The Adsorption of H2 and C2H2 on Ge-Doped and Cr-Doped Graphene Structures: A DFT Study
Abstract
:1. Introduction
2. Computation Methods
3. Results and Discussions
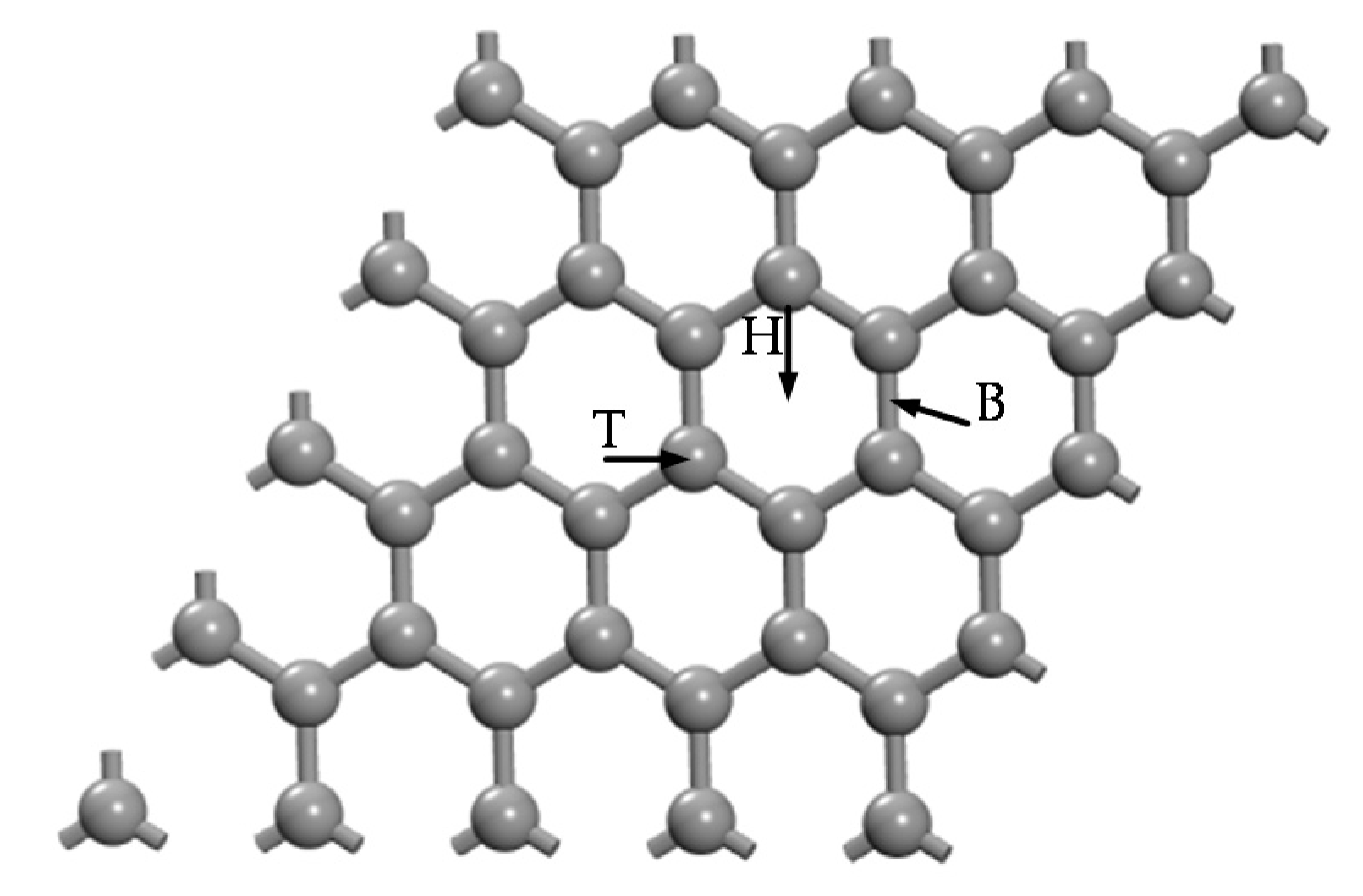
3.1. Structures of H2 and C2H2 Molecules and IG
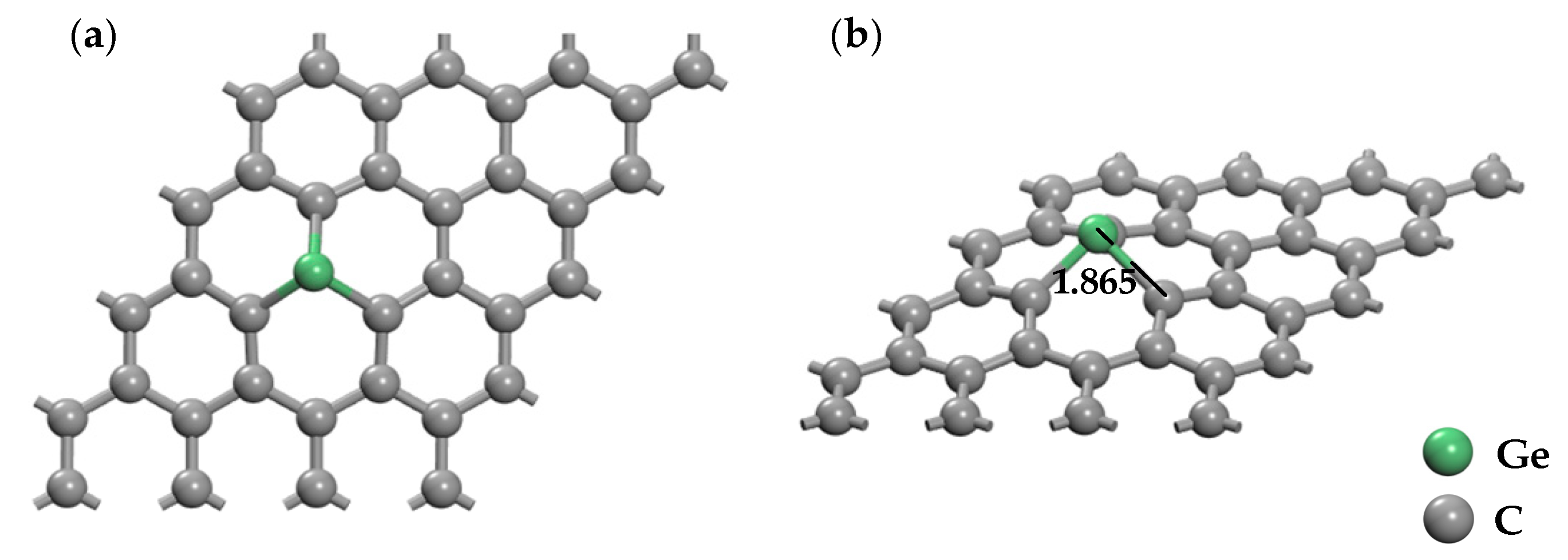
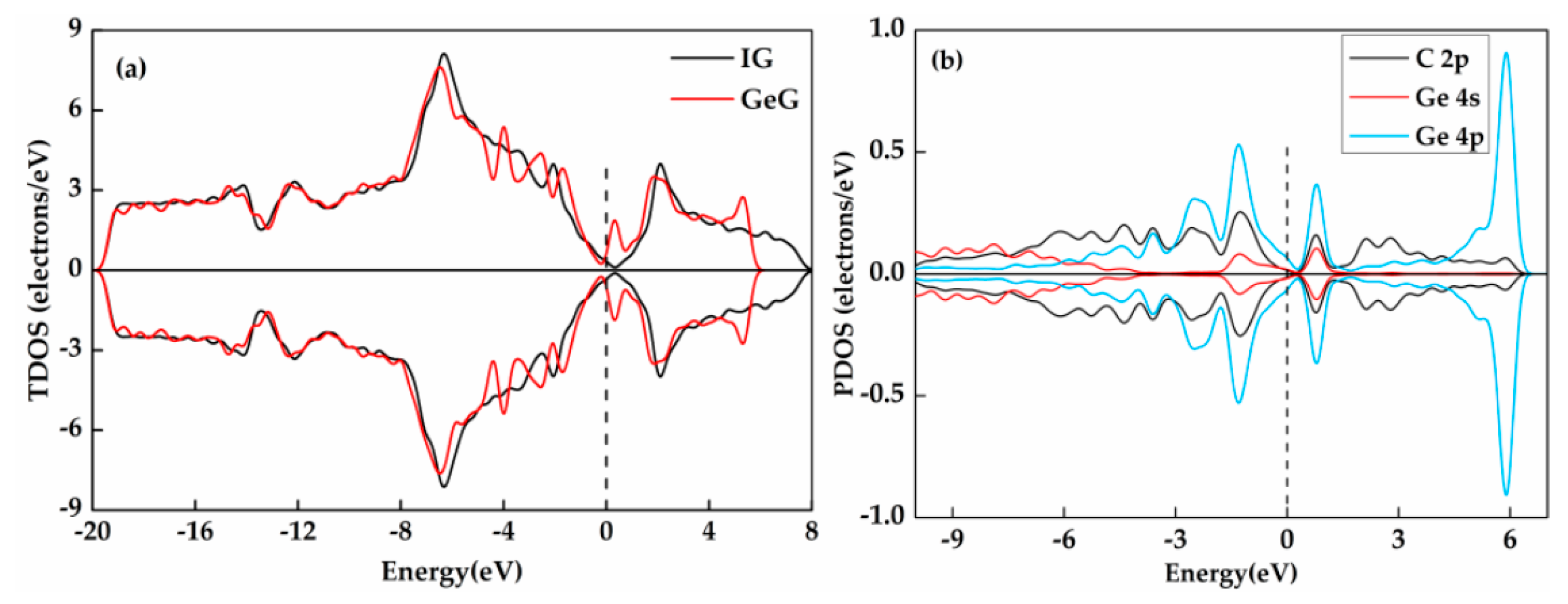
3.2. Ge and Cr Doping on Graphene
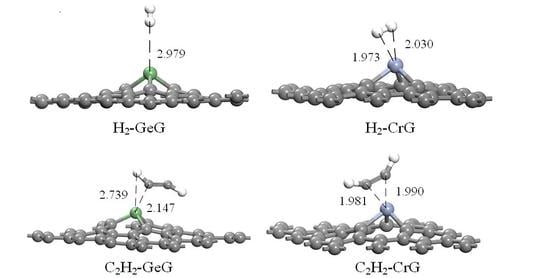
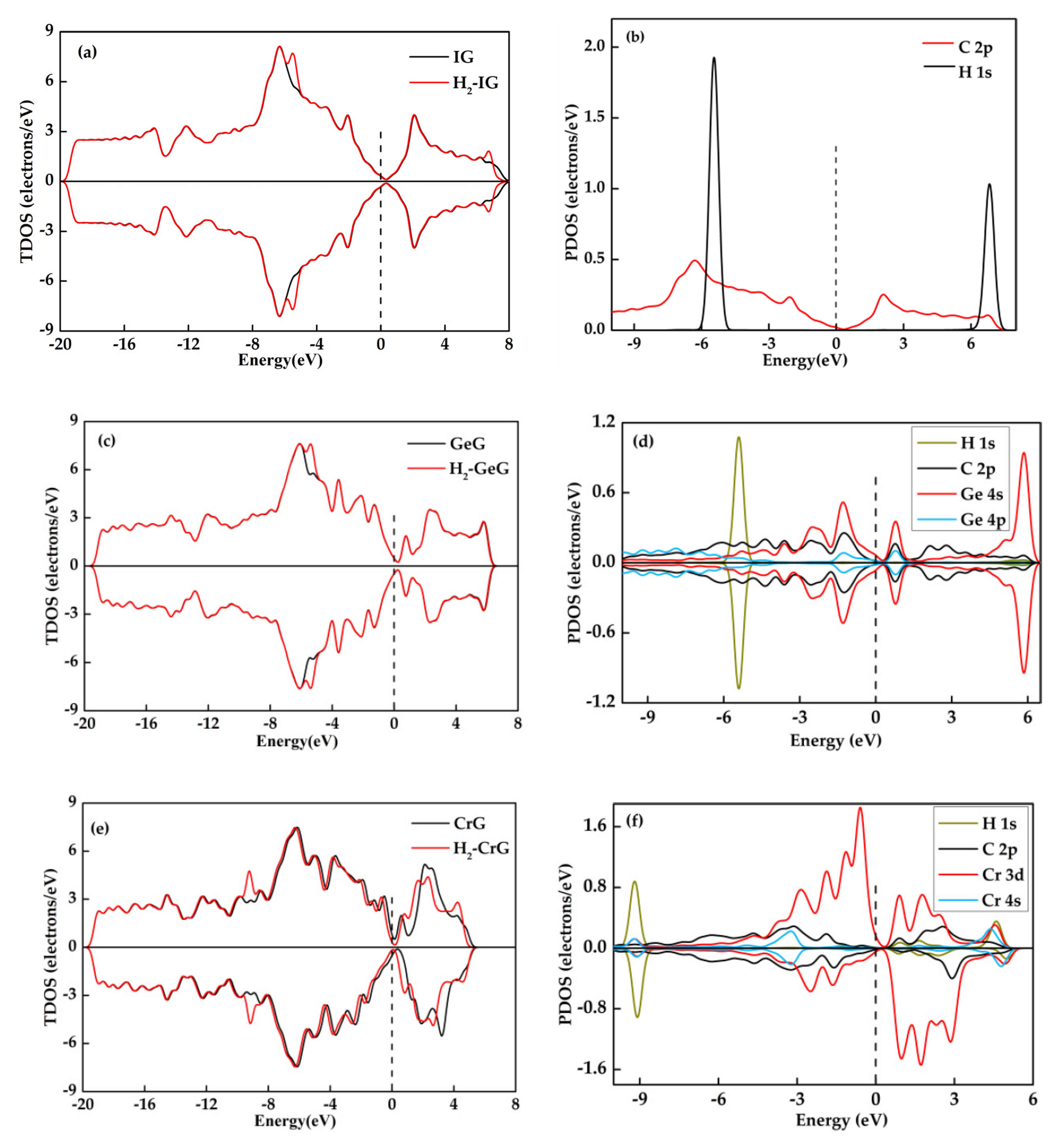
3.3. H2 Adsorbed on IG, GeG, and CrG Systems
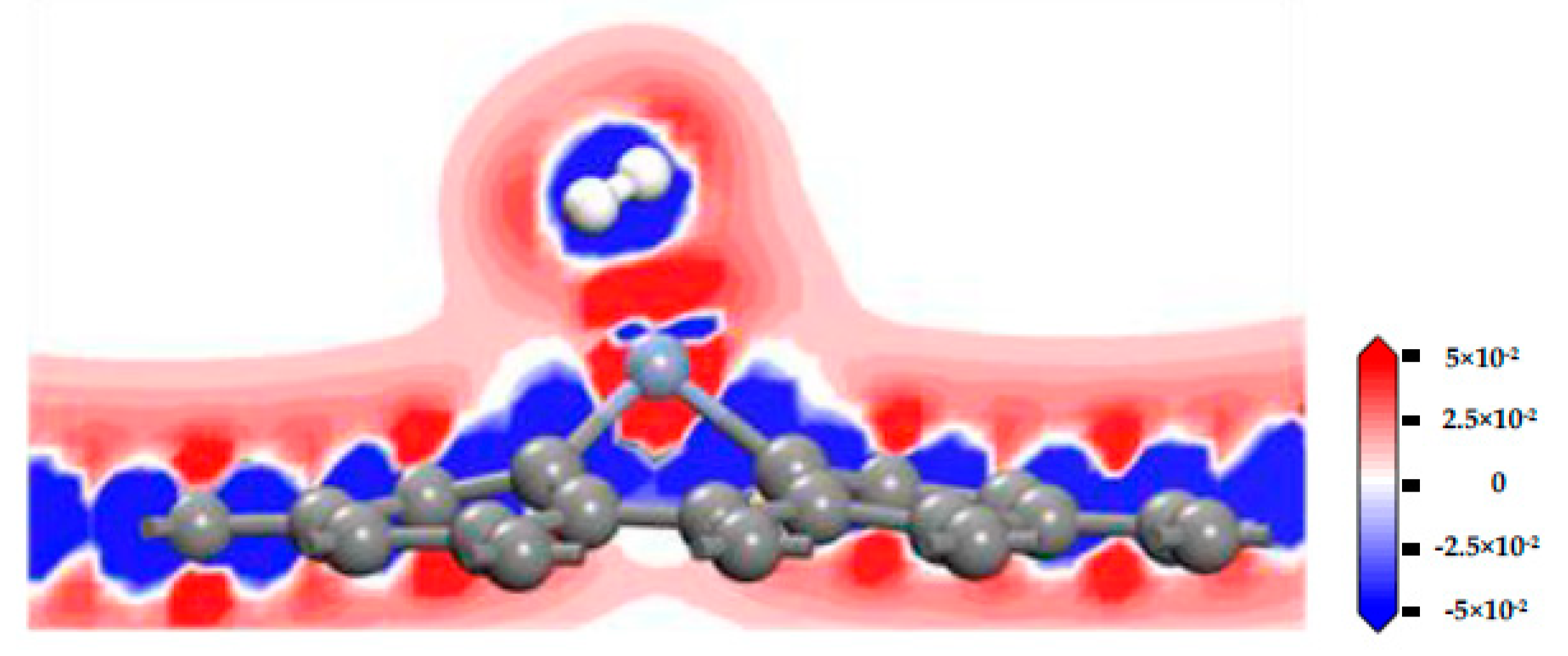
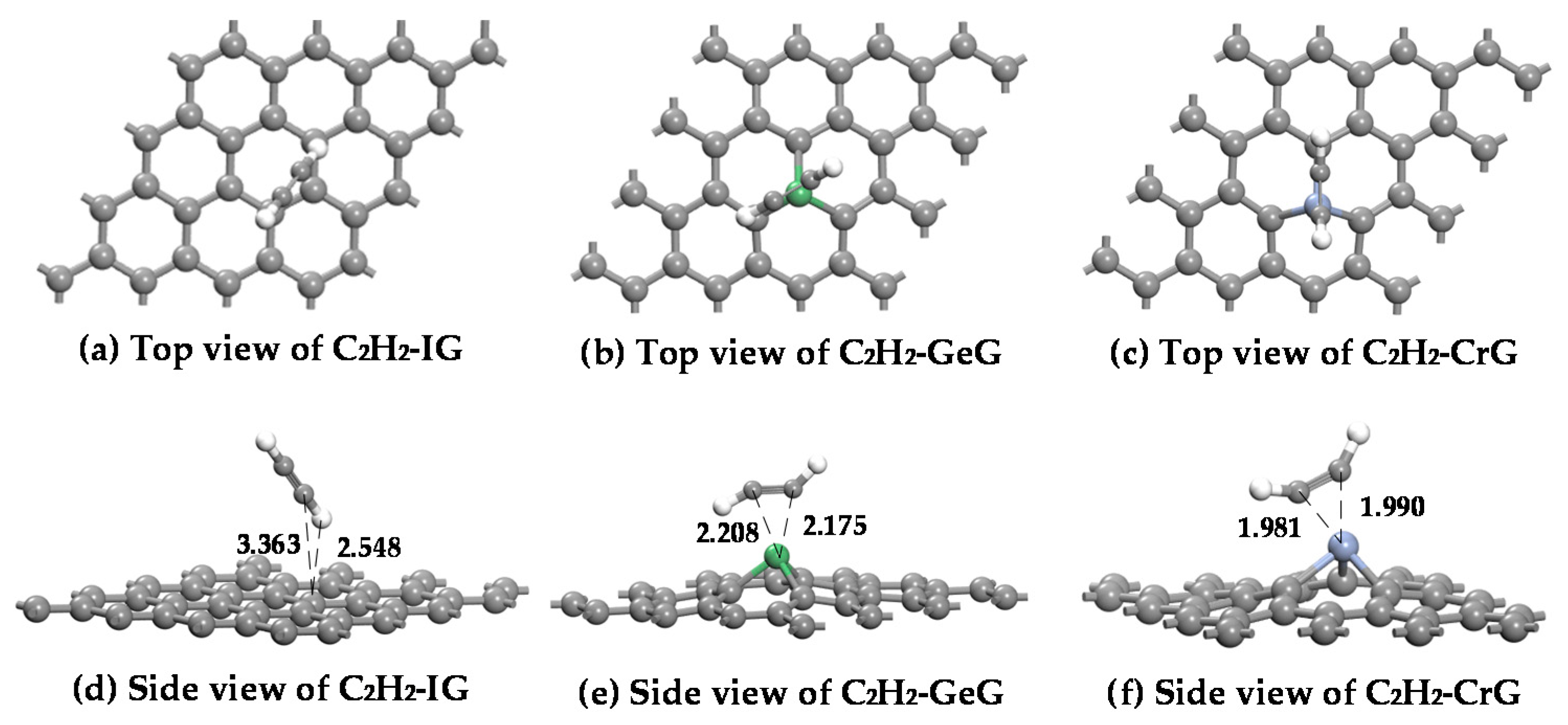
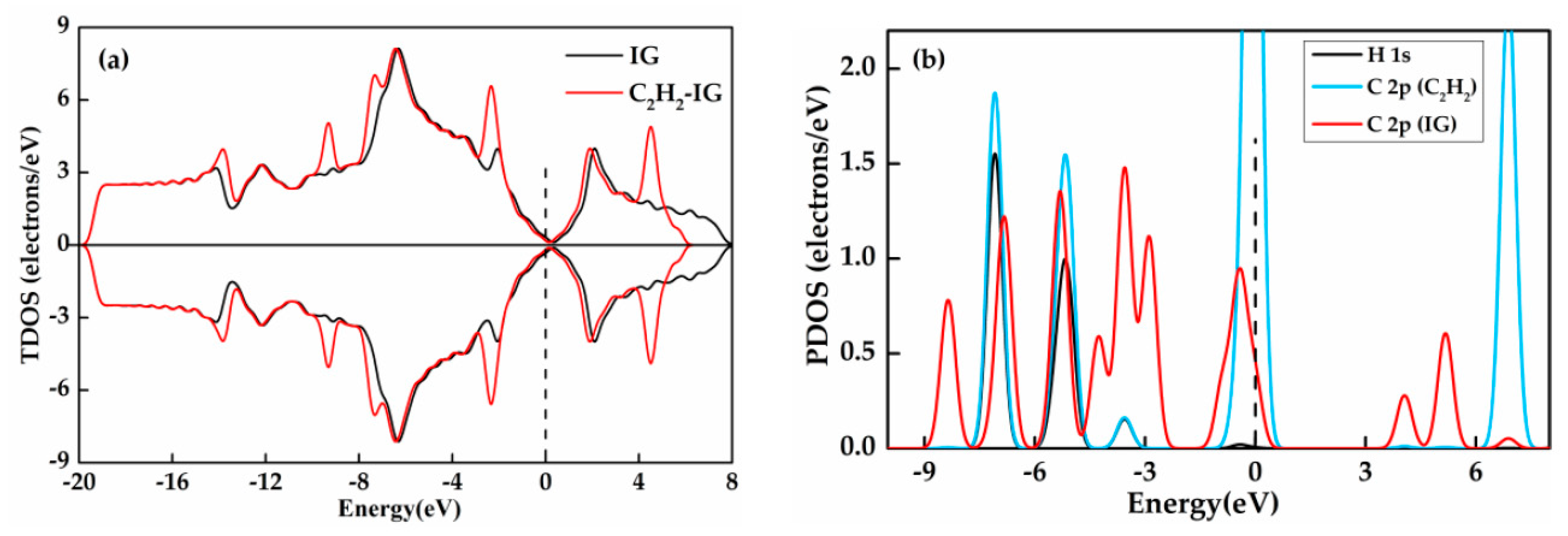
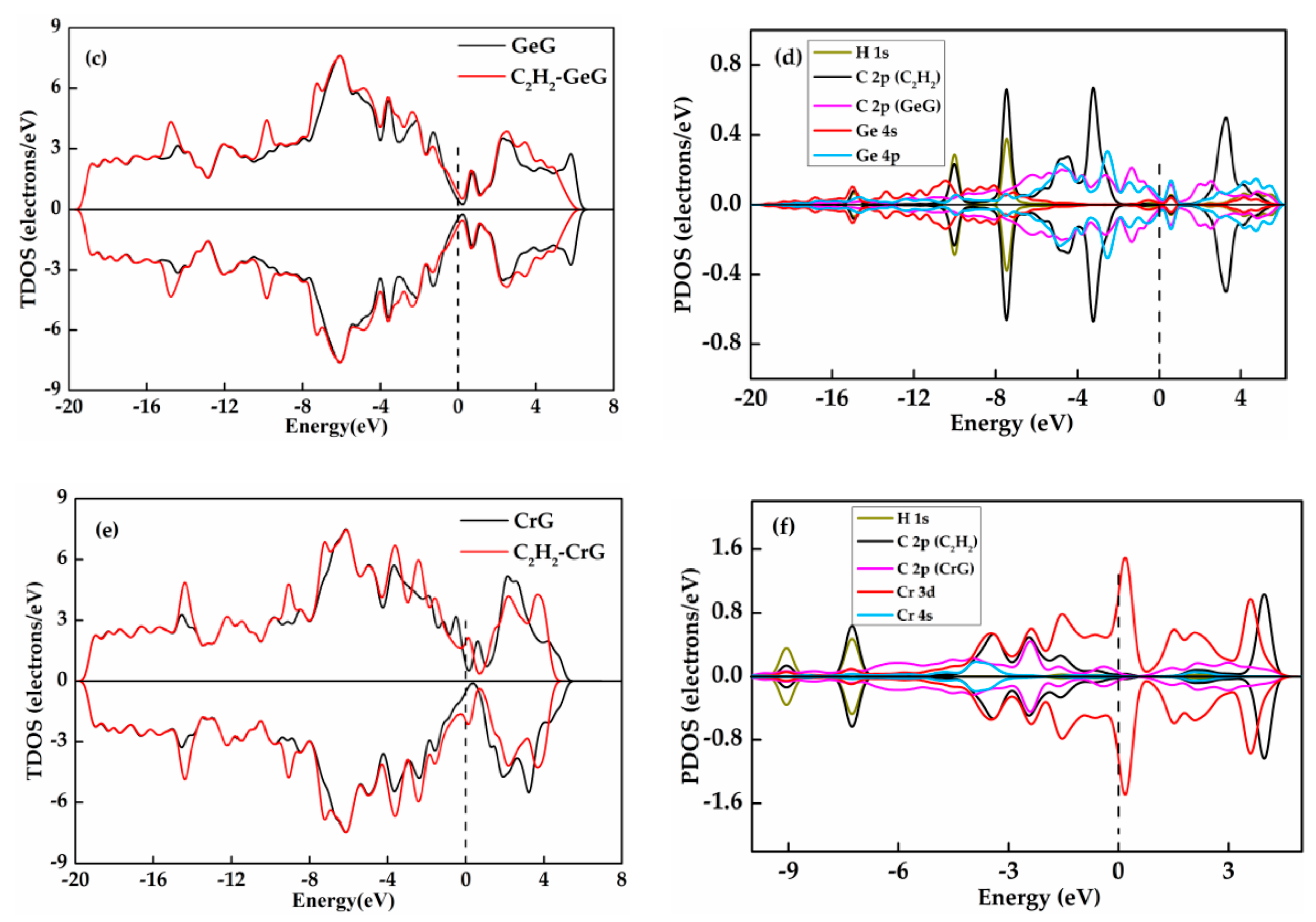
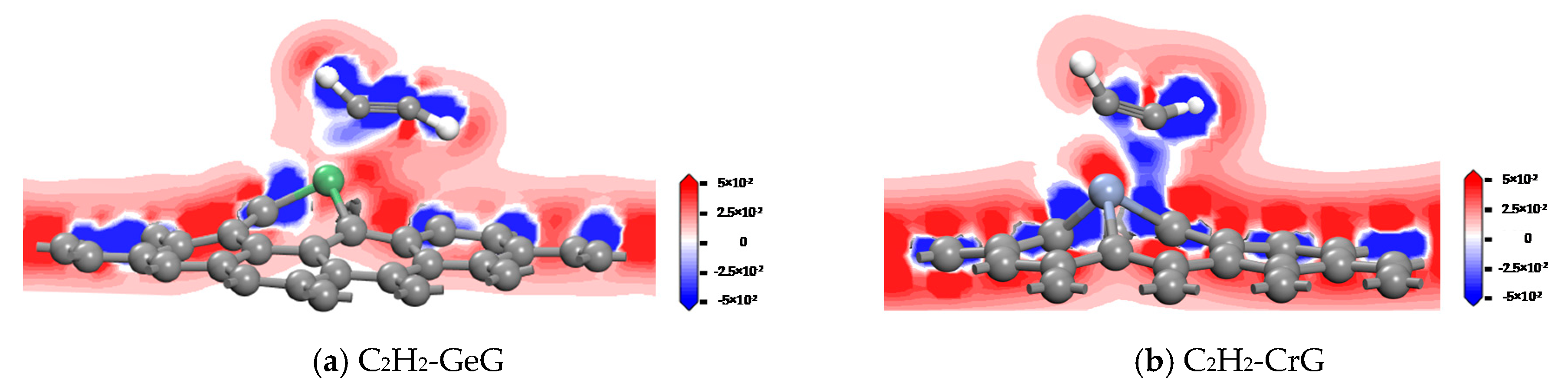
3.4. C2H2 Adsorbed on IG, GeG, and CrG Systems
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tra, V.; Bach, B.P.; Kim, J.M. Improving diagnostic performance of a power transformer using an adaptive over-sampling method for imbalanced Data. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 1325–1333. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, R.Y.; Chen, M.; Wang, W.H.; Zhang, C.H. Dynamic fault prediction of power transformers based on hidden markov model of dissolved gases analysis. IEEE Trans. Power Deliv. 2019, 34, 1393–1400. [Google Scholar] [CrossRef]
- Yan, C.; Li, M.X.; Liu, W. Transformer fault diagnosis based on BP-Adaboost and PNN series connection. Math. Probl. Eng. 2019, 2019, 1019845. [Google Scholar] [CrossRef] [Green Version]
- Suryavanshi, H.; Velandy, J.; Sakthivel, M. Wavelet power ratio signature spectrum analysis for prediction of winding insulation defects in transformer and shunt reactor. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2649–2659. [Google Scholar] [CrossRef]
- Zhou, Q.; Tang, C.; Zhu, S.P.; Chen, W.G.; Li, J. Synthesis, characterisation and sensing properties of Sm2O3 doped SnO2 nanorods to C2H2 gas extracted from power transformer oil. Mater. Technol. 2016, 31, 364–370. [Google Scholar] [CrossRef]
- Zheng, H.B.; Zhang, Y.Y.; Liu, J.F.; Wei, H.; Zhao, J.H.; Liao, R.J. A novel model based on wavelet LS-SVM integrated improved PSO algorithm for forecasting of dissolved gas contents in power transformers. Electr. Power Syst. Res. 2018, 155, 196–205. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, W.G.; Xu, L.N.; Kumarc, R.; Gui, Y.G.; Zhao, Z.Y.; Tang, C.; Zhu, S.P. Highly sensitive carbon monoxide (CO) gas sensors based on Ni and Zn doped SnO2 nanomaterials. Ceram. Int. 2018, 44, 4392–4399. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, X.X.; Wu, X.Q.; Dai, Z.Q.; Zhang, J.B. A Ni-doped carbon nanotube sensor for detecting oil-dissolved gases in transformers. Sensors 2015, 15, 13522–13532. [Google Scholar] [CrossRef] [Green Version]
- Faiz, J.; Soleimani, M. Assessment of computational intelligence and conventional dissolved gas analysis methods for transformer fault diagnosis. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1798–1806. [Google Scholar] [CrossRef]
- Ding, J.F.; Li, X.M.; Jian, C.; Sheng, L.Y.; Yin, L.Z.; Xu, X.M. New sensor for gases dissolved in transformer oil based on solid oxide fuel cell. Sens. Actuators B Chem. 2014, 202, 232–239. [Google Scholar] [CrossRef]
- Guo, M.M.; Cheng, R.L.; Mu, R.D.; Wang, L. High sensitive and reliable fiber Bragg grating hydrogen sensor for fault detection of power transformer. Sens. Actuators. B Chem. 2012, 169, 195–198. [Google Scholar]
- Yang, Z.; Zhou, Q.; Wu, X.D.; Zhao, Z.Y. A novel measuring method of interfacial tension of transformer oil combined PSO optimized SVM and multi frequency ultrasonic technology. IEEE Access 2019, 17, 182624. [Google Scholar] [CrossRef]
- Morais, D.R.; Rolim, J.G. A hybrid tool for detection of incipient faults in transformers based on the dissolved gas analysis of insulating oil. IEEE Trans. Power Deliv. 2006, 21, 673–680. [Google Scholar] [CrossRef]
- Li, Z.N.; Gadipelli, S.; Yang, Y.C.; He, G.J.; Guo, J.; Lia, J.T.; Lu, Y.; Howard, C.A.; Brett, D.J.L.; Parkin, I.P.; et al. Exceptional supercapacitor performance from optimized oxidation of graphene-oxide. Energy Storage Mater. 2018, 17, 12–21. [Google Scholar] [CrossRef]
- Contreras, E.M.C.; Oliveira, G.A.; Filho, E.P.B. Experimental analysis of the therymohydraulic performance of graphene and silver nanofluids in automotive cooling systems. Int. J. Heat Mass Transf. 2019, 132, 375–387. [Google Scholar] [CrossRef]
- Tian, H.L.; Fan, H.Q.; Ma, J.W.; Liu, Z.Y.; Ma, L.T.; Lei, S.H.; Fang, J.W.; Long, C.B. Pt-decorated zinc oxide nanorod arrays with graphitic carbon nitride nanosheets for highly efficient dual-functional gas sensing. J. Hazard Mater. 2018, 341, 102–111. [Google Scholar] [CrossRef]
- Dong, X.C.; Zhang, X.X.; Cui, H.; Zhang, J. A first principle simulation of competitive adsorption of SF6 decomposition components on nitrogen-doped anatase TiO2 (101) surface. Appl. Surf. Sci. 2017, 422, 331–338. [Google Scholar] [CrossRef]
- Diego, C.A.; Nery, V.E.; Daniela, E.O. Fe-doped graphene nanosheet as an adsorption platform of harmful gas molecules (CO, CO2, SO2 and H2S), and the co-adsorption in O2 environments. Appl. Surf. Sci. 2018, 427, 227–236. [Google Scholar]
- Zhang, C.P.; Li, B.; Shao, Z.G. First-principle investigation of CO and CO2 absorption on Fe-doped penta-graphene. Appl. Surf. Sci. 2019, 469, 641–646. [Google Scholar] [CrossRef]
- Yang, B.; Li, D.B.; Qi, L.; Li, T.B.; Yang, P. Thermal properties of triangle nitrogen-doped graphene nanoribbons. Phys. Lett. A 2019, 383, 1–4. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Saeidi, N.; Nematollahi, P. Si-doped graphene: A promising metal-freecatalyst for oxidation of SO2. Chem. Phys. Lett. 2016, 649, 37–43. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Hwang, Y.; Chung, Y.C. Modulating magnetic characteristics of Pt embedded graphene by gas adsorption (N2, O2, NO2, SO2). Appl. Surf. Sci. 2014, 289, 445–449. [Google Scholar] [CrossRef]
- Yang, S.L.; Lei, G.; Xu, H.X.; Xu, B.; Li, H.P.; Lan, Z.G.; Wang, Z. A DFT study of CO adsorption on the pristine, defective, In-doped and Sb-doped graphene and the effect of applied electric field. Appl. Surf. Sci. 2019, 480, 205–211. [Google Scholar] [CrossRef]
- Struzzi, C.; Sezen, H.; Amati, M.; Gregoratti, L.; Reckinger, N.; Colomer, J.F.; Snyders, R.; Bittencourt, C.; Scardamaglia, M. Fluorine and sulfur simultaneously Co-doped suspended graphene. Appl. Surf. Sci. 2017, 422, 104–110. [Google Scholar] [CrossRef]
- Bo, Z.; Guo, X.; Wei, X.; Yang, H.; Yan, J.; Cen, K. Density functional theory calculations of NO2 and H2S adsorption on the group 10 transition metal (Ni, Pd and Pt) decorated grapheme. Physica E 2019, 109, 156–163. [Google Scholar] [CrossRef]
- Zhou, Q.; Umar, A.; Sodki, E.M.; Amine, A.; Xu, L.N.; Gui, Y.G.; Ibrahim, A.A.; Kumar, R.; Baskoutas, S. Fabrication and characterization of highly sensitive and selective sensors based on porous NiO nanodisks. Sens. Actuators B Chem. 2018, 259, 604–615. [Google Scholar] [CrossRef]
- Shukri, M.S.M.; Saimin, M.N.S.; Yaakob, M.K.; Yahya, M.Z.A.; Taib, M.F.M. Structural and electronic properties of CO and NO gas molecules on Pd-doped vacancy graphene: A first principles study. Appl. Surf. Sci. 2019, 494, 817–828. [Google Scholar] [CrossRef]
- Zheng, Z.Q.; Wang, H.L. Different elements doped graphene sensor for CO2 greenhouse gases detection: The DFT study. Chem. Phys. Lett. 2019, 721, 33–37. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Q.; Wang, J.X.; Xu, L.N.; Zeng, W. Adsorption of SO2 molecule on Ni-doped and Pd-doped graphene based on first-principle study. Appl. Surf. Sci. 2020, 517, 146180. [Google Scholar] [CrossRef]
- Gönüllü, Y.; Haidry, A.A.; Saruhan, B. Nanotubular Cr-doped TiO2 for use as high-temperature NO2 gas sensor. Sens. Actuators B Chem. 2015, 271, 78–87. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Nematollahi, P.; Abdollahpour, H. A comparative DFT study on the CO oxidation reaction over Al- and Ge-embedded graphene as efficient metal-free catalysts. Appl. Surf. Sci. 2016, 378, 418–425. [Google Scholar] [CrossRef]
- Roohi, H.; Ardehjani, N.A. Theoretical investigation of nitric oxide adsorption on the surface of pure and metal (Ti, Cr, Fe, Ni and Zn) doped gallium nitride nanosheets. Phys. E 2020, 120, 114075. [Google Scholar] [CrossRef]
- Bunpang, K.; Wisitsoraatc, A.; Tuantranont, A.; Singkammo, S.; Phanichphant, S.; Liewhirana, C. Highly selective and sensitive CH4 gas sensors based on flame-spray-made Cr-doped SnO2 particulate films. Sens. Actuators B Chem. 2019, 291, 177–191. [Google Scholar] [CrossRef]
- Rahman, M.M. Selective and sensitive 4-Aminophenol chemical sensor development based on low-dimensional Ge-doped ZnO nanocomposites by electrochemical method. Microchem. J. 2020, 157, 104945. [Google Scholar] [CrossRef]
- Zhou, Q.X.; Ju, W.W.; Yong, Y.L.; Su, X.Y.; Li, X.H.; Fu, Z.B.; Wang, C.Y. DFT study on the adsorption sensitivity of graphane doped with Cr and Mn toward H2CO molecule. Phys. E 2018, 95, 16–21. [Google Scholar] [CrossRef]
- Wang, Z.L.; Liu, W.J.; Hu, Y.M.; Guan, M.L.; Xu, L.; Li, H.P.; Bao, J.; Li, H.M. Cr-doped CoFe layered double hydroxides: Highly efficient and robust bifunctional electrocatalyst for the oxidation of water and urea. Appl. Catal. B Environ. 2020, 272, 118959. [Google Scholar] [CrossRef]
- Gecim, G.; Ozekmekci, M.; Fellah, M.F. Ga and Ge-doped graphene structures: A DFT study of sensor applications for methanol. Comput. Theor. Chem. 2020, 1180, 112828. [Google Scholar] [CrossRef]
- Wicaksono, Y.; Teranishi, S.; Nishiguchi, K.; Kusakabe, K. Tunable induced magnetic moment and in-plane conductance of graphene in Ni/graphene/Ni nano-spin-valve-like structure: A first principles study. Carbon 2019, 143, 828–836. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Zhou, Q.; Wang, J.X.; Xu, L.N.; Zeng, W. Performance of intrinsic and modified grapheme for the adsorption of H2S and CH4: A DFT study. Nanomaterials 2020, 10, 299. [Google Scholar] [CrossRef] [Green Version]
- Yoosefian, M.; Zahedi, M.; Mola, A.; Naserian, S. A DFT comparative study of single and double SO2 adsorption on Pt-doped and Au-doped single-walled carbon nanotube. Appl. Surf. Sci. 2015, 349, 864–869. [Google Scholar] [CrossRef]
- Pakornchote, T.; Ektarawong, A.; Alling, B.; Pinsook, U.; Tancharakorn, S.; Busayaporn, W.; Bovornratanaraks, T. Phase stabilities and vibrational analysis of hydrogenated diamondized bilayer graphenes: A first principles investigation. Carbon 2019, 146, 468–475. [Google Scholar] [CrossRef]
- Mashhadzadeh, A.H.; Ahangari, M.G.; Dadrasi, A.; Fathalian, M. Theoretical studies on the mechanical and electronic properties of 2D and 3D structures of Beryllium-Oxide graphene and graphene nanobud. Appl. Surf. Sci. 2019, 476, 36–48. [Google Scholar] [CrossRef]
- Wang, J.X.; Zhou, Q.; Lu, Z.R.; Wei, Z.J.; Zeng, W. Gas sensing performances and mechanism at atomic level of Au-MoS2 microspheres. Appl. Surf. Sci. 2019, 490, 124–136. [Google Scholar] [CrossRef]
- Rafiee, M.; Nitzsche, F.; Laliberte, J.; Hind, S.; Robitaille, F.; Labrosse, M.R. Thermal properties of doubly reinforced fiberglass/epoxy composites with graphene nanoplatelets, graphene oxide and reduced-graphene oxide. Compos. Part. B Eng. 2019, 164, 1–9. [Google Scholar] [CrossRef]
- Ni, J.; Quintana, M.; Song, S. Adsorption of small gas molecules on transition metal (Fe, Ni and Co, Cu) doped graphene: A systematic DFT study. Physica E 2020, 116, 113768. [Google Scholar] [CrossRef]
- Aghaei, S.M.; Monshi, M.M.; Torres, I.; Zeidi, S.M.J.; Calizo, I. DFT study of adsorption behavior of NO, CO, NO2, and NH3 molecules on graphene-like BC3: A search for highly sensitive molecular sensor. Appl. Surf. Sci. 2018, 427, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.X.; Ju, W.W.; Sua, X.Y.; Yong, Y.L.; Li, X.H. Adsorption behavior of SO2 on vacancy-defected graphene: A DFT study. J. Phys. Chem. Solids 2017, 109, 40–45. [Google Scholar] [CrossRef]
- Li, B.S.; Yong, P.W.; Hai, K.D. First-principle study of structural, electronic, vibrational and magnetic properties of HCN adsorbed graphene doped with Cr, Mn and Fe. Appl. Surf. Sci. 2015, 329, 330–336. [Google Scholar]
- Tabari, L.; Farmanzadeh, D. Yttrium doped graphene oxide as a new adsorbent for H2O, CO, and ethylene molecules: Dispersion-corrected DFT calculations. Appl. Surf. Sci. 2020, 500, 144029. [Google Scholar] [CrossRef]
- Cui, H.; Chen, D.C.; Zhang, Y.; Zhang, X.X. Dissolved gas analysis in transformer oil using Pd catalyst decorated MoSe2 monolayer: A first-principles theory. Sustain. Mater. Technol. 2019, 20, e00094. [Google Scholar] [CrossRef]




| Site | Mulliken Charge (e) | ||
|---|---|---|---|
| GT | 1.865 | 4.725 | −0.525 |
| GH | 2.119 | 2.301 | 0.135 |
| GB | 2.279 | 1.095 | 0.032 |
| Site | Mulliken Charge (e) | ||
|---|---|---|---|
| GT | 1.856 | 4.732 | −0.252 |
| GH | 2.111 | 2.031 | 0.211 |
| GB | 2.442 | 1.386 | 0.236 |
| System | (Å) | Mulliken Charge (e) | ||
|---|---|---|---|---|
| IG | H-C 3.026 | −0.153 | −0.012 | C −0.014 H −0.006 H −0.006 |
| GeG | Ge-H 2.908 | −0.117 | −0.010 | Ge −0.529 H 0.005 H −0.015 |
| CrG | Cr-H1 2.030 Cr-H2 1.973 | −0.390 | 0.052 | Cr −0.317 H 0.107 H −0.055 |
| System | (Å) | Mulliken Charge (e) | ||
|---|---|---|---|---|
| IG | H-C 2.548 | −0.066 | −0.008 | CIG 0.024 C −0.092 C −0.092 H 0.096 H 0.096 |
| GeG | Ge-C 2.175 Ge-C 2.208 | −0.287 | 0.063 | Ge −0.463 C −0.198 C −0.052 H 0.165 H 0.148 |
| CrG | Cr-C1 1.990 Cr-C2 1.981 | −1.436 | 0.079 | Cr −0.317 C −0.137 C 0.018 H 0.095 H 0.103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.; Peng, R.; Peng, S.; Zeng, W.; Zhou, Q. The Adsorption of H2 and C2H2 on Ge-Doped and Cr-Doped Graphene Structures: A DFT Study. Nanomaterials 2021, 11, 231. https://doi.org/10.3390/nano11010231
Liao Y, Peng R, Peng S, Zeng W, Zhou Q. The Adsorption of H2 and C2H2 on Ge-Doped and Cr-Doped Graphene Structures: A DFT Study. Nanomaterials. 2021; 11(1):231. https://doi.org/10.3390/nano11010231
Chicago/Turabian StyleLiao, Yiming, Ruochen Peng, Shudi Peng, Wen Zeng, and Qu Zhou. 2021. "The Adsorption of H2 and C2H2 on Ge-Doped and Cr-Doped Graphene Structures: A DFT Study" Nanomaterials 11, no. 1: 231. https://doi.org/10.3390/nano11010231
APA StyleLiao, Y., Peng, R., Peng, S., Zeng, W., & Zhou, Q. (2021). The Adsorption of H2 and C2H2 on Ge-Doped and Cr-Doped Graphene Structures: A DFT Study. Nanomaterials, 11(1), 231. https://doi.org/10.3390/nano11010231