First Phenol Carboxylation with CO2 on Carbon Nanostructured C@Fe-Al2O3 Hybrids in Aqueous Media under Mild Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Analytical Methods
2.3. Phenol Conversion
3. Results
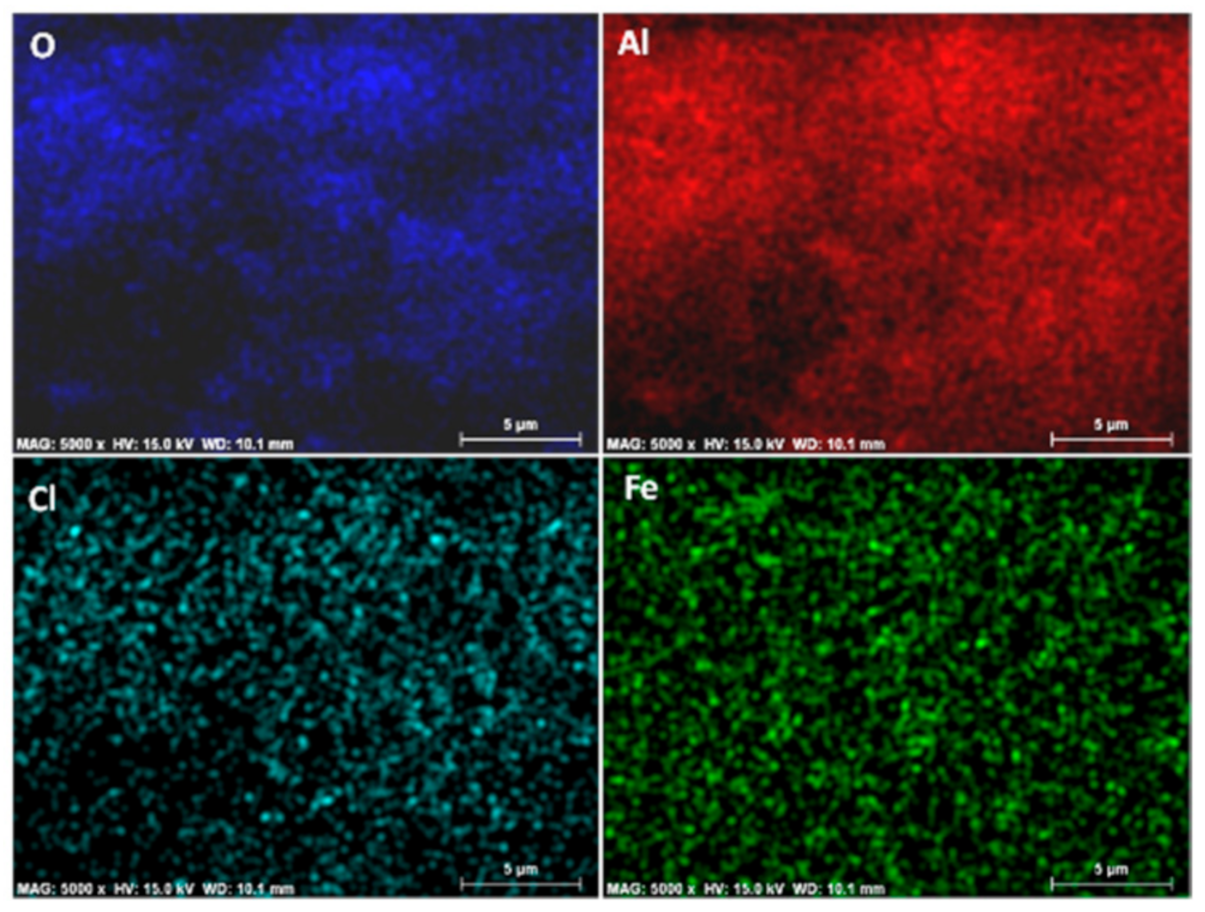
3.1. Characterization of the Hybrid Precursors
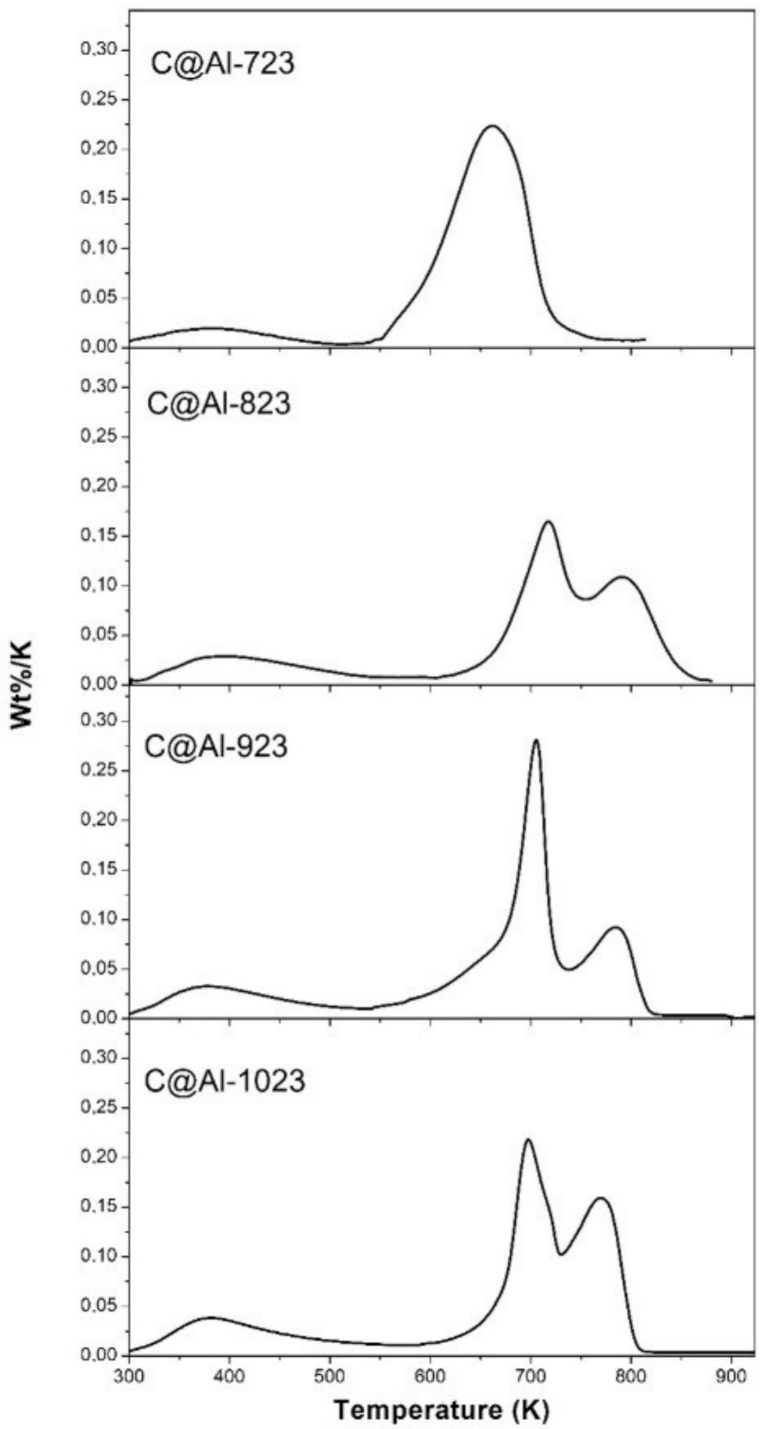
3.2. Characterization of the C@Fe–Al2O3 Hybrids
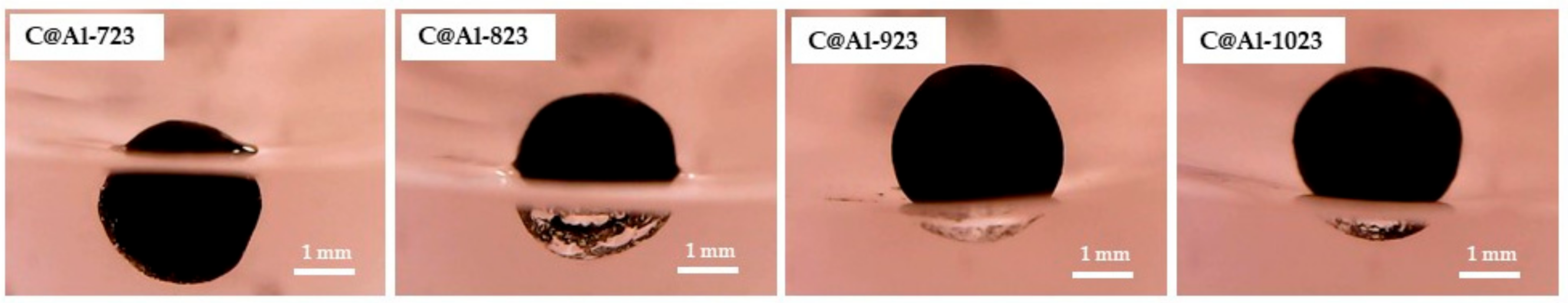
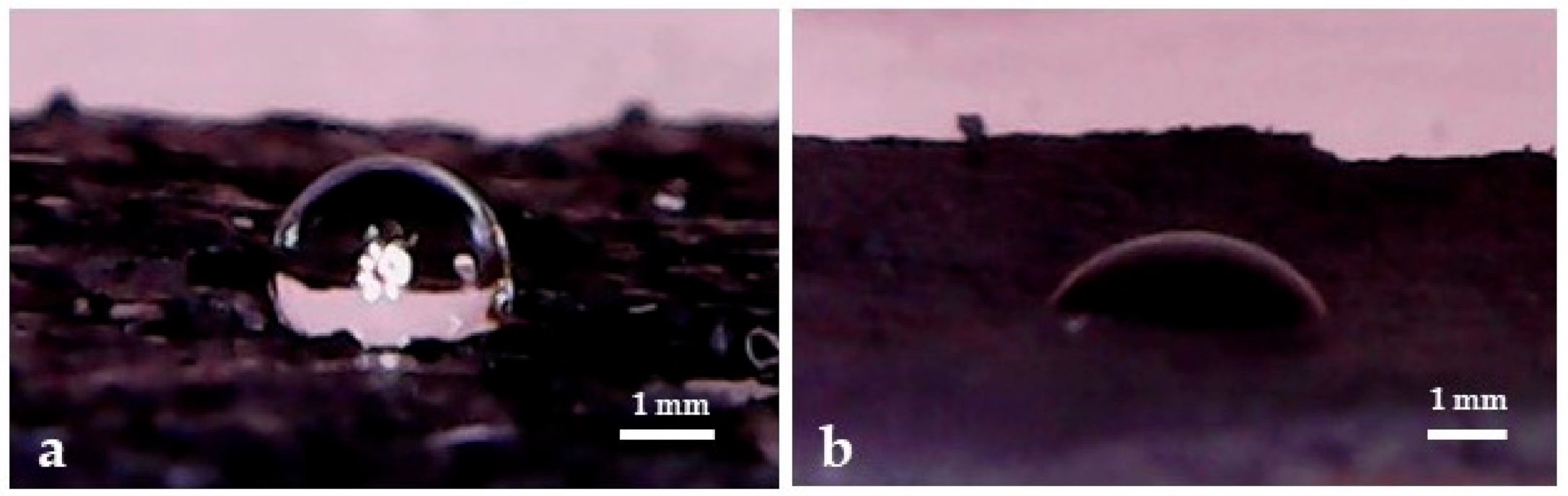
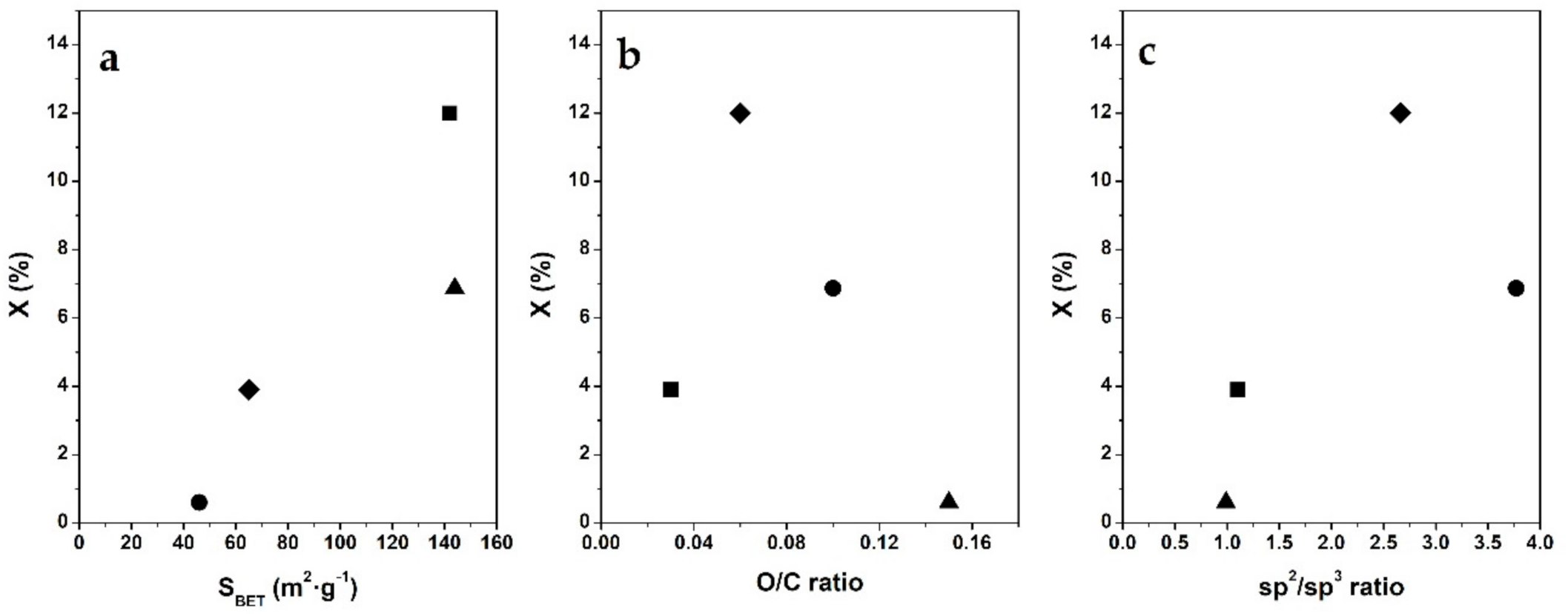
3.3. Phenol Conversion in C@Fe–Al2O3 Hybrids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dabral, S.; Schaub, T. The use of carbon dioxide (CO2) as a building block in organic synthesis from an industrial perspective. Adv. Synth. Catal. 2019, 361, 223–246. [Google Scholar] [CrossRef]
- Tanaka, R.; Yamashita, M.; Nozaki, K. Catalytic hydrogenation of carbon dioxide using Ir(III)—Princes complexes. J. Am. Chem. Soc. 2009, 131, 14168–14169. [Google Scholar] [CrossRef] [PubMed]
- Van der Ham, L.; van der Berg, H.; Benneker, A.; Simmelink, G.; Timmer, J.; Van Weerden, S. Hydrogenation of carbon dioxide for methanol production. Chem. Eng. Trans. 2012, 29, 181–186. [Google Scholar] [CrossRef]
- Fujiwara, M.; Sakurai, H.; Shiokawa, K.; Iizuka, Y. Synthesis of C2+ hydrocarbons by CO2 hydrogenation over the composite catalyst Cu-Zn-Al oxide and HB zeolite using two-stage reactor system under low pressure. Catal. Today 2015, 242, 255–260. [Google Scholar] [CrossRef]
- Li, C.; Yuan, X.; Fujimoto, K. Direct synthesis of LPG from carbon dioxide over hybrid catalyst comprising modified methanol synthesis catalyst and beta-type. App. Catal. A 2014, 475, 155–160. [Google Scholar] [CrossRef]
- Li, C.; Fujimoto, K. Efficient conversion of carbon dioxide to non-methane light hydrocarbons—Two stage process with intercooler. Fuel Proc. Technol. 2015, 136, 50–55. [Google Scholar] [CrossRef]
- Omae, I. Recent developments in carbon dioxide utilization for the production of organic chemicals. Coord. Chem. Rev. 2012, 256, 1384–1405. [Google Scholar] [CrossRef]
- Rabie, A.M.; Betiha, M.A.; Park, S.-E. Direct synthesis of acetic acid by simultaneous co-activation of methane and CO2 over Cu-exchaged ZSM-5 catalyst. Appl. Catal. B Environ. 2017, 215, 50–59. [Google Scholar] [CrossRef]
- Pescarmona, P.; Taherimehr, M. Challenges in the catalytic synthesis of cyclic and polymeric carbonates from epoxides and CO2. Catal. Sci. Technol. 2012, 20, 2169–2187. [Google Scholar] [CrossRef]
- Guo, C.-X.; Ma, R.; He, L.-N. Metal-promoted synthesis of cyclic carbonates from 1,2-diols and carbon dioxide. Open Org. Chem. J. 2014, 8, 6–14. [Google Scholar] [CrossRef]
- Kikuchi, S.; Yoshida, S.; Sugawara, Y.; Yamada, W.; Cheng, H.-M.; Fukui, K.; Kohei, S.; Izumi, I.; Taketo, I.; Tohru, Y. Silver-catalyzed carbon dioxide incorporation and rearrangement on propargylic derivates. Bull. Chem. Soc. Jpn. 2011, 7, 698–717. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Moncada, A.I.; Choi, W.; Reibenspies, J.H. Mechanistic studies of the copolymerization reaction od oxetane and carbon dioxide to provide aliphatic polycarbonates catalyzed by (salen) CrX complexes. J. Am. Chem. Soc. 2008, 130, 6523–6533. [Google Scholar] [CrossRef] [PubMed]
- Omae, I. Aspects of carbon dioxide utilization. Catal. Today 2006, 115, 33–52. [Google Scholar] [CrossRef]
- Arayachukiat, S.; Yingcharoen, P.; Vummaleti Sai, V.C.; Cavallo, L.; Poater, A.; D’Elia, V. Cycloaddition of CO2 to challenging N-tosyl aziridines using a halogen-free niobium complex: Catalytic activity and mechanistic insights. Mol. Catal. 2017, 443, 280–285. [Google Scholar] [CrossRef]
- Kolbe, E.; Lautemann, H. Ueber die Constitution und Basicitat der Salicyl saüre. Ann. Chem. Pharm. 1860, 113, 157–206. [Google Scholar] [CrossRef]
- Schmitt, R. Beitrag zur Kenntuiss der Kolbe’schen Salicyl-säure-Synthese. J. Pratk. Chem. 1885, 31, 397–411. [Google Scholar] [CrossRef]
- Baine, O.; Adamson, G.F.; Barton, J.W.; Fitch, J.L.; Swayampati, D.R.; Jeskey, H. A study of the Kolbe- Schmitt reaction. II The carbonation of phenols. J. Org. Chem. 1953, 19, 510–514. [Google Scholar] [CrossRef]
- Lindsey, A.S.; Jeskey, H. The Kolbe Schmitt Reaction. Chem. Rev. 1957, 57, 583–620. [Google Scholar] [CrossRef]
- Peters, G.P.; Le Quéré, C.; Andrew, R.M.; Canadell, J.G.; Friedlingstein, P.; Ilyina, T.; Joos, F.; Korsbakken, J.I.; McKinley, G.A.; Sitch, S.; et al. Towards real-time verification of CO2 emissions. Nat. Clim. Chang. 2017, 7, 848–850. [Google Scholar] [CrossRef]
- Jackson, R.B.; Le Quéré, C.; Andrew, R.M.; Canadell, J.G.; Peters, G.P.; Roy, J.; Wu, L. Warning signs for stabilizing global CO2 emissions. Environ. Res. Lett. 2017, 12, 1–4. [Google Scholar] [CrossRef]
- Ullmann, F. Enciclopedia de Química Industrial Vol III: Urea; Gustavo Gili: Sao Paulo, Brazil, 1950. [Google Scholar]
- Alper, E.; Yuksel Orhan, O. CO2 utilization: Developments in conversion processes. Petroleum 2017, 3, 109–126. [Google Scholar] [CrossRef]
- Alvarez-Rodriguez, J.; Soria-Sanchez, M.; Calvo-Castañera, F.; Maroto-Valiente, A. Selection of iron precursors for preparation of 3D-solids of hydrophobic composites with γ-alumina and carbon nanostructures materials. J. Clean. Prod. 2019, 234, 290–297. [Google Scholar] [CrossRef]
- ASTM E394-00. Standard Test Method for Iron in Trace Quantities Using 1,12-Phenanthroline Method; American Society for Testing and Materials: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschk, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectra analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Jorio, A.; Coelho, L.N.; Magalhães-Paniago, R.; Pimenta, M.A. General equation for the determination of the crystallite size La of nanographite by Raman spectroscopy. App. Phys. Lett. 2006, 88, 1631061–1631063. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Pawlyta, M.; Rouzaud, J.-N.; Duber, S. Raman microespectroscopy characterization of carbon blacks: Spectral analysis and structural information. Carbon 2015, 84, 479–490. [Google Scholar] [CrossRef]
- Fairley, N. Casa Software Version 2.3.23 Ltd. 2020. Available online: http://www.casaxps.com (accessed on 1 November 2020).
- Cejka, J. Organized mesoporous alumina: synthesis, structure and potential in catalysis. Appl. Catal. A 2003, 254, 327–338. [Google Scholar] [CrossRef]
- Cava, S.; Tebcherani, S.M.; Souza, I.A.; Pianaro, S.A.; Paskocimas, C.A.; Longo, E.; Valera, J.A. Structural characterization of phase transition of Al2O3 nanopowders obtained by polymeric precursor method. Mater. Chem. Phys. 2007, 103, 394–399. [Google Scholar] [CrossRef]
- Souza Santos, P.; Souza Santos, H.; Toledo, S.P. Standard transition aluminas. Electron microscopy studies. Mater. Res. 2000, 3, 104–114. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Dillon, R.O.; Woolland, J. Use the Raman scattering to investigate disorder and crystallite formation in as-deposited and annealed carbon films. Phys. Rev. B 1984, 29, 3482–3489. [Google Scholar] [CrossRef]
- López-Díaz, D.; López Holgado, M.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Cai, J.; Naraghi, M. Non-intertwined graphitic domains leads to super strong and tough continuous 1D nanostructures. Carbon 2018, 137, 242–251. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, Y.; Duan, X.; Zhou, S.; Niu, Y.; Sun, H.; Zhi, L.; Wang, S. Unzipping carbon nanotubes to nanoribbons for revealing the mechanism of nonradical oxidation by carbocatalysis. Appl. Catal. B Environ. 2020, 276, 119146. [Google Scholar] [CrossRef]
- Yokoyama, K.; Sato, Y.; Yamamoto, M.; Nishida, T.; Itoh, T.; Motomiya, K.; Sato, Y. Functionalization of primary amine groups to single-walled carbon nanotubes by reacting fluorinated SWCNTs with ammonia gas at a low temperature. Carbon 2021, 172, 360–371. [Google Scholar] [CrossRef]
- Jawhari, T.; Roid, A.; Casado, J. Raman spectroscopy characterization of some commercially available carbon black materials. Carbon 1995, 33, 1561–1565. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martínez-Alonso, A.; Tascón, J.M.D. Raman microprobe studies on carbon materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Leiro, J.A.; Heinonen, M.H.; Laiho, T.; Batirev, I.G. Core-level XPS spectra of fullerene, highly oriented pyrolitic graphite, and glassy carbon. J. Electron. Spectros. Relat. Phenom. 2003, 128, 205–213. [Google Scholar] [CrossRef]
- Blume, R.; Rosenthal, D.; Tessonnier, J.-P.; Li, H.; Knop-Gericke, A.; Schlögl, R. Characterizing graphitic carbon with x-ray photoelectron spectroscopy: A step-by-step approach. ChemCatChem 2015, 7, 2871–2881. [Google Scholar] [CrossRef]
- Fujimoto, A.; Yamada, Y.; Koinuma, M.; Sato, S. Origins of sp3C peaks in C1s X-ray photoelectron spectra of carbon materials. Anal. Chem. 2016, 88, 6110–6114. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, A.; Jones, D.; Dell’Elce, S.; Treossi, E.; Liscio, A.; Palermo, V. Accurate chemical analysis of oxygenated graphene-based materials using X-ray photoelectron spectroscopy. Carbon 2019, 143, 268–275. [Google Scholar] [CrossRef]
- Soria-Sánchez, M.; Maroto-Valiente, A.; Álvarez-Rodríguez, J.; Muñoz-Andrés, V.; Rodríguez-Ramos, I.; Guerrero-Ruíz, A. Carbon nanostrutured materials as direct catalysts for phenol oxidation in aqueous phase. Appl. Catal. B Environ. 2011, 104, 101–109. [Google Scholar] [CrossRef]
- Maestro, A.; Bonales, L.J.; Ritacco, H.; Rubio, R.G.; Ortega, F. Effect of the spreading solvent on the three-phase contact angle of microparticles attached at fluid interfaces. Phys. Chem. Chem. Phys. 2010, 12, 14115–14120. [Google Scholar] [CrossRef]
- Maestro, A.; Guzmán, E.; Ortega, F.; Rubio, R.G. Contact angle of micro-and nanoparticles at fluid interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 355–367. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Tran, H.N.; Plamondon, C.O.; Tuduri, L.; Vo, D.-V.N.; Nanda, S.; Mishra, A.; Chao, H.-P.; Bajpai, A.K. Recent progress in the preparation, properties and applications of superhydrophobic nano-based coatings and surfaces: A review. Prog. Org. Coat. 2019, 132, 235–256. [Google Scholar] [CrossRef]
- Celia, E.; Darmanin, T.; de Givenchy, E.T.; Amigoni, S.; Guittard, F. Recent advances in designing superhydrophobic surfaces. J. Colloid Interface Sci. 2013, 402, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhang, W.; Lan, X.; Lin, H. CO2 Reduction to Methanol in the Liquid Phase: A Review. ChemSusChem 2020, 13, 1–20. [Google Scholar] [CrossRef]
- Bonetto, R.; Crisanti, F.; Sartorel, A. Carbon Dioxide Reduction Mediated by Iron Catalysts: Mechanism and Intermediates That Guide Selectivity. ACS Omega 2020, 5, 21309–21319. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.; Jeskey, H.; Baine, A. The Kolbe-Schmitt reaction. I. Variations in the carbonation of p-cresol. J. Org. Chem. 1950, 15, 233–236. [Google Scholar] [CrossRef]
- Cason, J.; Dyke, G.O., Jr. Preparation of 2,3-Dihydroxybenzoic Acid. J. Am. Chem. Soc. 1950, 72, 621–622. [Google Scholar] [CrossRef]
- Luo, J.; Preciado, S.; Xie, P.; Larrosa, I. Carboxylation of phenols with CO2 at atmospheric pressure. Chem. Eur. J. 2016, 22, 6798–6802. [Google Scholar] [CrossRef] [PubMed]
- Hales, J.L.; Idris Jones, J.; Lindsey, A.S. Mechanism of the Kolbe–Schmitt reaction. Part I. Infra-red studies. J. Chem. Soc. 1954, 3145–3151. [Google Scholar] [CrossRef]
- Liu, M.; Yi, Y.; Wang, L.; Guo, H.; Bogaerts, A. Hydrogenation of carbon dioxide to value-added chemicals by heterogeneous catalysis and plasma catalysis. Catalysts 2019, 9, 275. [Google Scholar] [CrossRef]




| Sample | Average Pore Size 1 (nm) | Pore Volume 1 (cm3 g−1) | SBET (cm3 g−1) |
|---|---|---|---|
| γ-Al2O3 | 4.6 | 0.42 | 293 |
| FeAl | 5.0 | 0.40 | 247 |
| FeAlR-723 | 4.8 | 0.38 | 255 |
| FeAlR-823 | 4.7 | 0.40 | 255 |
| FeAlR-923 | 5.0 | 0.39 | 221 |
| FeAlR-1023 | 5.7 | 0.41 | 193 |
| FeAlR-1123 | 7.3 | 0.42 | 155 |
| Sample | SBET (cm3 g−1) | Carbon 1 (wt%) | T1 (K) | Carbon (wt%) | FWHM (K) | T2 (K) | Carbon (wt%) | FWHM (K) |
|---|---|---|---|---|---|---|---|---|
| C@Al-723 | 46 | 22.4 | 692 | 100 | 85.7 | - | - | - |
| C@Al-823 | 66 | 19.4 | 745 | 64.2 | 59.7 | 817 | 35.8 | 60.0 |
| C@Al-923 | 142 | 18.0 | 731 | 64.3 | 29.1 | 811 | 35.7 | 57.8 |
| C@Al-1023 | 145 | 12.6 | 721 | 54.7 | 60.9 | 809 | 45.3 | 56.2 |
| Hybrid Sample | D1 1 | D2 | D3 | D | G 2 | ID3/IG | La 3 nm | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cm−1 | FWHM | cm−1 | FWHM | cm−1 | FWHM | cm−1 | FWHM | cm−1 | FWHM | |||
| C@Al-723 | 1233 | 98 | 1611 | 42 | 1500 | 174 | 1343 | 161 | 1588 | 50 | 0.62 | 5.19 |
| C@Al-823 | 1180 | 89 | 1611 | 40 | 1508 | 159 | 1341 | 161 | 1589 | 66 | 0.38 | 7.24 |
| C@Al-923 | 1197 | 100 | 1611 | 31 | 1508 | 144 | 1338 | 127 | 1594 | 56 | 0.38 | 7.58 |
| C@Al-1023 | 1200 | 112 | 1612 | 21 | 1504 | 142 | 1342 | 130 | 1589 | 62 | 0.28 | 8.11 |
| Hybrid | C1s (wt%) | sp2/sp3 | O/C | ||
|---|---|---|---|---|---|
| Sample | C=C | C–C | C–O | Ratio | Ratio |
| C@Al-723 | 41.40 | 44.53 | 14.07 | 0.99 | 0.15 |
| C@Al-823 | 44.41 | 52.41 | 3.18 | 1.10 | 0.03 |
| C@Al-923 | 68.08 | 26.25 | 5.68 | 2.66 | 0.06 |
| C@Al-1023 | 72.11 | 19.11 | 8.78 | 3.77 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Castañera, F.; Álvarez-Rodríguez, J.; Candela, N.; Maroto-Valiente, Á. First Phenol Carboxylation with CO2 on Carbon Nanostructured C@Fe-Al2O3 Hybrids in Aqueous Media under Mild Conditions. Nanomaterials 2021, 11, 190. https://doi.org/10.3390/nano11010190
Calvo-Castañera F, Álvarez-Rodríguez J, Candela N, Maroto-Valiente Á. First Phenol Carboxylation with CO2 on Carbon Nanostructured C@Fe-Al2O3 Hybrids in Aqueous Media under Mild Conditions. Nanomaterials. 2021; 11(1):190. https://doi.org/10.3390/nano11010190
Chicago/Turabian StyleCalvo-Castañera, Feliciano, Jesús Álvarez-Rodríguez, Nuria Candela, and Ángel Maroto-Valiente. 2021. "First Phenol Carboxylation with CO2 on Carbon Nanostructured C@Fe-Al2O3 Hybrids in Aqueous Media under Mild Conditions" Nanomaterials 11, no. 1: 190. https://doi.org/10.3390/nano11010190
APA StyleCalvo-Castañera, F., Álvarez-Rodríguez, J., Candela, N., & Maroto-Valiente, Á. (2021). First Phenol Carboxylation with CO2 on Carbon Nanostructured C@Fe-Al2O3 Hybrids in Aqueous Media under Mild Conditions. Nanomaterials, 11(1), 190. https://doi.org/10.3390/nano11010190







