Niobium Modification of Au/CeO2 for Enhanced Catalytic Performance over Benzene Combustion
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Catalyst Characterization
2.3. Catalytic Activity Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Piumetti, M.; Fino, D.; Russo, N. Mesoporous manganese oxides prepared by solution combustion synthesis as catalysts for the total oxidation of VOCs. Appl. Catal. B Environ. 2015, 163, 277–287. [Google Scholar] [CrossRef]
- Yang, C.; Miao, G.; Pi, Y.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019, 370, 128–1153. [Google Scholar] [CrossRef]
- Jiang, W.; Feng, Y.; Zeng, Y.; Yao, Y.; Gu, L.; Sun, H.; Ji, W.; Arandiyan, H.; Au, C.-T. Establishing high-performance Au/cobalt oxide interfaces for low-temperature benzene combustion. J. Catal. 2019, 375, 171–182. [Google Scholar] [CrossRef]
- Li, J.; Zuo, S.; Qi, C. Preparation and high performance of rare earth modified Co/USY for benzene catalytic combustion. Catal. Commun. 2017, 91, 30–33. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Li, S.; Shan, X.; Liu, G.; Chen, Y. Co-nanocasting synthesis of mesoporous Cu–Mn composite oxides and their promoted catalytic activities for gaseous benzene removal. Appl. Catal. B Environ. 2015, 162, 110–121. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, Y.; Li, C.; Shen, Y.; Li, H.; Hu, Z.; Di, X.; Liu, Z. Creation of three-dimensionally ordered macroporous Au/CeO2 catalysts with controlled pore sizes and their enhanced catalytic performance for formaldehyde oxidation. Appl. Catal. B Environ. 2009, 91, 11–20. [Google Scholar] [CrossRef]
- Tabakova, T.; Dimitrov, D.; Manzoli, M.; Vindigni, F.; Petrova, P.; Ilieva, L.; Zanella, R.; Ivanov, K. Impact of metal doping on the activity of Au/CeO2 catalysts for catalytic abatement of VOCs and CO in waste gases. Catal. Commun. 2013, 35, 51–58. [Google Scholar] [CrossRef]
- Xie, S.; Deng, J.; Zang, S.; Yang, H.; Guo, G.; Arandiyan, H.; Dai, H. Au–Pd/3DOM Co3O4: Highly active and stable nanocatalysts for toluene oxidation. J. Catal. 2015, 322, 38–48. [Google Scholar] [CrossRef]
- Kaminski, P.; Ziolek, M. Zr-oxides and its impact on total oxidation of methanol. Appl. Catal. B Environ. 2016, 187, 328–341. [Google Scholar] [CrossRef]
- Topka, P.; Delaigle, R.; Kaluža, L.; Gaigneaux, E.M. Performance of platinum and gold catalysts supported on ceria–zirconia mixed oxide in the oxidation of chlorobenzene. Catal. Today 2015, 253, 172–177. [Google Scholar] [CrossRef]
- Carltonbird, M.; Eaimsumang, S.; Pongstabodee, S.; Boonyuen, S.; Smith, S.M.; Luengnaruemitchai, A. Effect of the exposed ceria morphology on the catalytic activity of gold/ceria catalysts for the preferential oxidation of carbon monoxide. Chem. Eng. J. 2018, 344, 545–555. [Google Scholar] [CrossRef]
- Araiza, D.G.; Gómez-Cortés, A.; Díaz, G. Effect of ceria morphology on the carbon deposition during steam reforming of ethanol over Ni/CeO2 catalysts. Catal. Today. 2020, 349, 235–243. [Google Scholar] [CrossRef]
- Nedyalkova, R.; Ilieva, L.; Bernard, M.C.; Hugot-Le Goff, A.; Andreeva, D. Gold supported catalysts on titania and ceria, promoted by vanadia or molybdena for complete benzene oxidation. Mater. Chem. Phys. 2009, 116, 214–218. [Google Scholar] [CrossRef]
- Ying, F.; Wang, S.; Au, C.-T.; Lai, S.Y. Highly active and stable mesoporous Au/CeO2 catalysts prepared from MCM-48 hard-template. Microporous Mesoporous Mater. 2011, 142, 308–315. [Google Scholar] [CrossRef]
- Yang, S.M.; Liu, D.M.; Liu, S.Y. Catalytic combustion of benzene over Au supported on ceria and vanadia promoted ceria. Top. Catal. 2008, 47, 101–108. [Google Scholar] [CrossRef]
- Andreeva, D.; Nedyalkova, R.; Ilieva, L.; Abrashev, M.V. Nanosize gold-ceria catalysts promoted by vanadia for complete benzene oxidation. Appl. Catal. A Gen. 2003, 246, 29–38. [Google Scholar] [CrossRef]
- Xie, Q.; Chen, L.; Weng, W.; Wan, H. Preparation of MoVTe(Sb)Nb mixed oxide catalysts using a slurry method for selective oxidative dehydrogenation of ethane. J. Mol. Catal. A Chem. 2005, 240, 191–196. [Google Scholar] [CrossRef]
- Rocha, A.S.; Forrester, A.M.S.; de la Cruz, M.H.C.; da Silva, C.T.; Lachter, E.R. Comparative performance of niobium phosphates in liquid phase anisole benzylation with benzyl alcohol. Catal. Commun. 2008, 9, 1959–1965. [Google Scholar] [CrossRef]
- Tanabe, K.; Okazaki, S. Various reactions catalyzed by niobium compounds and materials. Appl. Catal. A Gen. 1995, 133, 191–218. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, L.; Gao, Y.; Huang, W. CeO morphology-dependent NbO–CeO interaction, structure and catalytic performance of NbO/CeO catalysts in oxidative dehydrogenation of propane. Appl. Catal. B Environ. 2016, 197, 214–221. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Y.; Li, S.; Li, W. Promotional synergistic effect of Cu and Nb doping on a novel Cu/Ti-Nb ternary oxide catalyst for the selective catalytic reduction of NO with NH. Appl. Catal. B Environ. 2018, 220, 234–250. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, X.; Si, Z.; Weng, D.; Ma, J.; Xu, T. Impacts of niobia loading on active sites and surface acidity in NbOx/CeO2–ZrO2 NH3–SCR catalysts. Appl. Catal. B Environ. 2015, 179, 380–394. [Google Scholar] [CrossRef]
- Qu, R.; Gao, X.; Cen, K.; Li, J. Relationship between structure and performance of a novel cerium-niobium binary oxide catalyst for selective catalytic reduction of NO with NH. Appl. Catal. B Environ. 2013, 142–143, 290–297. [Google Scholar] [CrossRef]
- Gong, J. Structure and Surface Chemistry of Gold-Based Model Catalysts. Chem. Rev. 2012, 112, 2987–3054. [Google Scholar] [CrossRef] [PubMed]
- Kosacki, I.; Suzuki, T.; Anderson, H.U.; Colomban, P. Raman scattering and lattice defects in nanocrystalline CeO2 thin films. Solid State Ion. 2002, 149, 99–105. [Google Scholar] [CrossRef]
- Graham, G.W.; Weber, W.H.; Peters, C.R.; Usmen, R. Empirical method for determining CeO2-particle size in catalysts by raman spectroscopy. J. Catal. 1991, 130, 310–313. [Google Scholar] [CrossRef]
- Wachs, I.E.; Chen, Y.; Jehng, J.M.; Briand, L.E.; Tanaka, T. Molecular structure and reactivity of the Group V metal oxides. Catal. Today 2003, 78, 13–24. [Google Scholar] [CrossRef]
- Wu, Z.L.; Li, M.; Overbury, S.H. On the structure dependence of CO oxidation over CeO2 nanocrystals with well-defined surface planes. J. Catal. 2012, 285, 61–73. [Google Scholar] [CrossRef]
- Wisniewska, J.; Ziolek, M.; Artioli, N.; Daturi, M. The effect of niobium and tantalum on physicochemical and catalytic properties of silver and platinum catalysts based on MCF mesoporous cellular foams. J. Catal. 2016, 336, 58–74. [Google Scholar] [CrossRef]
- Martínez-Méndez, S.; Henríquez, Y.; Domínguez, O.; D’Ornelas, L.; Krentzien, H. Catalytic properties of silica supported titanium, vanadium and niobium oxide nanoparticles towards the oxidation of saturated and unsaturated hydrocarbons. J. Mol. Catal. A Chem. 2006, 252, 226–234. [Google Scholar] [CrossRef]
- Boffa, V.; ten Elshof, J.E.; Garcia, R.; Blank, D.H.A. Microporous niobia–silica membranes: Influence of sol composition and structure on gas transport properties. Microporous Mesoporous Mater. 2009, 118, 202–209. [Google Scholar] [CrossRef]
- Paparazzo, E.; Ingo, G.M.; Zacchetti, N. X-ray induced reduction effects at CeO2 surfaces: An x-ray photoelectron spectroscopy study. J. Vac. Sci. Technol. A 1991, 9, 1416–1420. [Google Scholar] [CrossRef]
- López, J.M.; Arenal, R.; Puértolas, B.; Mayoral, Á.; Taylor, S.H.; Solsona, B.; García, T. Au deposited on CeO prepared by a nanocasting route: A high activity catalyst for CO oxidation. J. Catal. 2014, 317, 167–175. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Longo, A.; Martorana, A.; Prestianni, A.; Venezia, A.M. XPS study of supported gold catalysts: The role of Au0 and Au+δ species as active sites. Surf. Interface Anal. 2006, 38, 215–218. [Google Scholar] [CrossRef]
- Li, H.F.; Zhang, N.; Chen, P.; Luo, M.F.; Lu, J.Q. High surface area Au/CeO2 catalysts for low temperature formaldehyde oxidation. Appl. Catal. B Environ. 2011, 110, 279–285. [Google Scholar] [CrossRef]
- Haruta, M. Size- and support-dependency in the catalysis of gold. Catal. Today 1997, 36, 153–166. [Google Scholar] [CrossRef]
- Huang, H.B.; Xu, Y.; Feng, Q.Y.; Leung, D.Y.C. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
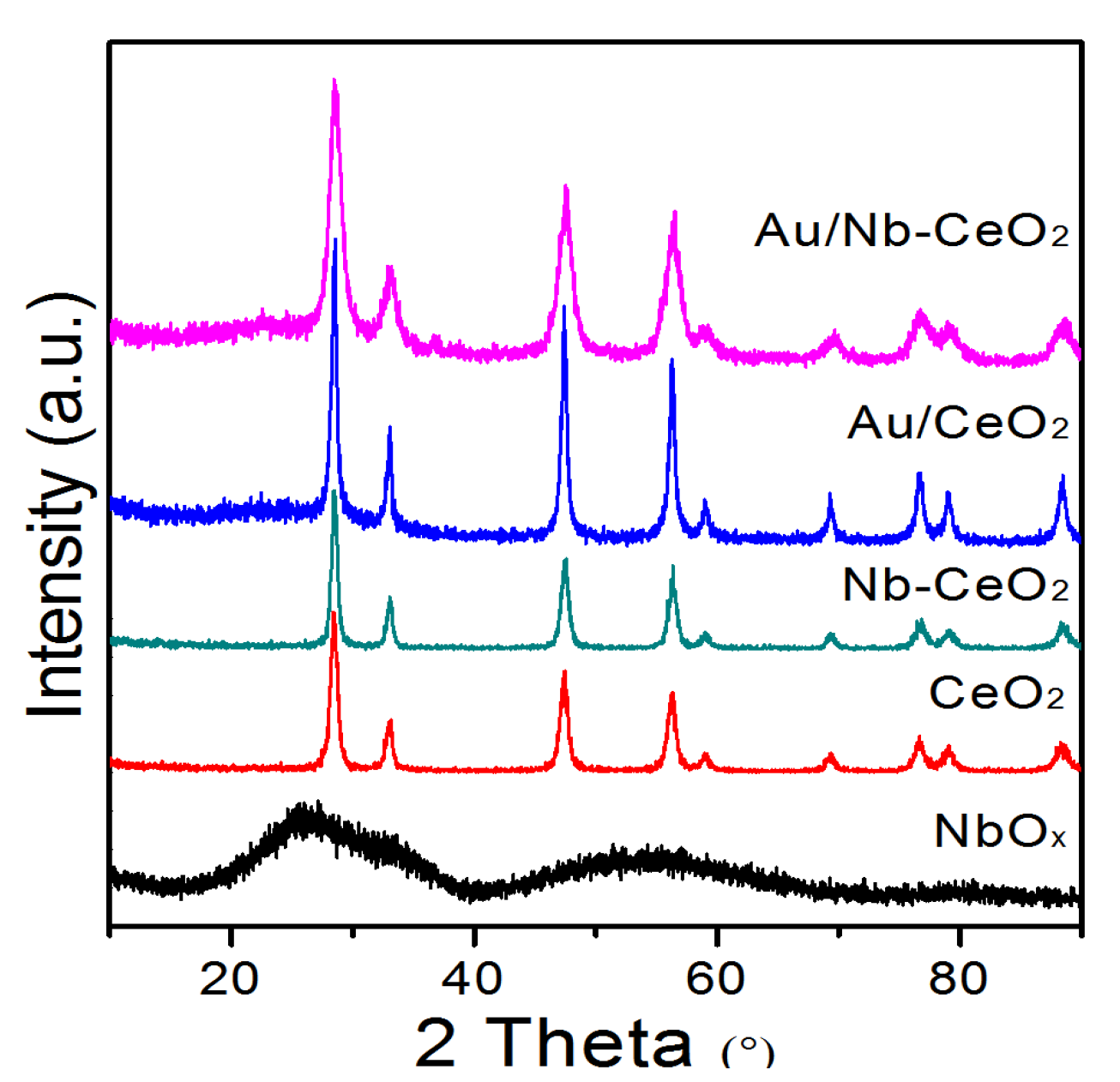

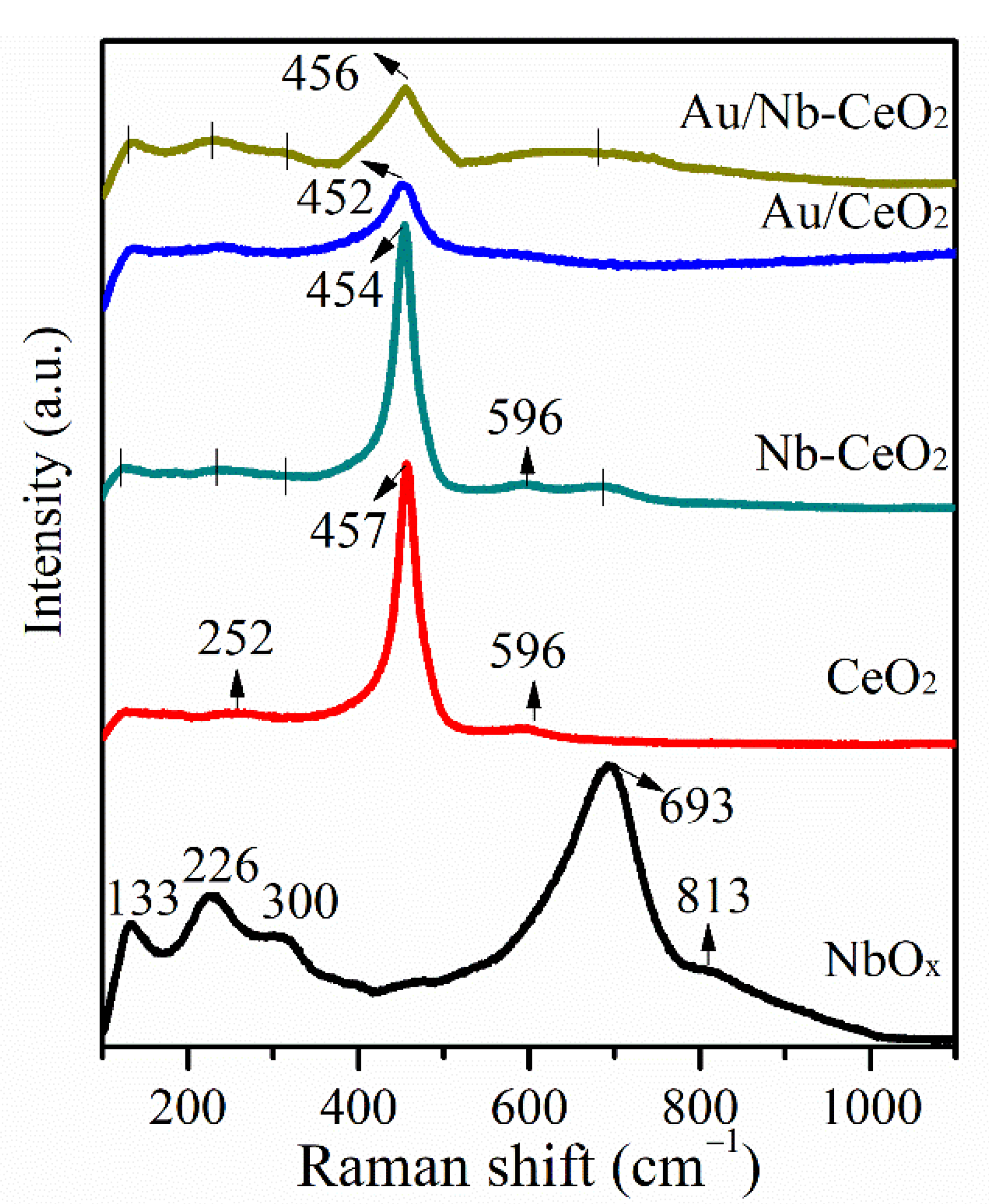


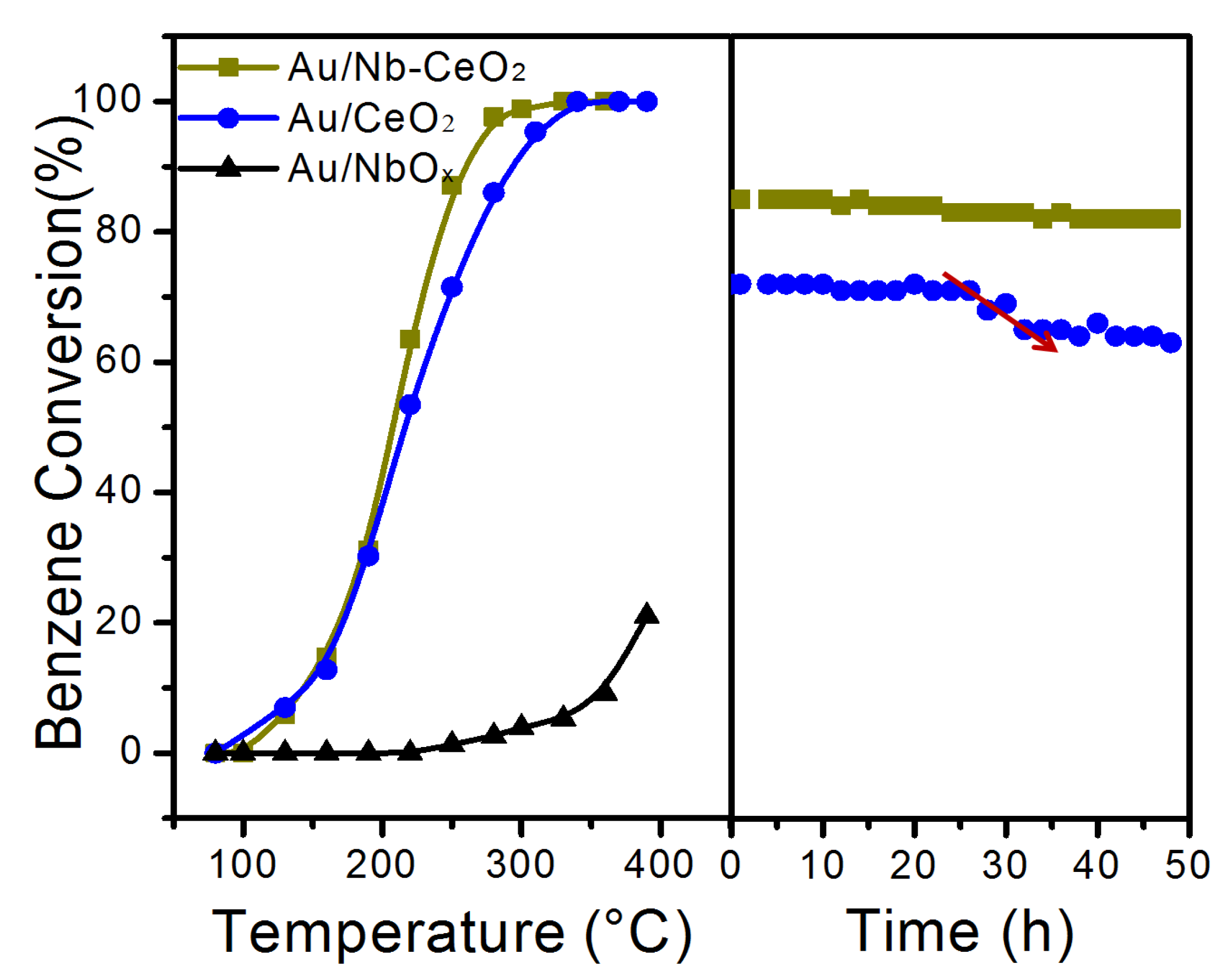
| Catalyst | Crystalline Size (nm) a | SBET (m2 g−1) b | H2 Consumption (mmol g−1) c | Au (wt%) d | XPS Data | |||
|---|---|---|---|---|---|---|---|---|
| Auδ+/Au | Ce3+/Ce4+ | Osup/Olatt | Nb/Ce | |||||
| Au/CeO2 | 12 | 71 | 5.13 | 0.92 | 0.32 | 0.31 | 0.70 | - |
| Au/Nb-CeO2 | 7 | 203 | 5.79 | 0.99 (0.07) e | 0.41 | 0.32 | 1.33 | 0.37 |
| Au/NbOx | - | 44 | - | - | 0.12 | - | 0.65 | - |
| Nb-CeO2 | - | - | - | - | - | 0.17 | 2.21 | 0.67 |
| Catalyst | Frequency (cm−1) | FWHM (cm−1) |
|---|---|---|
| CeO2 | 457 | 34 |
| Nb-CeO2 | 454 | 32 |
| Au/CeO2 | 452 | 51 |
| Au/Nb-CeO2 | 456 | 65 |
| Catalysts | Au/CeO2 | Au/Nb-CeO2 | Au/CeO2-1st a | Au/Nb-CeO2-1st | Au/Nb-CeO2-48 h b |
|---|---|---|---|---|---|
| Au dispersion (%) | 68 | 72 | 54 | 72 | 71 |
| Au particle size c (nm) | 1.72 | 1.63 | 2.15 | 1.63 | 1.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zhang, X.; Cai, T.; Yuan, J.; Zhao, K.; Lu, W.; He, D. Niobium Modification of Au/CeO2 for Enhanced Catalytic Performance over Benzene Combustion. Nanomaterials 2021, 11, 189. https://doi.org/10.3390/nano11010189
Liu Z, Zhang X, Cai T, Yuan J, Zhao K, Lu W, He D. Niobium Modification of Au/CeO2 for Enhanced Catalytic Performance over Benzene Combustion. Nanomaterials. 2021; 11(1):189. https://doi.org/10.3390/nano11010189
Chicago/Turabian StyleLiu, Zhe, Xiaolan Zhang, Ting Cai, Jing Yuan, Kunfeng Zhao, Wenquan Lu, and Dannong He. 2021. "Niobium Modification of Au/CeO2 for Enhanced Catalytic Performance over Benzene Combustion" Nanomaterials 11, no. 1: 189. https://doi.org/10.3390/nano11010189
APA StyleLiu, Z., Zhang, X., Cai, T., Yuan, J., Zhao, K., Lu, W., & He, D. (2021). Niobium Modification of Au/CeO2 for Enhanced Catalytic Performance over Benzene Combustion. Nanomaterials, 11(1), 189. https://doi.org/10.3390/nano11010189




