres. Nat. Mater. 2015, 14, 763. [Google Scholar] [CrossRef] [PubMed]
- Sannasi, V.; Maheswari, K.U.; Karthikeyan, C.; Karuppuchamy, S. H2O2-assisted microwave synthesis of NiO/CNT nanocomposite material for supercapacitor applications. Ionics 2020, 26, 4067–4079. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Nagamuthu, S.; Muralidharan, G. Porous NiO/C Nanocomposites as Electrode Material for Electrochemical Supercapacitors. ACS Sustain. Chem. Eng. 2013, 1, 1110–1118. [Google Scholar] [CrossRef]
- Wu, S.R.; Liu, J.B.; Wang, H.; Yan, H. NiO@graphite carbon nanocomposites derived from Ni-MOFs as supercapacitor electrodes. Ionics 2019, 25, 1–8. [Google Scholar] [CrossRef]
- Wang, L.; Tian, H.; Wang, D.; Qin, X.; Shao, G. Preparation and electrochemical characteristic of porous NiO supported by sulfonated graphene for supercapacitors. Electrochim. Acta 2015, 151, 407–414. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Zhu, D.; Li, L.; Duan, H.; Xu, Z.; Wang, Z.; Gan, L. Encapsulation of NiO nanoparticles in mesoporous carbon nanospheres for advanced energy storage. Chem. Eng. J. 2017, 308, 240–247. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernysheva, D.; Pudova, L.; Popov, Y.; Smirnova, N.; Maslova, O.; Allix, M.; Rakhmatullin, A.; Leontyev, N.; Nikolaev, A.; Leontyev, I. Non-Isothermal Decomposition as Efficient and Simple Synthesis Method of NiO/C Nanoparticles for Asymmetric Supercapacitors. Nanomaterials 2021, 11, 187. https://doi.org/10.3390/nano11010187
Chernysheva D, Pudova L, Popov Y, Smirnova N, Maslova O, Allix M, Rakhmatullin A, Leontyev N, Nikolaev A, Leontyev I. Non-Isothermal Decomposition as Efficient and Simple Synthesis Method of NiO/C Nanoparticles for Asymmetric Supercapacitors. Nanomaterials. 2021; 11(1):187. https://doi.org/10.3390/nano11010187
Chicago/Turabian StyleChernysheva, Daria, Ludmila Pudova, Yuri Popov, Nina Smirnova, Olga Maslova, Mathieu Allix, Aydar Rakhmatullin, Nikolay Leontyev, Andrey Nikolaev, and Igor Leontyev. 2021. "Non-Isothermal Decomposition as Efficient and Simple Synthesis Method of NiO/C Nanoparticles for Asymmetric Supercapacitors" Nanomaterials 11, no. 1: 187. https://doi.org/10.3390/nano11010187
APA StyleChernysheva, D., Pudova, L., Popov, Y., Smirnova, N., Maslova, O., Allix, M., Rakhmatullin, A., Leontyev, N., Nikolaev, A., & Leontyev, I. (2021). Non-Isothermal Decomposition as Efficient and Simple Synthesis Method of NiO/C Nanoparticles for Asymmetric Supercapacitors. Nanomaterials, 11(1), 187. https://doi.org/10.3390/nano11010187




