Electrochemical Sensors Based on Conducting Polymers for the Aqueous Detection of Biologically Relevant Molecules
Abstract
:1. Introduction
2. Fundamentals of the Electrochemical Sensors
3. Electrochemical Sensors Based on Conducting Polymers
3.1. Neurotransmitters
3.1.1. Dopamine (DA)
3.1.2. Epinephrine (EP)
3.1.3. Serotonin (SER)
3.2. Uric Acid (UA)
3.3. Ascorbic Acid (AA)
3.4. Glucose
3.4.1. Enzymatic Sensors
3.4.2. Non-Enzymatic Sensors
3.5. Hydrogen Peroxide
3.5.1. Enzymatic Sensors
3.5.2. Non-Enzymatic Sensors
3.6. Diverse Pharmaceuticals
3.6.1. Metronidazole (MNZ)
3.6.2. 2-Amino-9-[(2-Hydroxyethoxy) Methyl]-6,9-Dihydro-3H-Purin-6-One (Acyclovir)
3.6.3. Ciprofloxacin (CFX)
3.6.4. 17-β-Estradiol (E2)
3.6.5. Paracetamol (PR)
3.6.6. Other Drugs
3.7. Hydrazine
3.8. Nitrites
3.9. Phenolic Compounds
3.10. Nitroaromatic Compounds
4. Conclusions
5. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1:5-DAN | 1,5-Diaminonaphthalene |
| 35DT | 3,5-Diamino-1,2,4-Triazole |
| 3-TBA | 3-Thiophene boronic acid |
| AA | Ascorbic acid |
| AcA | Acrylic Acid |
| AdSDPV | Adsorptive differential pulse stripping voltammetry |
| afGQDs | Amino-functionalized graphene quantum dots |
| AFM | Atomic force microscopy |
| AGCE | Anodized glassy carbon electrode |
| AgNCs | Silver nanocrystals |
| AHMP | Poly-4-Amino-6-hydroxy-2-mercaptopyrimidine |
| AMTEOS | anilinomethyltriethoxysilane |
| APS | Ammonium persulphate |
| APTMS | 3-aminopropyltriethoxysilane |
| ASA | Acetylsalicylic Acid |
| Ath | 3-Aminothiophene |
| AuNPs | Gold Nanoparticles |
| BDD | Boron-Doped Diamond |
| BSA | Bovine serum albumin |
| C# | Carbon-coated mesoporous |
| CA | Chronoamperometry |
| CC | Catechol |
| CD | Cyclic dextrin |
| CE | Counter electrode |
| CFX | Ciprofloxacin |
| CNT | Carbon nanotubes |
| CoNS | Cobalt Nanostructures |
| CPs | Conducting polymers |
| CPE | Carbon paste electrode |
| CQDs | Carbon Quantum Dots |
| CS | Chitosan |
| CV | Cyclic voltammetry |
| DA | Dopamine |
| DMF | N,N-Dimethylmethanamide |
| DNT | 2,4-Dinitrotoluene |
| DPASV | Differential Pulse Anodic Striping Voltammetry |
| DPV | Differential pulse voltammetry |
| E | Voltage |
| E2 | 17-β-Estradiol |
| EB | Electron Beam |
| EBT | Eriochrome black T |
| EDOT | (3,4-ethylenedioxythiophene) |
| EGDE | Ethylene Glycol Diglycidyl Ether |
| ENPPy | Nano Poly (Pyrrole) |
| EP | Epinephrine |
| ERGO | Electrochemical reduced graphene oxide |
| FA | Poly-fuchsine acid |
| FESEM | Field emission scanning electron microscopy |
| f-MWCNTs | Functionalized multi-walled carbon nanotubes |
| f-SWCNTs | Functionalized Single-Walled Carbon Nanotubes |
| FTD | Furaltadone |
| FTIR | Fourier-transformed infrared spectroscopy |
| FTO | Fluorine Tin Oxide |
| FZD | Furazolidone |
| GCE | Glassy Carbon Electrode |
| GO | Graphene oxide |
| GOx | Glucose Oxidase |
| GP | Graphene |
| GS | graphene carbon spheres |
| HQ | Hydroquinone |
| HRP | Horseradish Peroxidase |
| HXA | Hypoxanthine |
| i | Current |
| IL | Ionic liquid |
| ITO | Indium thin oxide |
| IUPAC | International Union of Pure and Applied chemistry |
| LOD | Limit of detection |
| LSG | Laser scribed graphene |
| LSV | Linear swipe voltammetry |
| MIP | Molecular Imprinted Polymer |
| MNZ | Metronidazole |
| MOF | Metal- organic framework |
| MPrPt | Mesoporous Platinum |
| MS | Mass spectroscopy |
| MWCNT | Multi Wallet carbon nanotubes |
| Nf | Nano fiber |
| NFT | Nitrofurantoin |
| NFZ | Nitrofurazone |
| NG B | Naphthol Green B |
| nHAp | Nano-sized Hydroxyapatite |
| NP | Nitrophenol |
| NPs | Nanoparticles |
| OPPy | Overoxidized electropolymerized polypyrrole |
| p(P3CA) | Poly (pyrrole-3-carboxylic acid) |
| P3-TBA | Poly 3-Thiophene boronic acid |
| P6-TG | Poly(6-thioguanine) |
| p-ABSA | p-Aminobenzene Sulfonic Acid |
| p-AHNSA | Poly 4-amino-3-hydroxy-1-naphthalenesulfonic acid |
| PAMT | Poly (2-amino-5-mercapto-1, 3, 4-thiadiazole) |
| PANI | Polyaniline |
| PANI-co-PoAN | Poly (aniline-co-o-anisidine) |
| PAPBA | Poly (3-aminophenylboronic acid) |
| PAR | Poly (Alizarin Red) |
| PBCB | Poly (brilliant cresyl blue) |
| PBS | Phosphate buffer solution |
| PCC | Poly-catechol |
| PDA | Phenylenediamine |
| PdNPs | Palladium Nanoparticles |
| PDNs | Polydopamine nanospheres |
| PEB | Poly (Evans Blue) |
| pEBT | Poly (eriochrome black T) |
| PEDOT | Poly (3,4-ethylenedioxythiophene) |
| PEDOT:PSS | Poly (3,4-Ethylenedioxythiophene):Polystyrene Sulfonate |
| PEDOTM | Poly (Hydroxymethylated-3,4-Ethylenedioxythiophene) |
| PEDOT-SH | Poly (Thiomethyl 3,4-Ethylenedioxythiophene) |
| PGBHA | Poly (glyoxal-bis(2-hydro- xyanil) |
| PGE | Pencil Graphite Electrode |
| pHQ | Poly (hydroquinone) |
| PMB | Poly (Methylene Blue) |
| PME | Poly (Melamine) |
| PNEDA | Poly (N-(Naphthyl) ethylenediamine dihydrochloride) nanofibers |
| Pol | 4,7-bis(5-(3,4- Ethylenedioxythiophene)thiophen-2-yl)Benzothiadiazole |
| poly 2AB | Poly (2-aminophenylbenzimidazole) |
| Poly(BCG) | Poly (bromocresol green) |
| poly(p-ABSA) | Poly (p-amino benzene sulfonic acid) |
| Poly(TB) | Polytoluidine blue |
| poly-TrB | Poly-Trypan Blue |
| POMA | Poly (o-methoxyaniline) |
| PPR | Poly (Phenol Red) |
| p-ProH | Poly (procaterol hydrochloride) |
| PPy | Polypirrrole |
| PPy3C | Poly (Pyrrole-3-Carboxylic Acid) |
| PR | Paracetamol |
| PS | Polysudan III |
| PSA | Poly (sulfosalicylic acid) |
| PTH | Polythionine |
| p-TPP | Polytetraphenylporphyrin |
| pTSA | p-toluene sulphonic acids |
| PVP | Polyvinylpyrrolidone |
| RSD | Relative standard deviation |
| RC | Resorcinol |
| RE | Reference electrode |
| rGO | Reduced Graphene Oxide |
| SBP | Soybean Seed Coat Peroxidase |
| SDS | Sodium Dodecyl Sulphate |
| SEM | Scanning electron microscopy |
| SER | Serotonin |
| SPCs | Screen printed carbon sensor |
| SWV | Square Wave Voltammetry |
| SβCD | Sulfonated β-cyclodextrin |
| TBA-TFB | tetrabutylammonium tetra- fluoroborate |
| TEM | Transmission electron microscopy |
| TEOS | tetraethyl orthosilicate |
| Tetryl | 2,4,6-Trinitrophenylmethylnitramine |
| TNT | 2,4,6-Trinitrotoluene |
| UA | Uric acid |
| WAXD | Wide angle X-Ray diffraction |
| WE | Working electrode |
| XA | Xanthine |
| ZNRs | Zinc Nano rods |
| ZNTs | Zinc nanotubes |
| γ-PGA | Poly (γ-Glutamic Acid) |
References
- Sinha, K.; Das Mukhopadhyay, C. Quantitative detection of neurotransmitter using aptamer: From diagnosis to therapeutics. J. Biosci. 2020, 45, 1–12. [Google Scholar] [CrossRef]
- Si, B.; Song, E. Recent Advances in the Detection of Neurotransmitters. Chemosensors 2018, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Si, B.; Song, E. Molecularly imprinted polymers for the selective detection of multi-analyte neurotransmitters. Microelectron. Eng. 2018, 187–188, 58–65. [Google Scholar] [CrossRef]
- Komoto, Y.; Ohshiro, T.; Yoshida, T.; Tarusawa, E.; Yagi, T.; Washio, T.; Taniguchi, M. Time-resolved neurotransmitter detection in mouse brain tissue using an artificial intelligence-nanogap. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Dai, X.; Fang, X.; Zhang, C.; Xu, R.; Xu, B. Determination of serum uric acid using high-performance liquid chromatography (HPLC)/isotope dilution mass spectrometry (ID-MS) as a candidate reference method. J. Chromatogr. B 2007, 857, 287–295. [Google Scholar] [CrossRef]
- Wang, J.; Chang, Y.; Wu, W.B.; Zhang, P.; Lie, S.Q.; Huang, C. Label-free and selective sensing of uric acid with gold nanoclusters as optical probe. Talanta 2016, 152, 314–320. [Google Scholar] [CrossRef]
- Badihi-Mossberg, M.; Buchner, V.; Rishpon, J. Electrochemical Biosensors for Pollutants in the Environment. Electroanalysis 2007, 19, 2015–2028. [Google Scholar] [CrossRef]
- Xie, Z.; Ebinghaus, R. Analytical methods for the determination of emerging organic contaminants in the atmosphere. Anal. Chim. Acta 2008, 610, 156–178. [Google Scholar] [CrossRef]
- Honeychurch, K.C.; Hart, J.P. Screen-printed electrochemical sensors for monitoring metal pollutants. TrAC Trends Anal. Chem. 2003, 22, 456–469. [Google Scholar] [CrossRef]
- Dirtu, A.C.; Eede, N.V.D.; Malarvannan, G.; Ionas, A.C.; Covaci, A. Analytical methods for selected emerging contaminants in human matrices—A review. Anal. Bioanal. Chem. 2012, 404, 2555–2581. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Balamurugan, A.; Lai, Y.-H.; Ho, K.-C. A novel poly(3,4-ethylenedioxythiophene)/iron phthalocyanine/multi-wall carbon nanotubes nanocomposite with high electrocatalytic activity for nitrite oxidation. Talanta 2010, 82, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Yanagida, Y.; Hatsuzawa, T. Quantitative determination of hydrogen peroxide using polymer coated Ag nanoparticles. Measurement 2008, 41, 1045–1053. [Google Scholar] [CrossRef]
- Tohidinia, M.; Farsadrooh, M.; Bahmanzadeh, S.; Sabbaghi, N.; Noroozifar, M. Poly(quercetin)-bismuth nanowires as a new modifier for simultaneous voltammetric determination of dihydroxybenzene isomers and nitrite. RSC Adv. 2018, 8, 1237–1245. [Google Scholar] [CrossRef] [Green Version]
- Kou, L.-J.; Liang, R.-N.; Wang, X.; Chen, Y.; Qin, W. Potentiometric sensor for determination of neutral bisphenol A using a molecularly imprinted polymer as a receptor. Anal. Bioanal. Chem. 2013, 405, 4931–4936. [Google Scholar] [CrossRef]
- Goodson, K.L.; Pitt, R.; Subramaniam, S.; Clark, S. The Effect of Increased Flows on the Treatability of Emerging Contaminants at a Wastewater Treatment Plant during Rain Events. Proc. Water Environ. Fed. 2012, 2012, 7224–7237. [Google Scholar] [CrossRef]
- Arciuli, M.; Palazzo, G.; Gallone, A.; Mallardi, A. Bioactive paper platform for colorimetric phenols detection. Sens. Actuators B Chem. 2013, 186, 557–562. [Google Scholar] [CrossRef]
- Baek, S.; Lee, Y.; Son, Y. Amperometric Phenol Sensors Employing Conducting Polymer Microtubule Structure. Mol. Cryst. Liq. Cryst. 2010, 519, 69–76. [Google Scholar] [CrossRef]
- Prehn, R.; Gonzalo-Ruiz, J.; Cortina-Puig, M. Electrochemical Detection of Polyphenolic Compounds in Foods and Beverages. Curr. Anal. Chem. 2012, 8, 472–484. [Google Scholar] [CrossRef]
- El-Kosasy, A.M.; Riad, S.M.; El-Fattah, L.E.A.; Ahmad, S.A.E.-K. Novel poly (vinyl chloride) matrix membrane electrodes for the determination of phenolic pollutants in waste water. Water Res. 2003, 37, 1769–1775. [Google Scholar] [CrossRef]
- Yang, C.; Xu, J.; Hu, S. Development of a novel nitrite amperometric sensor based on poly (toluidine blue) film electrode. J. Solid State Electrochem. 2006, 11, 514–520. [Google Scholar] [CrossRef]
- Qu, J.; Dong, Y.; Wang, Y.; Lou, T.; Du, X.; Qu, J. Novel Nanofilm Sensor Based on Poly-(Alizarin Red)/Fe3O4 Magnetic Nanoparticles-Multiwalled Carbon Nanotubes Composite Material for Determination of Nitrite. J. Nanosci. Nanotechnol. 2016, 16, 2731–2736. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, P.-H.; Cai, H.-H.; Cai, J. Constructions of polyaniline nanofiber-based electrochemical sensor for specific detection of nitrite and sensitive monitoring of ascorbic acid scavenging nitrite. Synth. Met. 2012, 162, 326–331. [Google Scholar] [CrossRef]
- Dong, X.M.; Cui, Y.Z.; Li, T.D. Synthesis of Carbazole Modified Glass Plate for Detecting Nitroaromatic Compounds. Adv. Mater. Res. 2012, 557, 1074–1077. [Google Scholar] [CrossRef]
- Green, L.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Bernal, J.; Del Nozal, M.J.; Martin, M.; Jiménez, J. Possibilities of gas chromatography-atomic emission detection in pesticide multiresidue analysis Application to herbicide analysis in soils. J. Chromatogr. A 1996, 754, 245–256. [Google Scholar] [CrossRef]
- Jenkins, A.L.; Yin, R.; Jensen, J.L. Molecularly imprinted polymer sensors for pesticide and insecticide detection in water. Anal. Camb. UK 2001, 126, 798–802. [Google Scholar] [CrossRef]
- Badea, M.; Amine, A.; Palleschi, G.; Moscone, D.; Volpe, G.; Curulli, A. New electrochemical sensors for detection of nitrites and nitrates. J. Electroanal. Chem. 2001, 509, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Toal, S.J.; Trogler, W.C. Polymer sensors for nitroaromatic explosives detection. J. Mater. Chem. 2006, 16, 2871–2883. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Bonetto, M.C.; Muñoz, F.F.; Diz, V.E.; Sacco, N.J.; Cortón, E. Fused and unzipped carbon nanotubes, electrochemically treated, for selective determination of dopamine and serotonin. Electrochim. Acta 2018, 283, 338–348. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Li, M.; Zhu, Z. A novel electrochemical sensor based on carbon nanotubes array for selective detection of dopamine or uric acid. Talanta 2019, 201, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.; Dorrajia, P.S.; Ehsani, A. Recent Progress in the Development of Conducting Polymer-Based Nanocomposites for Electrochemical Biosensors Applications: A Mini-Review. Chem. Rec. 2018, 18, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Song, N.-N.; Wang, Y.-Z.; Yang, X.-Y.; Zong, H.-L.; Chen, Y.-X.; Ma, Z.; Chen, C.-X. A novel electrochemical biosensor for the determination of dopamine and ascorbic acid based on graphene oxide /poly(aniline-co-thionine) nanocomposite. J. Electroanal. Chem. 2020, 873, 114352. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Fujii, N.; Wang, A. π-Conjugated polymer with Alloxazine-6,9-diyl unit in the Main chain: Synthesis, chemical properties, and sensing ability for metal ions and nucleosides. React. Funct. Polym. 2020, 155, 104691. [Google Scholar] [CrossRef]
- Samanta, S.; Roy, P.; Kar, P. Sensing of ethanol and other alcohol contaminated ethanol by conducting functional poly(o-phenylenediamine). Mater. Sci. Eng. B 2020, 256, 114541. [Google Scholar] [CrossRef]
- Moon, J.-M.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shim, Y.-B. Conducting polymer-based electrochemical biosensors for neurotransmitters: A review. Biosens. Bioelectron. 2018, 102, 540–552. [Google Scholar] [CrossRef]
- Guinovart, T.; Parrilla, M.; Crespo, G.A.; Rius, F.X.; Andrade, F.J. Potentiometric sensors using cotton yarns, carbon nanotubes and polymeric membranes. Analyst 2013, 138, 5208–5215. [Google Scholar] [CrossRef]
- Coyle, S.; Curto, V.F.; Benito-Lopez, F.; Florea, L.; Diamond, D. Wearable Bio and Chemical Sensors; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 65–83. [Google Scholar]
- Ghosh, S.K.; Calizo, I.; Teweldebrhan, D.; Pokatilov, E.P.; Nika, D.L.; Balandin, A.A.; Bao, W.; Miao, F.; Lau, C.N. Extremely high thermal conductivity of graphene: Prospects for thermal management applications in nanoelectronic circuits. Appl. Phys. Lett. 2008, 92, 151911. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Stetter, J.R.; Penrose, W.R.; Yao, S. Sensors, Chemical Sensors, Electrochemical Sensors, and ECS. J. Electrochem. Soc. 2003, 150, S11–S16. [Google Scholar] [CrossRef]
- Stradiotto, N.R.; Yamanaka, H.; Zanoni, M.V.B. Electrochemical sensors: A powerful tool in analytical chemistry. J. Braz. Chem. Soc. 2003, 14, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, A.; Han, Y.; Li, T. Sensors based on conductive polymers and their composites: A review. Polym. Int. 2020, 69, 7–17. [Google Scholar] [CrossRef]
- Domínguez-Renedo, O.; Alonso-Lomillo, M.A.; Arcos-Martínez, M.J. Determination of Metals Based on Electrochemical Biosensors. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1042–1073. [Google Scholar] [CrossRef]
- Skotheim, T.A.; Reynolds, J.R. Handbook of Conducting Polymers: Conjugated Polymers Processing and Applications; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9781420043587. [Google Scholar]
- Rattan, S.; Singhal, P.; Verma, A. Synthesis of PEDOT: PSS (poly(3,4-ethylenedioxythiophene))/poly(4-styrene sulfonate))/ngps (nanographitic platelets) nanocomposites as chemiresistive sensors for detection of nitroaromatics. Polym. Eng. Sci. 2013, 53, 2045–2052. [Google Scholar] [CrossRef]
- Wang, G.; Morrin, A.; Li, M.; Liu, N.; Luo, X. Nanomaterial-doped conducting polymers for electrochemical sensors and biosensors. J. Mater. Chem. B 2018, 6, 4173–4190. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Rincón, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical–Chiral Sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef]
- Park, C.S.; Lee, C.-S.; Kwon, O.S. Conducting Polymer Based Nanobiosensors. Polym. Basel 2016, 8, 249. [Google Scholar] [CrossRef]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.-B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Lobnik, A.; Turel, M.; Korent, P. Optical Chemical Sensors: Design and Applications. In Advances in Chemical Sensors; IntechOpen: London, UK, 2012. [Google Scholar]
- Scheller, F.; Hintsche, R.; Pfeiffer, D.; Schubert, F.; Riedel, K.; Kindervater, R. Biosensors: Fundamentals, applications and trends. Sens. Actuators B Chem. 1991, 4, 197–206. [Google Scholar] [CrossRef]
- Kreysa, G.; Ota, K.; Savinell, R.F. (Eds.) Encyclopedia of Applied Electrochemistry; Springer: New York, NY, USA, 2014; ISBN 978-1-4419-6995-8. [Google Scholar]
- Wang, J. Analytical Electrochemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; ISBN 9780471790303. [Google Scholar]
- Bard, A.J. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Bard, A.J., Faulkner, L.R., Eds.; John Wiley: New York, NY, USA, 2001; ISBN 0471043729. [Google Scholar]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Evans, D.H. Review of Voltammetric Methods for the Study of Electrode Reactions. In Microelectrodes: Theory and Applications; Springer Science and Business Media LLC: Berlin, Germany, 1991; pp. 17–32. [Google Scholar]
- Lenik, J. Cyclodextrins Based Electrochemical Sensors for Biomedical and Pharmaceutical Analysis. Curr. Med. Chem. 2017, 24, 2359–2391. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, P.T.; Heineman, W.R. Cyclic voltammetry. J. Chem. Educ. 1983, 60, 702. [Google Scholar] [CrossRef]
- Mabbott, G.A. An introduction to cyclic voltammetry. J. Chem. Educ. 1983, 60, 697. [Google Scholar] [CrossRef]
- Kawagoe, K.T.; Zimmerman, J.B.; Wightman, R. Principles of voltammetry and microelectrode surface states. J. Neurosci. Methods 1993, 48, 225–240. [Google Scholar] [CrossRef]
- Henstridge, M.C.; Laborda, E.; Rees, N.V.; Compton, R.G. Marcus—Hush—Chidsey theory of electron transfer applied to voltammetry: A review. Electrochim. Acta 2012, 84, 12–20. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods—Fundamentals and Applications; Wiley: New York, NY, USA, 1980; p. 718. [Google Scholar]
- Keteklahijani, Y.Z. Development of Electrochemical Sensors Based on Polymer Nanocomposites for Sensing of Neurotransmitter Dopamine. Ph.D. Dissertation, University of Calgary, Calgary, AB, Canada, 2020. [Google Scholar]
- Rifkin, S.C.; Evans, D.H. General equation for voltammetry with step-functional potential changes applied to differential pulse voltammetry. Anal. Chem. 1976, 48, 1616–1618. [Google Scholar] [CrossRef]
- Simões, F.R.; Xavier, M. Electrochemical Sensors. In Nanoscience and its Applications; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 155–178. ISBN 9780323497800. [Google Scholar]
- Dias, L.G.; Meirinho, S.G.; Veloso, A.C.; Rodrigues, L.R.; Peres, A.M. Electronic Tongues and Aptasensors; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 371–402. ISBN 9780081007464. [Google Scholar]
- Franklin, R.; Martin, S.; Strong, T.; Brown, R. Chemical and Biological Systems: Chemical Sensing Systems for Liquids; Elsevier BV: Amsterdam, The Netherlands, 2016; ISBN 9780128035818. [Google Scholar]
- Scott, K. Electrochemical principles and characterization of bioelectrochemical systems. In Microbial Electrochemical and Fuel Cells; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 29–66. ISBN 9781782423966. [Google Scholar]
- Marx, Í.M.G.; Veloso, A.C.; Dias, L.G.; Casal, S.; Pereira, J.A.; Peres, A.M. Electrochemical Sensor-Based Devices for Assessing Bioactive Compounds in Olive Oils: A Brief Review. Electronics 2018, 7, 387. [Google Scholar] [CrossRef] [Green Version]
- Adeloju, S. Amperometry. In Encyclopedia of Analytical Science; Elsevier BV: Amsterdam, The Netherlands, 2004; pp. 70–79. ISBN 9780123693976. [Google Scholar]
- Gaudin, V. Chapter 11: Receptor-based electrochemical biosensors for the detection of contaminants in food products. In Electrochemical Biosensors; Ensafi, A.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 307–365. [Google Scholar] [CrossRef]
- Delahay, P.; Mamantov, G. Voltammetry at Constant Current: Review of Theoretical Principles. Anal. Chem. 1955, 27, 478–483. [Google Scholar] [CrossRef]
- Westbroek, P. Electrochemical methods. In Analytical Electrochemistry in Textiles; Elsevier BV: Amsterdam, The Netherlands, 2005; pp. 37–69. ISBN 9781855739192. [Google Scholar]
- Amine, A.; Mohammadi, H. Amperometry. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier BV: Amsterdam, The Netherlands, 2018; ISBN 9780081019832. [Google Scholar]
- Roy, N.; Yasmin, S.; Jeon, S. Effective electrochemical detection of dopamine with highly active molybdenum oxide nanoparticles decorated on 2, 6 diaminopyridine/reduced graphene oxide. Microchem. J. 2020, 153, 104501. [Google Scholar] [CrossRef]
- Ramachandran, R.; Leng, X.; Zhao, C.; Xu, Z.-X.; Wang, F. 2D siloxene sheets: A novel electrochemical sensor for selective dopamine detection. Appl. Mater. Today 2020, 18, 100477. [Google Scholar] [CrossRef]
- Lu, Z.; Li, Y.; Liu, T.; Wang, G.; Sun, M.; Jiang, Y.; He, H.; Wang, Y.; Zou, P.; Wang, X.; et al. A dual-template imprinted polymer electrochemical sensor based on AuNPs and nitrogen-doped graphene oxide quantum dots coated on NiS2/biomass carbon for simultaneous determination of dopamine and chlorpromazine. Chem. Eng. J. 2020, 389, 124417. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Tao, L.; Min, Q.; Xiang, J.; Wang, Q.; Xie, J.; Yue, Y.; Wu, S.; Li, X.; et al. A disposable electrochemical sensor for simultaneous determination of norepinephrine and serotonin in rat cerebrospinal fluid based on MWNTs-ZnO/chitosan composites modified screen-printed electrode. Biosens. Bioelectron. 2015, 65, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Poh, E.Z.; Hahne, D.; Moretti, J.; Harvey, A.R.; Clarke, M.; Rodger, J. Simultaneous quantification of dopamine, serotonin, their metabolites and amino acids by LC-MS/MS in mouse brain following repetitive transcranial magnetic stimulation. Neurochem. Int. 2019, 131, 104546. [Google Scholar] [CrossRef]
- Kemp, S.F.; Lockey, R.F.; Simons, F.E.R. On behalf of the World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis Epinephrine: The drug of choice for anaphylaxis—A statement of the World Allergy Organization. Allergy 2008, 63, 1061–1070. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Shen, D.; Li, X.; Chen, Y.; Hou, L.; Zhang, Y.; Sha, J. Molybdenum oxide-based metal-organic framework/polypyrrole nanocomposites for enhancing electrochemical detection of dopamine. Talanta 2020, 209, 120507. [Google Scholar] [CrossRef]
- Jain, R.; Jadon, N.; Pawaiya, A. Polypyrrole based next generation electrochemical sensors and biosensors: A review. TrAC Trends Anal. Chem. 2017, 97, 363–373. [Google Scholar] [CrossRef]
- Qian, T.; Yu, C.; Zhou, X.; Wu, S.; Shen, J. Au nanoparticles decorated polypyrrole/reduced graphene oxide hybrid sheets for ultrasensitive dopamine detection. Sens. Actuators B Chem. 2014, 193, 759–763. [Google Scholar] [CrossRef]
- Adhikari, A.; De, S.; Halder, A.; Pattanayak, S.; Dutta, K.; Mondal, D.; Rana, D.; Ghosh, R.; Bera, N.K.; Chattopadhyay, S.; et al. Biosurfactant tailored synthesis of porous polypyrrole nanostructures: A facile approach towards CO2 adsorption and dopamine sensing. Synth. Met. 2018, 245, 209–222. [Google Scholar] [CrossRef]
- Harley, C.C.; Annibaldi, V.; Yu, T.; Breslin, C.B. The selective electrochemical sensing of dopamine at a polypyrrole film doped with an anionic β-cyclodextrin. J. Electroanal. Chem. 2019, 855, 113614. [Google Scholar] [CrossRef]
- Chen, X.; Li, D.; Ma, W.; Yang, T.; Zhang, Y.; Zhang, D. Preparation of a glassy carbon electrode modified with reduced graphene oxide and overoxidized electropolymerized polypyrrole, and its application to the determination of dopamine in the presence of ascorbic acid and uric acid. Microchim. Acta 2019, 186, 407. [Google Scholar] [CrossRef] [PubMed]
- Demirkan, B.; Bozkurt, S.; Cellat, K.; Arıkan, K.; Yılmaz, M.; Şavk, A.; Çalımlı, M.H.; Nas, M.S.; Atalar, M.N.; Alma, M.H.; et al. Palladium supported on polypyrrole/reduced graphene oxide nanoparticles for simultaneous biosensing application of ascorbic acid, dopamine, and uric acid. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Wang, H.-H.; Chen, X.-J.; Li, W.-T.; Zhou, Z.; Guo, X.-C.; Kang, W.-Y.; Kou, D.-X.; Zhou, Z.-J.; Meng, Y.-N.; Tian, Q.; et al. ZnO nanotubes supported molecularly imprinted polymers arrays as sensing materials for electrochemical detection of dopamine. Talanta 2018, 176, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Hsu, W.-F.; Wu, T.-M. Electrochemical determination of dopamine using a conductive polypyrrole/carbon-coated mesoporous silica composite electrode. J. Appl. Electrochem. 2020, 50, 311–319. [Google Scholar] [CrossRef]
- Eom, G.; Oh, C.; Moon, J.; Kim, H.; Kim, M.K.; Kim, K.; Seo, J.-W.; Kang, T.; Lee, H.J. Highly sensitive and selective detection of dopamine using overoxidized polypyrrole/sodium dodecyl sulfate-modified carbon nanotube electrodes. J. Electroanal. Chem. 2019, 848, 113295. [Google Scholar] [CrossRef]
- Adhikari, A.; De, S.; Rana, D.; Nath, J.; Ghosh, D.; Dutta, K.; Chakraborty, S.; Chattopadhyay, S.; Chakraborty, M.; Chattopadhyay, D. Selective sensing of dopamine by sodium cholate tailored polypyrrole-silver nanocomposite. Synth. Met. 2020, 260, 116296. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, X.; Shi, J.; Zou, X.; Huang, X.; Tahir, H.E.; ZhiHua, L.; Xucheng, Z.; Jiyong, S.; Xiaobo, Z.; et al. Preparation of conducting polyaniline/protoporphyrin composites and their application for sensing VOCs. Food Chem. 2019, 276, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, S.; Shokry, A.; Khalil, M.M.A.; Ibrahim, H.; Soliman, M. Polyaniline/Ag nanoparticles/graphene oxide nanocomposite fluorescent sensor for recognition of chromium (VI) ions. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Alfano, B.; Massera, E.; De Maria, A.; De Girolamo, A.; Di Francia, G.; Veneri, P.D.; Napolitano, T.; Borriello, A. Polyaniline proton doping for sensor application. In Proceedings of the 2015 XVIII AISEM Annual Conference, Trento, Italy, 3–5 February 2015; pp. 1–4. [Google Scholar]
- Ashraf, P.M.; Lalitha, K.; Edwin, L. Synthesis of polyaniline hybrid composite: A new and efficient sensor for the detection of total volatile basic nitrogen molecules. Sens. Actuators B Chem. 2015, 208, 369–378. [Google Scholar] [CrossRef]
- Mahalakshmi, S.; Sridevi, V. Conducting, crystalline and electroactive polyaniline-Au nanocomposites through combined acid and oxidative doping pathways for biosensing applications: Detection of dopamine. Mater. Chem. Phys. 2019, 235, 121728. [Google Scholar] [CrossRef]
- Chang, Y.H.; Abdullah, J.; Alias, Y.B. The selective electrochemical detection of dopamine in the presence of ascorbic acid and uric acid using electro-polymerised-β-cyclodextrin incorporated f-MWCNTs/polyaniline modified glassy carbon electrode. Microchem. J. 2019, 148, 322–330. [Google Scholar] [CrossRef]
- Sangamithirai, D.; Munusamy, S.; Narayanan, V.; Stephen, A. A voltammetric biosensor based on poly(o-methoxyaniline)-gold nanocomposite modified electrode for the simultaneous determination of dopamine and folic acid. Mater. Sci. Eng. C 2018, 91, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Ganash, A.A.; AlQarni, S.A.; Hussein, M.A. Poly(aniline-co-o-anisidine)/graphene oxide Au nanocomposites for dopamine electrochemical sensing application. J. Appl. Electrochem. 2018, 49, 179–194. [Google Scholar] [CrossRef]
- Rahman, M.; Ahmed, A.; Lee, J.-J. A Conducting Poly(N-(1-Naphthyl)ethylenediamine dihydrochloride) Nanofibers for the Sensitive and Interference-Free Detection of Dopamine. J. Electrochem. Soc. 2018, 165, B89–B95. [Google Scholar] [CrossRef]
- Dükar, N.; Tunç, S.; Öztürk, K.; Demirci, S.; Dumangöz, M.; Çelebi, M.S.; Kuralay, F. Highly sensitive and selective dopamine sensing in biological fluids with one-pot prepared graphene/poly(o-phenylenediamine) modified electrodes. Mater. Chem. Phys. 2019, 228, 357–362. [Google Scholar] [CrossRef]
- Kannan, A.; Sevvel, R. A highly selective and simultaneous determination of paracetamol and dopamine using poly-4-amino-6-hydroxy-2-mercaptopyrimidine (Poly-AHMP) film modified glassy carbon electrode. J. Electroanal. Chem. 2017, 791, 8–16. [Google Scholar] [CrossRef]
- Ramya, R.; Sangaranarayanan, M.V. Electrochemical Sensing of Anesthetics using Polythiophene Coated Glassy Carbon Electrodes. ChemistrySelect 2019, 4, 9776–9783. [Google Scholar] [CrossRef]
- Dervisevic, M.; Senel, M.; Cevik, E. Novel impedimetric dopamine biosensor based on boronic acid functional polythiophene modified electrodes. Mater. Sci. Eng. C 2017, 72, 641–649. [Google Scholar] [CrossRef]
- Gueye, M.N.; Carella, A.; Faure-Vincent, J.; Demadrille, R.; Simonato, J.-P. Progress in understanding structure and transport properties of PEDOT-based materials: A critical review. Prog. Mater. Sci. 2020, 108, 100616. [Google Scholar] [CrossRef]
- Hui, Y.; Bian, C.; Xia, S.; Tong, J.; Wang, J. Synthesis and electrochemical sensing application of poly(3,4-ethylenedioxythiophene)-based materials: A review. Anal. Chim. Acta 2018, 1022, 1–19. [Google Scholar] [CrossRef]
- Meng, L.; Turner, A.P.; Mak, W.C. Tunable 3D nanofibrous and bio-functionalised PEDOT network explored as a conducting polymer-based biosensor. Biosens. Bioelectron. 2020, 159, 112181. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.L.; Zamora, M.L.; Marchesi, L.F.; Vidotti, M. Adsorption of catechol onto PEDOT films doped with gold nanoparticles: Electrochemical and spectroscopic studies. Electrochim. Acta 2019, 322, 134773. [Google Scholar] [CrossRef]
- Donahue, M.J.; Sanchez-Sanchez, A.; Inal, S.; Qu, J.; Owens, R.M.; Mecerreyes, D.; Malliaras, G.G.; Martin, D.C. Tailoring PEDOT properties for applications in bioelectronics. Mater. Sci. Eng. R Rep. 2020, 140, 100546. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Jarjes, Z.A.; Desprez, V.; Kilmartin, P.A.; Travas-Sejdic, J. Sensitive, selective, disposable electrochemical dopamine sensor based on PEDOT-modified laser scribed graphene. Biosens. Bioelectron. 2018, 107, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Rojas, A.P.; Cortés, M.T.; Hurtado, J. Electrochemical synthesis of poly(3,4-ethylenedioxythiophene) doped with a new bis(pyrazolyl)methane disulfonate and its behavior towards dopamine detection. J. Electroanal. Chem. 2019, 837, 200–207. [Google Scholar] [CrossRef]
- Ali, A.; Jamal, R.; Abdiryim, T.; Huang, X. Synthesis of monodispersed PEDOT/Au hollow nanospheres and its application for electrochemical determination of dopamine and uric acid. J. Electroanal. Chem. 2017, 787, 110–117. [Google Scholar] [CrossRef]
- Üğe, A.; Zeybek, D.K.; Zeybek, B. An electrochemical sensor for sensitive detection of dopamine based on MWCNTs/CeO 2 -PEDOT composite. J. Electroanal. Chem. 2018, 813, 134–142. [Google Scholar] [CrossRef]
- Tukimin, N.; Abdullah, J.; Sulaiman, Y. Electrodeposition of poly(3,4-ethylenedioxythiophene)/reduced graphene oxide/manganese dioxide for simultaneous detection of uric acid, dopamine and ascorbic acid. J. Electroanal. Chem. 2018, 820, 74–81. [Google Scholar] [CrossRef]
- Song, Z.; Sheng, G.; Cui, Y.; Li, M.; Song, Z.; Ding, C.; Luo, X. Low fouling electrochemical sensing in complex biological media by using the ionic liquid-doped conducting polymer PEDOT: Application to voltammetric determination of dopamine. Microchim. Acta 2019, 186, 220. [Google Scholar] [CrossRef]
- Qian, Y.; Ma, C.; Song, H.; Gao, J.; Liu, M.; Xie, K.; Wang, S.; Sun, K.; Song, H. High performance electrochemical electrode based on polymeric composite film for sensing of dopamine and catechol. Sens. Actuators B Chem. 2018, 255, 1655–1662. [Google Scholar] [CrossRef]
- Pananon, P.; Sriprachuabwong, C.; Wisitsoraat, A.; Chuysinuan, P.; Tuantranont, A.; Saparpakorn, P.; Dechtrirat, D. A facile one-pot green synthesis of gold nanoparticle-graphene-PEDOT:PSS nanocomposite for selective electrochemical detection of dopamine. RSC Adv. 2018, 8, 12724–12732. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, C.S.; Oliveira, M.M.; Bergamini, M.F.; Marcolino-Junior, L.H.; Zarbin, A.J.G. Facile synthesis and dopamine sensing application of three component nanocomposite thin films based on polythiophene, gold nanoparticles and carbon nanotubes. J. Electroanal. Chem. 2019, 840, 208–217. [Google Scholar] [CrossRef]
- Naik, T.S.K.; Mwaurah, M.M.; Swamy, B.K. Fabrication of poly (sudan III) modified carbon paste electrode sensor for dopamine: A voltammetric study. J. Electroanal. Chem. 2019, 834, 71–78. [Google Scholar] [CrossRef]
- Hsieh, M.-T.; Whang, T.-J. Mechanistic investigation on the electropolymerization of phenol red by cyclic voltammetry and the catalytic reactions toward acetaminophen and dopamine using poly(phenol red)-modified GCE. J. Electroanal. Chem. 2017, 795, 130–140. [Google Scholar] [CrossRef]
- Kong, D.; Zhuang, Q.; Han, Y.; Xu, L.; Wang, Z.; Jiang, L.; Su, J.; Lu, C.; Chi, Y. Simultaneous voltammetry detection of dopamine and uric acid in human serum and urine with a poly(procaterol hydrochloride) modified glassy carbon electrode. Talanta 2018, 185, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Kou, Y.; Jiang, X.; Wang, M.; Xue, Y.; Tian, B.; Tan, L. One-step preparation of poly(glyoxal-bis(2-hydroxyanil))-amino-functionalized graphene quantum dots-MnO2 composite on electrode surface for simultaneous determination of vitamin B2 and dopamine. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123652. [Google Scholar] [CrossRef]
- Li, X.; Lu, X.; Kan, X. 3D electrochemical sensor based on poly(hydroquinone)/gold nanoparticles/nickel foam for dopamine sensitive detection. J. Electroanal. Chem. 2017, 799, 451–458. [Google Scholar] [CrossRef]
- Edris, N.M.M.A.; Abdullah, J.; Kamaruzaman, S.; Saiman, M.I.; Sulaiman, Y. Electrochemical reduced graphene oxide-poly(eriochrome black T)/gold nanoparticles modified glassy carbon electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. Arab. J. Chem. 2018, 11, 1301–1312. [Google Scholar] [CrossRef]
- Palakollu, V.N.; Karpoormath, R. Enhanced electrochemical sensing of dopamine based on carboxylic acid functionalized multi-walled carbon nanotubes/poly(toluidine blue) composite. Synth. Met. 2018, 245, 87–95. [Google Scholar] [CrossRef]
- Arroquia, A.; Acosta, I.; Armada, M.P.G. Self-assembled gold decorated polydopamine nanospheres as electrochemical sensor for simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan. Mater. Sci. Eng. C 2020, 109, 110602. [Google Scholar] [CrossRef]
- Ramya, R.; Muthukumaran, P.; Wilson, J. Electron beam-irradiated polypyrrole decorated with Bovine serum albumin pores: Simultaneous determination of epinephrine and L-tyrosine. Biosens. Bioelectron. 2018, 108, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, K.; Hajian, A. Electrochemical characterization of Au/ZnO/PPy/RGO nanocomposite and its application for simultaneous determination of ascorbic acid, epinephrine, and uric acid. J. Electroanal. Chem. 2017, 801, 466–479. [Google Scholar] [CrossRef]
- Bonyadi, S.; Ghanbari, K.; Ghiasi, M. All-electrochemical synthesis of a three-dimensional mesoporous polymeric g-C3N4/PANI/CdO nanocomposite and its application as a novel sensor for the simultaneous determination of epinephrine, paracetamol, mefenamic acid, and ciprofloxacin. New J. Chem. 2020, 44, 3412–3424. [Google Scholar] [CrossRef]
- Tsele, T.P.; Adekunle, A.S.; Fayemi, O.E.; Ebenso, E.E. Electrochemical detection of Epinephrine using Polyaniline nanocomposite films doped with TiO2 and RuO2 Nanoparticles on Multi-walled Carbon Nanotube. Electrochim. Acta 2017, 243, 331–348. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, X.-T.; Zhou, J.-P.; Liu, G.-Z.; Zhang, S.-Y. Electrochemical preparation of surface molecularly imprinted poly(3-aminophenylboronic acid)/MWCNTs nanocomposite for sensitive sensing of epinephrine. Mater. Sci. Eng. C 2018, 91, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Kan, X. Conductive imprinted electrochemical sensor for epinephrine sensitive detection and double recognition. J. Electroanal. Chem. 2019, 836, 182–189. [Google Scholar] [CrossRef]
- Taei, M.; Hasanpour, F.; Tavakkoli, N.; Bahrameian, M. Electrochemical characterization of poly(fuchsine acid) modified glassy carbon electrode and its application for simultaneous determination of ascorbic acid, epinephrine and uric acid. J. Mol. Liq. 2015, 211, 353–362. [Google Scholar] [CrossRef]
- Ding, M.; Zhou, Y.; Liang, X.; Zou, H.; Wang, Z.; Wang, M.; Ma, J. An electrochemical sensor based on graphene/poly(brilliant cresyl blue) nanocomposite for determination of epinephrine. J. Electroanal. Chem. 2016, 763, 25–31. [Google Scholar] [CrossRef]
- Özcan, A.; Ilkbaş, S. Poly(pyrrole-3-carboxylic acid)-modified pencil graphite electrode for the determination of serotonin in biological samples by adsorptive stripping voltammetry. Sens. Actuators B Chem. 2015, 215, 518–524. [Google Scholar] [CrossRef]
- Ran, G.; Chen, C.; Gu, C. Serotonin sensor based on a glassy carbon electrode modified with multiwalled carbon nanotubes, chitosan and poly(p-aminobenzenesulfonate). Microchim. Acta 2015, 182, 1323–1328. [Google Scholar] [CrossRef]
- Raj, M.; Gupta, P.; Goyal, R.N.; Shim, Y.-B. Graphene/conducting polymer nano-composite loaded screen printed carbon sensor for simultaneous determination of dopamine and 5-hydroxytryptamine. Sens. Actuators B Chem. 2017, 239, 993–1002. [Google Scholar] [CrossRef]
- Ran, G.; Chen, X.; Xia, Y. Electrochemical detection of serotonin based on a poly(bromocresol green) film and Fe3O4 nanoparticles in a chitosan matrix. RSC Adv. 2017, 7, 1847–1851. [Google Scholar] [CrossRef] [Green Version]
- Al-Graiti, W.; Foroughi, J.; Liu, Y.; Chen, J. Hybrid Graphene/Conducting Polymer Strip Sensors for Sensitive and Selective Electrochemical Detection of Serotonin. ACS Omega 2019, 4, 22169–22177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.; Akhtar, M.H.; Benboudiaf, A.; Park, D.; Shim, Y.-B. A Sensor for Serotonin and Dopamine Detection in Cancer Cells Line Based on the Conducting Polymer−Pd Complex Composite. Electroanalysis 2020, 32, 520–527. [Google Scholar] [CrossRef]
- Soltani, Z.; Rasheed, K.; Kapusta, D.R.; Reisin, E. Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: Is it time for reappraisal? Curr. Hypertens. Rep. 2013, 15, 175–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, H.; Wang, N.; Wang, D.; Zhang, X.; Ma, H.; Lin, M. Voltammetric uric acid sensor based on a glassy carbon electrode modified with a nanocomposite consisting of polytetraphenylporphyrin, polypyrrole, and graphene oxide. Microchim. Acta 2016, 183, 3053–3059. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Basirun, W.; Sookhakian, M.; Abdullah, J.; Zalnezhad, E.; Hazarkhani, H.; Alias, Y. Synthesis and characterization of α-Fe2O3/polyaniline nanotube composite as electrochemical sensor for uric acid detection. Adv. Powder Technol. 2019, 30, 384–392. [Google Scholar] [CrossRef]
- Rajabi, H.; Noroozifar, M. New synthesis of poly ortho-methoxyaniline nanostructures and its application to construct modified multi-wall carbon nanotube/graphite paste electrode for simultaneous determination of uric acid and folic acid. Mater. Sci. Eng. C 2017, 75, 791–797. [Google Scholar] [CrossRef]
- Özcan, A.; Ilkbaş, S. Preparation of poly(3,4-ethylenedioxythiophene) nanofibers modified pencil graphite electrode and investigation of over-oxidation conditions for the selective and sensitive determination of uric acid in body fluids. Anal. Chim. Acta 2015, 891, 312–320. [Google Scholar] [CrossRef]
- Huang, X.; Shi, W.; Li, J.; Bao, N.; Yu, C.; Gu, H. Determination of salivary uric acid by using poly(3,4-ethylenedioxythipohene) and graphene oxide in a disposable paper-based analytical device. Anal. Chim. Acta 2020, 1103, 75–83. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, M.; Liu, W.; Yu, S.; Niu, L.; Li, G.; Li, H.; Liu, W. Electrochemical sensor based on molecularly imprinted polymer/reduced graphene oxide composite for simultaneous determination of uric acid and tyrosine. J. Electroanal. Chem. 2018, 813, 75–82. [Google Scholar] [CrossRef]
- Yan, H.; Xiao, H.; Xie, Q.; Liu, J.; Sun, L.; Zhou, Y.; Zhang, Y.; Chao, L.; Chen, C.; Yao, S. Simultaneous electroanalysis of isoniazid and uric acid at poly(sulfosalicylic acid)/electroreduced carboxylated graphene modified glassy carbon electrode. Sens. Actuators B Chem. 2015, 207, 167–176. [Google Scholar] [CrossRef]
- Taei, M.; Hasanpour, F.; Habibollahi, S.; Shahidi, L.; Habibolahi, S. Simultaneous electrochemical sensing of cysteine, uric acid and tyrosine using a novel Au-nanoparticles/poly-Trypan Blue modified glassy carbon electrode. J. Electroanal. Chem. 2017, 789, 140–147. [Google Scholar] [CrossRef]
- Lan, D.; Zhang, L. Electrochemical synthesis of a novel purine-based polymer and its use for the simultaneous determination of dopamine, uric acid, xanthine and hypoxanthine. J. Electroanal. Chem. 2015, 757, 107–115. [Google Scholar] [CrossRef]
- Shalini, A.; Paulraj, P.; Pandian, K.; Anbalagan, G.; Jaisankar, V. Single pot synthesis, characterization of PPy@C composites modified electrode for the electrocatalytic determination of ascorbic acid in commercial fruit samples. Surf. Interfaces 2019, 17, 100386. [Google Scholar] [CrossRef]
- Fang, Y.; Qi, J.; Deng, M.; Tian, Y.; Wen, Q.; Wang, M. Preparation in-situ of carbon nanotubes/polyaniline modified electrode and application for ascorbic acid detection. J. Electroanal. Chem. 2015, 755, 39–46. [Google Scholar] [CrossRef]
- Ji, W.-F.; Chu, C.-M.; Hsu, S.-C.; Lu, Y.-D.; Yu, Y.-C.; Santiago, K.S.; Yeh, J.-M. Synthesis and characterization of organo-soluble aniline oligomer-based electroactive doped with gold nanoparticles, and application to electrochemical sensing of ascorbic acid. Polym. Guildf 2017, 128, 218–228. [Google Scholar] [CrossRef]
- Devi, C.L.; Narayanan, S.S. Poly(amido amine) dendrimer and silver nanoparticle–multi-walled carbon nanotubes composite with poly(neutral red)-modified electrode for the determination of ascorbic acid. Bull. Mater. Sci. 2019, 42, 73. [Google Scholar] [CrossRef] [Green Version]
- Motshakeri, M.; Travas-Sejdic, J.; Phillips, A.R.; Kilmartin, P.A. Rapid electroanalysis of uric acid and ascorbic acid using a poly(3,4-ethylenedioxythiophene)-modified sensor with application to milk. Electrochim. Acta 2018, 265, 184–193. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Z.; Zhang, C.; Zhang, Y.; Chen, Q. One-step electrodeposition of poly (3,4-ethylenedioxythiophene) on carboxylated multi-wall carbon nanotubes and its application in ascorbic acid sensing. J. Electroanal. Chem. 2016, 782, 84–90. [Google Scholar] [CrossRef]
- Ergün, E.; Kart, Ş.; Zeybek, D.K.; Zeybek, B. Simultaneous electrochemical determination of ascorbic acid and uric acid using poly(glyoxal-bis(2-hydroxyanil)) modified glassy carbon electrode. Sens. Actuators B Chem. 2016, 224, 55–64. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.S.; Baik, S.; Hyeon, T.; Kim, D.-H. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv. Healthc. Mater. 2018, 7, e1701150. [Google Scholar] [CrossRef] [Green Version]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Sabu, C.; Henna, T.; Raphey, V.; Nivitha, K.; Pramod, K. Advanced biosensors for glucose and insulin. Biosens. Bioelectron. 2019, 141, 111201. [Google Scholar] [CrossRef]
- Thunyakontirakun, W.; Sriwichai, S.; Phanichphant, S.; Janmanee, R. Fabrication of poly(pyrrole-3-carboxylic acid)/graphene oxide composite thin film for glucose biosensor. Mater. Today Proc. 2019, 17, 2070–2077. [Google Scholar] [CrossRef]
- Fang, L.; Liang, B.; Yang, G.; Hu, Y.; Zhu, Q.; Ye, X. A needle-type glucose biosensor based on PANI nanofibers and PU/E-PU membrane for long-term invasive continuous monitoring. Biosens. Bioelectron. 2017, 97, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Wang, P.; Zhong, A.; Yang, M.; Xu, Q.; Hao, S.; Hu, X. Development of a glucose biosensor based on electrodeposited gold nanoparticles-polyvinylpyrrolidone-polyaniline nanocomposites. J. Electroanal. Chem. 2015, 756, 153–160. [Google Scholar] [CrossRef]
- Feng, X.; Cheng, H.; Pan, Y.; Zheng, H. Development of glucose biosensors based on nanostructured graphene-conducting polyaniline composite. Biosens. Bioelectron. 2015, 70, 411–417. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, M.; Yan, Z.; Chen, J. Highly selective and stable glucose biosensor based on incorporation of platinum nanoparticles into polyaniline-montmorillonite hybrid composites. Microchem. J. 2020, 152, 104266. [Google Scholar] [CrossRef]
- Sun, L.; Ma, Y.; Zhang, P.; Chao, L.; Huang, T.; Xie, Q.; Chen, C.; Yao, S. An amperometric enzyme electrode and its biofuel cell based on a glucose oxidase-poly(3-anilineboronic acid)-Pd nanoparticles bionanocomposite for glucose biosensing. Talanta 2015, 138, 100–107. [Google Scholar] [CrossRef]
- Li, J.; Bi, X.; Tamulevičius, S.; Erts, D.; Chang, C.-F.; Gu, Y. Fabrication of a biocompatible and continuous glucose biosensor with the poly(3,4-ethylenedioxythiophene) modified electrode. J. Taiwan Inst. Chem. Eng. 2019, 104, 1–7. [Google Scholar] [CrossRef]
- Tekbaşoğlu, T.Y.; Soganci, T.; Ak, M.; Koca, A.; Şener, M.K. Enhancing biosensor properties of conducting polymers via copolymerization: Synthesis of EDOT-substituted bis(2-pyridylimino)isoindolato-palladium complex and electrochemical sensing of glucose by its copolymerized film. Biosens. Bioelectron. 2017, 87, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Krzyczmonik, P.; Socha, E.; Skrzypek, S. Electrochemical Detection of Glucose in Beverage Samples Using Poly(3,4-ethylenedioxythiophene)-Modified Electrodes with Immobilized Glucose Oxidase. Electrocatalysis 2017, 9, 380–387. [Google Scholar] [CrossRef] [Green Version]
- Krzyczmonik, P.; Socha, E.; Skrzypek, S. Immobilization of glucose oxidase on modified electrodes with composite layers based on poly(3,4-ethylenedioxythiophene). Bioelectrochemistry 2015, 101, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Dilgin, D.G.; Ertek, B.; Dilgin, Y. A low-cost, fast, disposable and sensitive biosensor study: Flow injection analysis of glucose at poly-methylene blue-modified pencil graphite electrode. J. Iran. Chem. Soc. 2018, 15, 1355–1363. [Google Scholar] [CrossRef]
- Papi, M.A.P.; Caetano, F.R.; Bergamini, M.F.; Marcolino-Junior, L.H. Facile synthesis of a silver nanoparticles/polypyrrole nanocomposite for non-enzymatic glucose determination. Mater. Sci. Eng. C 2017, 75, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cho, M.; Pang, C.; Lee, Y. Highly sensitive non-enzymatic glucose sensor based on over-oxidized polypyrrole nanowires modified with Ni(OH)2 nanoflakes. Sens. Actuators B Chem. 2015, 211, 93–101. [Google Scholar] [CrossRef]
- Ahammad, A.J.S.; Al Mamun, A.; Akter, T.; Mamun, M.A.; Faraezi, S.; Monira, F.Z. Enzyme-free impedimetric glucose sensor based on gold nanoparticles/polyaniline composite film. J. Solid State Electrochem. 2016, 20, 1933–1939. [Google Scholar] [CrossRef]
- Sedghi, R.; Pezeshkian, Z. Fabrication of non-enzymatic glucose sensor based on nanocomposite of MWCNTs-COOH-Poly(2-aminothiophenol)-Au NPs. Sens. Actuators B Chem. 2015, 219, 119–124. [Google Scholar] [CrossRef]
- Ansari, S.A.; Ahmed, A.; Ferdousi, F.K.; Salam, A.; Shaikh, A.; Barai, H.R.; Lopa, N.S.; Rahman, M. Conducting poly(aniline blue)-gold nanoparticles composite modified fluorine-doped tin oxide electrode for sensitive and non-enzymatic electrochemical detection of glucose. J. Electroanal. Chem. 2019, 850, 113394. [Google Scholar] [CrossRef]
- Azharudeen, A.M.; Karthiga, R.; Rajarajan, M.; Suganthi, A. Fabrication, characterization of polyaniline intercalated NiO nanocomposites and application in the development of non-enzymatic glucose biosensor. Arab. J. Chem. 2020, 13, 4053–4064. [Google Scholar] [CrossRef]
- Hocevar, M.A.; Fabregat, G.; Armelin, E.; Ferreira, C.; Alemán, C. Nanometric polythiophene films with electrocatalytic activity for non-enzymatic detection of glucose. Eur. Polym. J. 2016, 79, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Amirzadeh, Z.; Javadpour, S.; Shariat, M.H.; Knibbe, R. Non-enzymatic glucose sensor based on copper oxide and multi-wall carbon nanotubes using PEDOT:PSS matrix. Synth. Met. 2018, 245, 160–166. [Google Scholar] [CrossRef]
- Huang, P.-C.; Shen, M.-Y.; Yu, H.-H.; Wei, S.-C.; Luo, S.-C. Surface Engineering of Phenylboronic Acid-Functionalized Poly(3,4-ethylenedioxythiophene) for Fast Responsive and Sensitive Glucose Monitoring. ACS Appl. Bio Mater. 2018, 1, 160–167. [Google Scholar] [CrossRef]
- Hui, N.; Wang, S.; Xie, H.; Xu, S.; Niu, S.; Luo, X. Nickel nanoparticles modified conducting polymer composite of reduced graphene oxide doped poly(3,4-ethylenedioxythiophene) for enhanced nonenzymatic glucose sensing. Sens. Actuators B Chem. 2015, 221, 606–613. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Amin-Sadrabadi, E.; Khoshroo, A. Enhanced activity for non-enzymatic glucose oxidation on nickel nanostructure supported on PEDOT:PSS. J. Electroanal. Chem. 2016, 775, 116–120. [Google Scholar] [CrossRef]
- Kim, D.-M.; Moon, J.-M.; Lee, W.-C.; Yoon, J.-H.; Choi, C.S.; Shim, Y.-B. A potentiometric non-enzymatic glucose sensor using a molecularly imprinted layer bonded on a conducting polymer. Biosens. Bioelectron. 2017, 91, 276–283. [Google Scholar] [CrossRef]
- Hui, N.; Wang, W.; Xu, G.; Luo, X. Graphene oxide doped poly(3,4-ethylenedioxythiophene) modified with copper nanoparticles for high performance nonenzymatic sensing of glucose. J. Mater. Chem. B 2014, 3, 556–561. [Google Scholar] [CrossRef]
- Wu, L.-N.; Zhong, J.-P.; Waqas, M.; Jiang, Z.; Fan, Y.; Sun, Y.; Li, J.; Chen, W. Controllable synthesis of six corner star-like Cu2O/PEDOT-MWCNT composites and their performance toward electrochemical glucose sensing. Electrochim. Acta 2019, 318, 837–846. [Google Scholar] [CrossRef]
- Wang, J.; Wang, M.; Guan, J.; Wang, C.; Wang, G. Construction of a non-enzymatic sensor based on the poly(o-phenylenediamine)/Ag-NPs composites for detecting glucose in blood. Mater. Sci. Eng. C 2017, 71, 844–851. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Lv, H.; Wang, G.; Hu, X.; Wang, C. Construction of a non-enzymatic glucose sensor based on copper nanoparticles/poly(o-phenylenediamine) nanocomposites. J. Solid State Electrochem. 2015, 19, 731–738. [Google Scholar] [CrossRef]
- Vilela, E.T.; Carvalho, R.D.C.S.; Neto, S.Y.; Luz, R.D.C.S.; Damos, F.S. Exploiting charge/ions compensating processes in PANI/SPANI/reduced graphene oxide composite for development of a high sensitive H2O2 sensor. J. Electroanal. Chem. 2015, 752, 75–81. [Google Scholar] [CrossRef]
- Luo, M.; Wang, W.; Zhao, Q.; Li, M.; Chen, Y.; Lu, Z.; Liu, K.; Wang, N. Chemiluminescence biosensor for hydrogen peroxide determination by immobilizing horseradish peroxidase onto PVA- co -PE nanofiber membrane. Eur. Polym. J. 2017, 91, 307–314. [Google Scholar] [CrossRef]
- Ojani, R.; Hamidi, P.; Raoof, J.B. Efficient nonenzymatic hydrogen peroxide sensor in acidic media based on Prussian blue nanoparticles-modified poly(o-phenylenediamine)/glassy carbon electrode. Chin. Chem. Lett. 2016, 27, 481–486. [Google Scholar] [CrossRef]
- Zhou, B.; Liang, L.-M.; Yao, J. Nanoflakes of an aminoacid-based chiral coordination polymer: Synthesis, optical and electrochemical properties, and application in electrochemical sensing of H2O2. J. Solid State Chem. 2015, 223, 152–155. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Chen, S.-M.; Kwak, C.H.; Hwang, S.-K.; Huh, Y.S.; Huh, Y.S. Immobilization of hemoglobin on functionalized multi-walled carbon nanotubes-poly-l-histidine-zinc oxide nanocomposites toward the detection of bromate and H2O2. Sens. Actuators B Chem. 2016, 224, 607–617. [Google Scholar] [CrossRef]
- Saidu, F.K.; Joseph, A.; Varghese, E.V.; Thomas, G.V. Silver nanoparticles-embedded poly(1-naphthylamine) nanospheres for low-cost non-enzymatic electrochemical H2O2 sensor. Polym. Bull. 2019, 77, 5825–5846. [Google Scholar] [CrossRef]
- Mercante, L.A.; Facure, M.H.; Sanfelice, R.C.; Migliorini, F.; Mattoso, L.H.; Correa, D.S. One-pot preparation of PEDOT:PSS-reduced graphene decorated with Au nanoparticles for enzymatic electrochemical sensing of H2O2. Appl. Surf. Sci. 2017, 407, 162–170. [Google Scholar] [CrossRef]
- Zhang, R.; Jiang, C.; Fan, X.; Yang, R.; Sun, Y.; Zhang, C. A gold electrode modified with a nanoparticulate film composed of a conducting copolymer for ultrasensitive voltammetric sensing of hydrogen peroxide. Microchim. Acta 2018, 185, 58. [Google Scholar] [CrossRef]
- Chen, C.; Hong, X.; Xu, T.; Chen, A.; Lu, L.; Gao, Y. Hydrogen peroxide biosensor based on the immobilization of horseradish peroxidase onto a poly(aniline-co-N-methylthionine) film. Synth. Met. 2016, 212, 123–130. [Google Scholar] [CrossRef]
- Torres, D.I.; Miranda, M.V.; Orto, V.C.D. One-pot preparation of SBP-PANI-PAA-ethylene glycol diglycidyl ether sensor for electrochemical detection of H2O2. Sens. Actuators B Chem. 2017, 239, 1016–1025. [Google Scholar] [CrossRef]
- Yusoff, F.; Muhamad, N.B. Synthesis and characterization of reduced graphene oxide/PEDOT composite as cathode materials for oxygen reduction reaction. Mater. Today Proc. 2019, 16, 2023–2029. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Cui, M.; Xu, S.; Luo, X. Enzymeless voltammetric hydrogen peroxide sensor based on the use of PEDOT doped with Prussian Blue nanoparticles. Microchim. Acta 2016, 184, 483–489. [Google Scholar] [CrossRef]
- Lete, C.; Marin, M.; Anghel, E.M.; Preda, L.; Matei, C.; Lupu, S. Sinusoidal voltage electrodeposition of PEDOT-Prussian blue nanoparticles composite and its application to amperometric sensing of H2O2 in human blood. Mater. Sci. Eng. C 2019, 102, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Teker, M.Ş.; Karaca, E.; Pekmez, N.Ö.; Tamer, U.; Pekmez, K. Şen; Karaca, E.; Pekmez, N. Özçiçek; Tamer, U.; Pekmez, K. An Enzyme-free H2O2 Sensor Based on Poly(2-Aminophenylbenzimidazole)/Gold Nanoparticles Coated Pencil Graphite Electrode. Electroanalysis 2018, 31, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Yuan, X.; Ren, Y.; Shi, Q.; Wang, S.; Dong, J.; Nan, Z.-D. Cost-effective three dimensional Ag/polymer dyes/graphene-carbon spheres hybrids for high performance nonenzymatic sensor and its application in living cell H2O2 detection. Bioelectrochemistry 2018, 123, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.-F.; Bai, Y.-F.; Wu, W.-W.; He, X.; Luong, J.H.T. Modification with mesoporous platinum and poly(pyrrole-3-carboxylic acid)-based copolymer on boron-doped diamond for nonenzymatic sensing of hydrogen peroxide. J. Electroanal. Chem. 2016, 766, 52–59. [Google Scholar] [CrossRef]
- Gu, Y.; Yan, X.; Liu, W.; Li, C.; Chen, R.; Tang, L.; Lu, N.; Yang, M. Biomimetic sensor based on copper-poly(cysteine) film for the determination of metronidazole. Electrochim. Acta 2015, 152, 108–116. [Google Scholar] [CrossRef]
- Xiao, N.; Deng, J.; Cheng, J.; Ju, S.; Zhao, H.; Xie, J.; Qian, D.; He, J. Carbon paste electrode modified with duplex molecularly imprinted polymer hybrid film for metronidazole detection. Biosens. Bioelectron. 2016, 81, 54–60. [Google Scholar] [CrossRef]
- Gu, Y.; Yan, X.; Li, C.; Zheng, B.; Li, Y.; Liu, W.; Lu, N.; Yang, M. Biomimetic sensor based on molecularly imprinted polymer with nitroreductase-like activity for metronidazole detection. Biosens. Bioelectron. 2016, 77, 393–399. [Google Scholar] [CrossRef]
- Yang, M.; Guo, M.; Feng, Y.; Lei, Y.; Cao, Y.; Zhu, D.; Yu, Y.; Ding, L. Sensitive Voltammetric Detection of Metronidazole Based on Three-Dimensional Graphene-Like Carbon Architecture/Polythionine Modified Glassy Carbon Electrode. J. Electrochem. Soc. 2018, 165, B530–B535. [Google Scholar] [CrossRef]
- Wang, P.; Gan, T.; Zhang, J.; Luo, J.; Zhang, S. Polyvinylpyrrolidone-enhanced electrochemical oxidation and detection of acyclovir. J. Mol. Liq. 2013, 177, 129–132. [Google Scholar] [CrossRef]
- Hamtaka, M.; Dorrajia, P.S.; Hosseinib, M.; Dorrajia, P.S. Improved Performance for Acyclovir Sensing in the Presence of Deep Eutectic Solvent and Nanostructures and Polymer. IEEE Sens. J. 2020, 20, 623–630. [Google Scholar] [CrossRef]
- Heli, H.; Zarghan, M.; Jabbari, A.; Parsaei, A.; Moosavi-Movahedi, A.A. Electrocatalytic oxidation of the antiviral drug acyclovir on a copper nanoparticles-modified carbon paste electrode. J. Solid State Electrochem. 2009, 14, 787–795. [Google Scholar] [CrossRef]
- Dorraji, P.S.; Jalali, F. Differential pulse voltammetric determination of nanomolar concentrations of antiviral drug acyclovir at polymer film modified glassy carbon electrode. Mater. Sci. Eng. C 2016, 61, 858–864. [Google Scholar] [CrossRef]
- Hamtak, M.; Dorrajia, P.S.; Hosseini, M.; Dorraji, P.S. Sensitive Determination of Acyclovir in Biological and Pharmaceutical Samples Based on Polymeric Film Decorated with Nanomaterials on Nanoporous Glassy Carbon Electrode. J. Electrochem. Soc. 2018, 165, B632–B637. [Google Scholar] [CrossRef]
- Shahrokhianab, S.; Azimzadeh, M.; Amini, M.K. Modification of glassy carbon electrode with a bilayer of multiwalled carbon nanotube/tiron-doped polypyrrole: Application to sensitive voltammetric determination of acyclovir. Mater. Sci. Eng. C 2015, 53, 134–141. [Google Scholar] [CrossRef]
- Ahmed, A.M.K.; Hanoon, I.T.; Ahmed, S.H. Determination of the Ciprofloxacin Hydrochloride Drug in Some Pharmaceuticals using Manufactured Membrane Selective Electrodes. Syst. Rev. Pharm. 2020, 11, 622–626. [Google Scholar] [CrossRef]
- Hatamluyi, B.; Zahed, F.M.; Es’Haghi, Z.; Darroudi, M. Carbon Quantum Dots Co-catalyzed with ZnO Nanoflowers and Poly (CTAB) Nanosensor for Simultaneous Sensitive Detection of Paracetamol and Ciprofloxacin in Biological Samples. Electroanal. 2020, 32, 1818–1827. [Google Scholar] [CrossRef]
- Pushpanjali, P.A.; Manjunatha, J.G.; Shreenivas, M.T. The Electrochemical Resolution of Ciprofloxacin, Riboflavin and Estriol Using Anionic Surfactant and Polymer-Modified Carbon Paste Electrode. ChemistrySelect 2019, 4, 13427–13433. [Google Scholar] [CrossRef]
- Chauhan, R.; Gill, A.A.; Nate, Z.; Karpoormath, R. Highly selective electrochemical detection of ciprofloxacin using reduced graphene oxide/poly(phenol red) modified glassy carbon electrode. J. Electroanal. Chem. 2020, 871, 114254. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, W.; Ye, J.; Zhan, S.; Xia, B.; Lv, J.; Xu, H.; Du, G.; Wang, L. A Label-Free Colorimetric Biosensor for 17β-Estradiol Detection Using Nanoparticles Assembled by Aptamer and Cationic Polymer. Aust. J. Chem. 2016, 69, 12–19. [Google Scholar] [CrossRef]
- Wang, A.; Ding, Y.; Li, L.; Duan, D.; Mei, Q.; Zhuang, Q.; Cui, S.; He, X. A novel electrochemical enzyme biosensor for detection of 17β-estradiol by mediated electron-transfer system. Talanta 2019, 192, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Spychalska, K.; Zając, D.; Cabaj, J. Electrochemical biosensor for detection of 17β-estradiol using semi-conducting polymer and horseradish peroxidase. RSC Adv. 2020, 10, 9079–9087. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Li, H.; Yu, S.; Zhang, J.; Zheng, W.; Niu, L.; Li, G. Poly(3,6-diamino-9-ethylcarbazole) based molecularly imprinted polymer sensor for ultra-sensitive and selective detection of 17-β-estradiol in biological fluids. Biosens. Bioelectron. 2018, 104, 79–86. [Google Scholar] [CrossRef]
- Chiam, E.; Weinberg, L.; Bellomo, R. Paracetamol: A review with specific focus on the haemodynamic effects of intravenous administration. Hear. lung Vessel. 2015, 7, 121–132. [Google Scholar]
- Li, M.; Wang, W.; Chen, Z.; Song, Z.; Luo, X. Electrochemical determination of paracetamol based on Au@graphene core-shell nanoparticles doped conducting polymer PEDOT nanocomposite. Sens. Actuators B Chem. 2018, 260, 778–785. [Google Scholar] [CrossRef]
- Wei, Y.; Zeng, Q.; Bai, S.; Wang, M.; Wang, L. Nanosized Difunctional Photo Responsive Magnetic Imprinting Polymer for Electrochemically Monitored Light-Driven Paracetamol Extraction. ACS Appl. Mater. Interfaces 2017, 9, 44114–44123. [Google Scholar] [CrossRef]
- Palakollu, V.N.; Chiwunze, T.E.; Liu, C.; Karpoormath, R. Electrochemical sensitive determination of acetaminophen in pharmaceutical formulations at iron oxide/graphene composite modified electrode. Arab. J. Chem. 2020, 13, 4350–4357. [Google Scholar] [CrossRef]
- Dai, Y.; Li, X.; Lu, X.; Kan, X. Voltammetric determination of paracetamol using a glassy carbon electrode modified with Prussian Blue and a molecularly imprinted polymer, and ratiometric read-out of two signals. Microchim. Acta 2016, 183, 2771–2778. [Google Scholar] [CrossRef]
- Kuskur, C.M.; Swamy, B.K.; Jayadevappa, H.; Ganesh, P.S. Poly (rhodamine B) sensor for norepinephrine and paracetamol: A voltammetric study. Ionics 2018, 24, 3631–3640. [Google Scholar] [CrossRef]
- Kuskur, C.M.; Swamy, B.K.; Jayadevappa, H. Poly (naphthol green B) modified carbon paste electrode for the analysis of paracetamol and norepinephrine. Ionics 2018, 25, 1845–1855. [Google Scholar] [CrossRef]
- Chitravathi, S.; Munichandraiah, N. Voltammetric determination of paracetamol, tramadol and caffeine using poly(Nile blue) modified glassy carbon electrode. J. Electroanal. Chem. 2016, 764, 93–103. [Google Scholar] [CrossRef]
- Li, C.; Si, W.; Lei, B.; Zhang, C.; Lei, W.; Hao, Q. Electrochemical Determination of Paracetamol at Poly(3-Methylthiophene)/Reduced Graphene Oxide Modified Glassy Carbon Electrode. Nano 2018, 13, 13. [Google Scholar] [CrossRef]
- Gholivand, M.-B.; Ahmadi, E. Square Wave Anodic Stripping Voltammetric Determination of Paracetamol at Poly Luminol/Functionalized Multi-Walled Carbon Nanotubes Modified Glassy Carbon Electrode. Russ. J. Electrochem. 2019, 55, 1151–1161. [Google Scholar] [CrossRef]
- Sipa, K.; Socha, E.; Skrzypek, S.; Krzyczmonik, P. Electrodes Modified with Composite Layers Based on Poly(3,4-ethylenedioxythiophene) as Sensors for Paracetamol. Anal. Sci. 2017, 33, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Bayram, E.; Akyilmaz, E. Development of a new microbial biosensor based on conductive polymer/multiwalled carbon nanotube and its application to paracetamol determination. Sens. Actuators B Chem. 2016, 233, 409–418. [Google Scholar] [CrossRef]
- Kaur, B.; Prathap, M.U.A.; Srivastava, R. Synthesis of Transition-Metal Exchanged Nanocrystalline ZSM-5 and Their Application in Electrochemical Oxidation of Glucose and Methanol. ChemPlusChem 2012, 77, 1119–1127. [Google Scholar] [CrossRef]
- Kaur, B.; Srivastava, R. Simultaneous determination of epinephrine, paracetamol, and folic acid using transition metal ion-exchanged polyaniline-zeolite organic-inorganic hybrid materials. Sens. Actuators B Chem. 2015, 211, 476–488. [Google Scholar] [CrossRef]
- Ghadimi, H.; Tehrani, R.M.A.; Basirun, W.J.; Ab Aziz, N.J.; Mohamed, N.; Ab Ghani, S. Electrochemical determination of aspirin and caffeine at MWCNTs-poly-4-vinylpyridine composite modified electrode. J. Taiwan Inst. Chem. Eng. 2016, 65, 101–109. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, Y.; Li, H.; Zhang, Y.; Xia, X.; Yan, Q. Frontal polymerization and characterization of interpenetrating polymer networks composed of poly(N-isopropylacrylamide) and polyvinylpyrrolidone. Colloid Polym. Sci. 2018, 296, 165–172. [Google Scholar] [CrossRef]
- Suriyanarayanan, S.; Mandal, S.; Ramanujam, K.; Nicholls, I.A. Electrochemically synthesized molecularly imprinted polythiophene nanostructures as recognition elements for an aspirin-chemosensor. Sens. Actuators B Chem. 2017, 253, 428–436. [Google Scholar] [CrossRef]
- Puangjan, A.; Chaiyasith, S.; Wichitpanya, S.; Daengduang, S.; Puttota, S. Electrochemical sensor based on PANI/MnO2-Sb2O3 nanocomposite for selective simultaneous voltammetric determination of ascorbic acid and acetylsalicylic acid. J. Electroanal. Chem. 2016, 782, 192–201. [Google Scholar] [CrossRef]
- Deiminiat, B.; Razavipanah, I.; Rounaghi, G.H.; Arbab-Zavar, M.H. A novel electrochemical imprinted sensor for acetylsalicylic acid based on polypyrrole, sol-gel and SiO2@Au core-shell nanoparticles. Sens. Actuators B Chem. 2017, 244, 785–795. [Google Scholar] [CrossRef]
- Huxtable, R.J. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huxtable, R.J.; Sebring, L.A. Towards a unifying theory for the actions of taurine. Trends Pharmacol. Sci. 1986, 7, 481–485. [Google Scholar] [CrossRef]
- Kupis-Rozmysłowicz, J.; Wagner, M.; Bobacka, J.; Lewenstam, A.; Migdalski, J. Biomimetic membranes based on molecularly imprinted conducting polymers as a sensing element for determination of taurine. Electrochim. Acta 2016, 188, 537–544. [Google Scholar] [CrossRef]
- Brogden, R.N.; Pinder, R.M.; Sawyer, P.R.; Speight, T.M.; Avery, G.S. Naproxen. Drugs 1975, 9, 326–363. [Google Scholar] [CrossRef]
- Eslami, M.R.; Alizadeh, N. Nanostructured conducting molecularly imprinted polypyrrole based quartz crystal microbalance sensor for naproxen determination and its electrochemical impedance study. RSC Adv. 2016, 6, 9387–9395. [Google Scholar] [CrossRef]
- Green, A.R.; Cross, A.J.; Goodwin, G.M. Review of the pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA or “Ecstasy”). Psychopharmacology 1995, 119, 247–260. [Google Scholar] [CrossRef]
- Couto, R.A.; Costa, S.S.; Mounssef, B.; Pacheco, J.G.; Fernandes, E.; Carvalho, F.; Rodrigues, C.M.; Delerue-Matos, C.; Braga, A.A.C.; Gonçalves, L.M.; et al. Electrochemical sensing of ecstasy with electropolymerized molecularly imprinted poly(o-phenylenediamine) polymer on the surface of disposable screen-printed carbon electrodes. Sens. Actuators B Chem. 2019, 290, 378–386. [Google Scholar] [CrossRef]
- Rothgery, E.F. Hydrazine and Its Derivatives. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2004; ISBN 9780471238966. [Google Scholar]
- Abbaspour, A.; Kamyabi, M.A. Electrocatalytic oxidation of hydrazine on a carbon paste electrode modified by hybrid hexacyanoferrates of copper and cobalt films. J. Electroanal. Chem. 2005, 576, 73–83. [Google Scholar] [CrossRef]
- Blaza, J.N.; Bridges, H.R.; Aragão, D.; Dunn, E.A.; Heikal, A.; Cook, G.M.; Nakatani, Y.; Hirst, J. The mechanism of catalysis by type-II NADH:quinone oxidoreductases. Sci. Rep. 2017, 7, 40165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rębiś, T.; Sobkowiak, M.; Milczarek, G. Electrocatalytic oxidation and detection of hydrazine at conducting polymer/lignosulfonate composite modified electrodes. J. Electroanal. Chem. 2016, 780, 257–263. [Google Scholar] [CrossRef]
- Afshari, M.; Dinari, M.; Momeni, M.M. The graphitic carbon nitride/polyaniline/silver nanocomposites as a potential electrocatalyst for hydrazine detection. J. Electroanal. Chem. 2019, 833, 9–16. [Google Scholar] [CrossRef]
- Zhang, T.; Asefa, T. Copper nanoparticles/polyaniline-derived mesoporous carbon electrocatalysts for hydrazine oxidation. Front. Chem. Sci. Eng. 2018, 12, 329–338. [Google Scholar] [CrossRef]
- Lashkenari, M.S.; Shahrokhi, B.; Ghorbani, M.; Falah, J.; Rostami, H. Polyrhodanine/NiFe2 O4 nanocomposite: A novel electrocatalyst for hydrazine oxidation reaction. Int. J. Hydrog. Energy 2018, 43, 11244–11252. [Google Scholar] [CrossRef]
- Ramaraj, S. A novel and Disposable Amperometric Hydrazine Sensor based on Polydimethyldiallylamine Stabilized Copper(II)hexacyanoferrate Nanocubes modified Screen- printed Carbon Electrode. Int. J. Electrochem. Sci. 2017, 12, 5567–5580. [Google Scholar] [CrossRef]
- Beduk, T.; Bihar, E.; Surya, S.G.; Castillo, A.N.; Inal, S.; Salama, K.N. A paper-based inkjet-printed PEDOT:PSS/ZnO sol-gel hydrazine sensor. Sens. Actuators B Chem. 2020, 306, 127539. [Google Scholar] [CrossRef]
- Faisal, M.; Harraz, F.A.; Al-Salami, A.; Al-Sayari, S.; Al-Hajry, A.; Al-Assiri, M. Polythiophene/ZnO nanocomposite-modified glassy carbon electrode as efficient electrochemical hydrazine sensor. Mater. Chem. Phys. 2018, 214, 126–134. [Google Scholar] [CrossRef]
- Xu, F.; Xie, S.; Liu, Y.; Wang, L. Electrochemical preparation of a three dimensional PEDOT–Cu x O hybrid for enhanced oxidation and sensitive detection of hydrazine. Anal. Methods 2016, 8, 316–325. [Google Scholar] [CrossRef]
- Karakaya, S. Development of an amperometric hydrazine sensor at a disposable poly(alizarin red S) modified pencil graphite electrode. Mon. Chem. Chem. Mon. 2019, 150, 1911–1920. [Google Scholar] [CrossRef]
- Peng, H.; Liang, C. Electrochemical determination of hydrazine based on polydopamine-reduced graphene oxide nanocomposite. Full Nanotub. Carbon Nanostruct. 2016, 25, 29–33. [Google Scholar] [CrossRef]
- Dai, J.; Deng, D.; Yuan, Y.; Zhang, J.; Deng, F.; He, S. Amperometric nitrite sensor based on a glassy carbon electrode modified with multi-walled carbon nanotubes and poly(toluidine blue). Microchim. Acta 2016, 183, 1553–1561. [Google Scholar] [CrossRef]
- Hui, N.; Chai, F.; Lin, P.; Song, Z.; Sun, X.; Li, Y.; Niu, S.; Luo, X. Electrodeposited Conducting Polyaniline Nanowire Arrays Aligned on Carbon Nanotubes Network for High Performance Supercapacitors and Sensors. Electrochim. Acta 2016, 199, 234–241. [Google Scholar] [CrossRef]
- Kannan, A.; Sivanesan, A.; Kalaivani, G.; Manivel, A.; Sevvel, R. A highly selective and simultaneous determination of ascorbic acid, uric acid and nitrite based on a novel poly-N-acetyl-l-methionine (poly-NALM) thin film. RSC Adv. 2016, 6, 96898–96907. [Google Scholar] [CrossRef]
- Dağcı, K.; Alanyalıoğlu, M. Preparation of Free-Standing and Flexible Graphene/Ag Nanoparticles/Poly(pyronin Y) Hybrid Paper Electrode for Amperometric Determination of Nitrite. ACS Appl. Mater. Interfaces 2016, 8, 2713–2722. [Google Scholar] [CrossRef]
- Liu, W.; Gu, Y.; Sun, G.; Na, K.; Li, C.; Tang, L.; Zhang, Z.; Yang, M. Poly(diallydimethylammonium chloride) Functionalized Graphene/Double-walled Carbon Nanotube Composite for Amperometric Determination of Nitrite. Electroanalysis 2016, 28, 484–492. [Google Scholar] [CrossRef]
- Jiao, M.; Li, Z.; Li, Y.; Cui, M.; Luo, X. Poly(3,4-ethylenedioxythiophene) doped with engineered carbon quantum dots for enhanced amperometric detection of nitrite. Microchim. Acta 2018, 185, 249. [Google Scholar] [CrossRef]
- Wang, G.; Han, R.; Feng, X.; Li, Y.; Lin, J.; Luo, X. A glassy carbon electrode modified with poly(3,4-ethylenedioxythiophene) doped with nano-sized hydroxyapatite for amperometric determination of nitrite. Microchim. Acta 2017, 1, 1721–1727. [Google Scholar] [CrossRef]
- Ge, Y.; Jamal, R.; Zhang, R.; Zhang, W.; Yu, Z.; Yan, Y.; Liu, Y.; Abdiryim, T. Electrochemical synthesis of multilayered PEDOT/PEDOT-SH/Au nanocomposites for electrochemical sensing of nitrite. Microchim. Acta 2020, 187, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Zhang, Z.; Jiao, J.; Ma, H.; Zhang, D.; Ma, H. Sensitive and selective nitrite sensor based on phosphovanadomolybdates H6[PMo9V3O40], poly(3,4-ethylenedioxythiophene) and Au nanoparticles. Sens. Actuators B Chem. 2016, 236, 418–424. [Google Scholar] [CrossRef]
- Shi, S. Electrochemically Co-Deposition of Palladium Nanoparticles and Poly(1, 5-diaminonaphthalene) onto Multiwalled Carbon Nanotubes (MWCNTs) Modified Electrode and its Application for Amperometric Determination of Nitrite. Int. J. Electrochem. Sci. 2019, 14, 7983–7994. [Google Scholar] [CrossRef]
- Wang, J.; Hui, N. A nanocomposite consisting of flower-like cobalt nanostructures, graphene oxide and polypyrrole for amperometric sensing of nitrite. Microchim. Acta 2017, 184, 2411–2418. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Amirian, P.; Mahvi, A.H.; Ansari-Moghaddam, A. Application of adsorption process for phenolic compounds removal from aqueous environments: A systematic review. Glob. Nest J. 2016, 18, 146–163. [Google Scholar]
- Abay, I.; Denizli, A.; Bişkin, E.; Salih, B. Removal and pre-concentration of phenolic species onto β-cyclodextrin modified poly(hydroxyethylmethacrylate–ethyleneglycoldimethacrylate) microbeads. Chemosphere 2005, 61, 1263–1272. [Google Scholar] [CrossRef]
- Bi, W.; Wang, M.; Yang, X.; Row, K.H. Facile synthesis of poly(ionic liquid)-bonded magnetic nanospheres as a high-performance sorbent for the pretreatment and determination of phenolic compounds in water samples. J. Sep. Sci. 2014, 37, 1632–1639. [Google Scholar] [CrossRef]
- Goswami, B.; Mahanta, D. Polyaniline coated nickel oxide nanoparticles for the removal of phenolic compounds: Equilibrium, kinetics and thermodynamic studies. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123843. [Google Scholar] [CrossRef]
- Khan, N.; Anwer, A.H.; Ahmad, A.; Sabir, S.; Sevda, S.; Khan, M.D. Investigation of CNT/PPy-Modified Carbon Paper Electrodes under Anaerobic and Aerobic Conditions for Phenol Bioremediation in Microbial Fuel Cells. ACS Omega 2019, 5, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Ozoner, S.K.; Yilmaz, F.; Celik, A.; Keskinler, B.; Erhan, E. A novel poly(glycine methacrylate-co-3-thienylmethyl methacrylate)-polypyrrole-carbon nanotube-horseradish peroxidase composite film electrode for the detection of phenolic compounds. Curr. Appl. Phys. 2011, 11, 402–408. [Google Scholar] [CrossRef]
- Khor, S.; Lee, Y.; Ahmad, M.; Karuppiah, N.; Sidek, H.; Abdullah, J. Poly(hydroxyl ethyl methacrylate) hydrogel matrix for phenol biosensor. In Proceedings of the Asian Conference on Sensors, Kebangsann, Malaysia, 18 July 2003; pp. 207–211. [Google Scholar]
- Wei, T.; Huang, X.; Zeng, Q.; Wang, L. Simultaneous electrochemical determination of nitrophenol isomers with the polyfurfural film modified glassy carbon electrode. J. Electroanal. Chem. 2015, 743, 105–111. [Google Scholar] [CrossRef]
- Zheng, H.; Yan, Z.; Wang, M.; Chen, J.; Zhang, X. Biosensor based on polyaniline-polyacrylonitrile-graphene hybrid assemblies for the determination of phenolic compounds in water samples. J. Hazard. Mater. 2019, 378, 120714. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Qin, W.; Shi, L. A Electrochemical Sensor based on Poly (Sulfosalicylic Acid) Film Modified Electrode and Application to Phenol Detection in Oilfield Wastewater. Int. J. Smart Home 2016, 10, 299–308. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, L.; Wen, Y.; Wang, Z.; Zhang, H.; Hu, D.; Xu, J.; Duan, X. Voltammetric determination of catechin using single-walled carbon nanotubes/poly(hydroxymethylated-3,4-ethylenedioxythiophene) composite modified electrode. Ionics 2015, 21, 2927–2936. [Google Scholar] [CrossRef]
- Tian, F.; Li, M.; Li, M.; Li, C.; Lei, Y.; Yang, B. Synthesis of one-dimensional poly(3,4-ethylenedioxythiophene)-graphene composites for the simultaneous detection of hydroquinone, catechol, resorcinol, and nitrite. Synth. Met. 2017, 226, 148–156. [Google Scholar] [CrossRef]
- Kuskur, C.M.; Swamy, B.K.; Jayadevappa, H. Poly (naphthol green B) modified carbon paste electrode sensor for catechol and hydroquinone. J. Electroanal. Chem. 2017, 804, 99–106. [Google Scholar] [CrossRef]
- Aravindan, N.; Preethi, S.; Sangaranarayanan, M.V. Non-Enzymatic Selective Determination of Catechol Using Copper Microparticles Modified Polypyrrole Coated Glassy Carbon Electrodes. J. Electrochem. Soc. 2017, 164, B274–B284. [Google Scholar] [CrossRef]
- Guo, Z.; Florea, A.; Cristea, C.; Bessueille, F.; Vocanson, F.; Goutaland, F.; Zhang, A.; Săndulescu, R.; Lagarde, F.; Jaffrezic-Renault, N. 1,3,5-Trinitrotoluene detection by a molecularly imprinted polymer sensor based on electropolymerization of a microporous-metal-organic framework. Sens. Actuators B Chem. 2015, 207, 960–966. [Google Scholar] [CrossRef]
- Giribabu, K.; Haldorai, Y.; Rethinasabapathy, M.; Jang, S.-C.; Suresh, R.; Cho, W.-S.; Huh, Y.S.; Roh, C.; Huh, Y.S.; Narayanan, V. Glassy carbon electrode modified with poly(methyl orange) as an electrochemical platform for the determination of 4-nitrophenol at nanomolar levels. Curr. Appl. Phys. 2017, 17, 1114–1119. [Google Scholar] [CrossRef]
- Marinovic, S.; Mudrinić, T.; Jović-Jovičić, N.; Ajduković, M.; Milutinović–Nikolić, A.; Bankovic, P.; Mojovic, Z. Non-toxic poly(vinyl alcohol)/clay composites as electrode material for detection of 4-chlorophenol and 4-nitrophenol. J. Electroanal. Chem. 2019, 848, 113280. [Google Scholar] [CrossRef]
- Sobkowiak, M.; Rebis, T.; Milczarek, G. Electrocatalytic sensing of poly-nitroaromatic compounds on multiwalled carbon nanotubes modified with alkoxysulfonated derivative of PEDOT. Mater. Chem. Phys. 2017, 186, 108–114. [Google Scholar] [CrossRef]
- Sağlam, Ş.; Üzer, A.; Erçağ, E.; Apak, M.R. Electrochemical Determination of TNT, DNT, RDX, and HMX with Gold Nanoparticles/Poly(Carbazole-Aniline) Film–Modified Glassy Carbon Sensor Electrodes Imprinted for Molecular Recognition of Nitroaromatics and Nitramines. Anal. Chem. 2018, 90, 7364–7370. [Google Scholar] [CrossRef] [PubMed]
- Dechtrirat, D.; Yingyuad, P.; Prajongtat, P.; Chuenchom, L.; Sriprachuabwong, C.; Tuantranont, A.; Tang, I.-M. A screen-printed carbon electrode modified with gold nanoparticles, poly(3,4-ethylenedioxythiophene), poly(styrene sulfonate) and a molecular imprint for voltammetric determination of nitrofurantoin. Microchim. Acta 2018, 185, 261. [Google Scholar] [CrossRef] [PubMed]
- Sağlam, Ş.; Üzer, A.; Tekdemir, Y.; Erçağ, E.; Apak, M.R. Electrochemical sensor for nitroaromatic type energetic materials using gold nanoparticles/poly(o-phenylenediamine–aniline) film modified glassy carbon electrode. Talanta 2015, 139, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, W.; Jiang, J.; Qian, L.; Zhang, X. Nitrofuran Determination in Aquaculture Water by Using Poly-Alizarin Red-Modified Electrode. J. Electrochem. Soc. 2019, 166, H425–H432. [Google Scholar] [CrossRef]
- Yao, C.; Sun, H.; Fu, H.-F.; Tan, Z.-C. Sensitive simultaneous determination of nitrophenol isomers at poly(p-aminobenzene sulfonic acid) film modified graphite electrode. Electrochim. Acta 2015, 156, 163–170. [Google Scholar] [CrossRef]
- Chiu, S.-H.; Su, Y.-L.; Le, A.V.T.; Cheng, S.-H. Nanocarbon material-supported conducting poly(melamine) nanoparticle-modified screen-printed carbon electrodes for highly sensitive determination of nitrofuran drugs by adsorptive stripping voltammetry. Anal. Bioanal. Chem. 2018, 410, 6573–6583. [Google Scholar] [CrossRef]
- Calam, T.T. Electrochemical Oxidative Determination and Electrochemical Behavior of 4-Nitrophenol Based on an Au Electrode Modified with Electro-polymerized 3,5-Diamino-1,2,4-triazole Film. Electroanalysis 2020, 32, 149–158. [Google Scholar] [CrossRef]
- Arulraj, A.D.; Vijayan, M.; Vasantha, V.S. Highly selective and sensitive simple sensor based on electrochemically treated nano polypyrrole-sodium dodecyl sulphate film for the detection of para-nitrophenol. Anal. Chim. Acta 2015, 899, 66–74. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Scherf, U. Electrogenerated Thin Films of Microporous Polymer Networks with Remarkably Increased Electrochemical Response to Nitroaromatic Analytes. ACS Appl. Mater. Interfaces 2015, 7, 11127–11133. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Preis, E.; Scherf, U. Silicon- or Carbon-Cored Multifunctional Carbazolyl Monomers for the Electrochemical Generation of Microporous Polymer Films. Macromolecules 2016, 49, 8041–8047. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Brunklaus, G.; Scherf, U. Thiophene-Based Microporous Polymer Networks via Chemical or Electrochemical Oxidative Coupling. Macromolecules 2015, 48, 6816–6824. [Google Scholar] [CrossRef] [Green Version]
- Palma-Cando, A.; Rendón-Enríquez, I.; Tausch, M.W.; Scherf, U. Thin Functional Polymer Films by Electropolymerization. Nanomaterials 2019, 9, 1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, S.; Hu, Q.; Zeng, Q.; Wang, M.; Wang, L. Variations in Surface Morphologies, Properties, and Electrochemical Responses to Nitro-Analyte by Controlled Electropolymerization of Thiophene Derivatives. ACS Appl. Mater. Interfaces 2018, 10, 11319–11327. [Google Scholar] [CrossRef]
- Ponnappa, S.P.; MacLeod, J.; Umer, M.; Soda, N.; Pannu, A.S.; Shiddiky, M.J.; Ayoko, G.A.; O’Mullane, A.P.; Sonar, P. Electropolymerized Porous Polymer Films on Flexible Indium Tin Oxide Using Trifunctional Furan Substituted Benzene Conjugated Monomer for Biosensing. ACS Appl. Polym. Mater. 2020, 2, 351–359. [Google Scholar] [CrossRef]
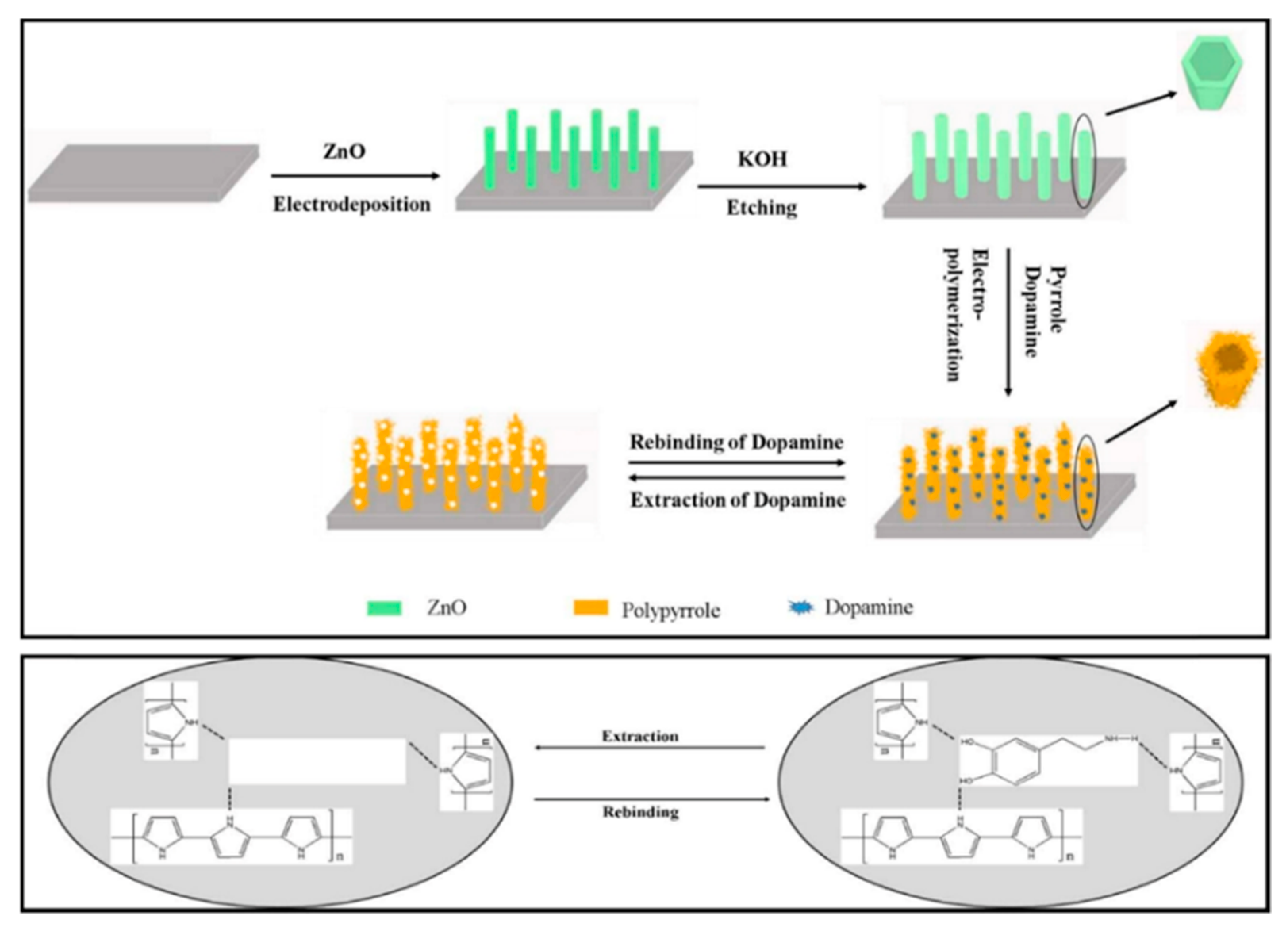
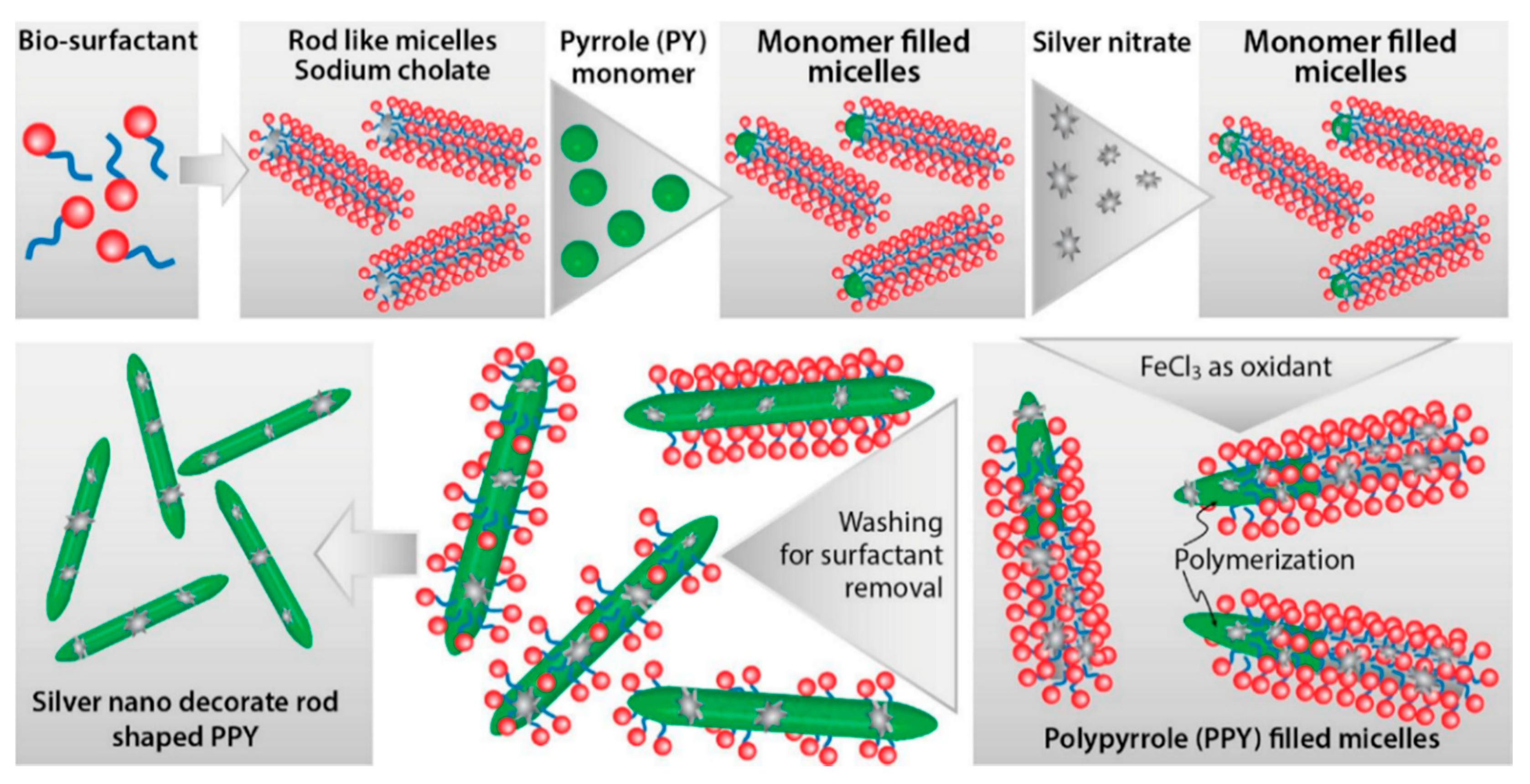
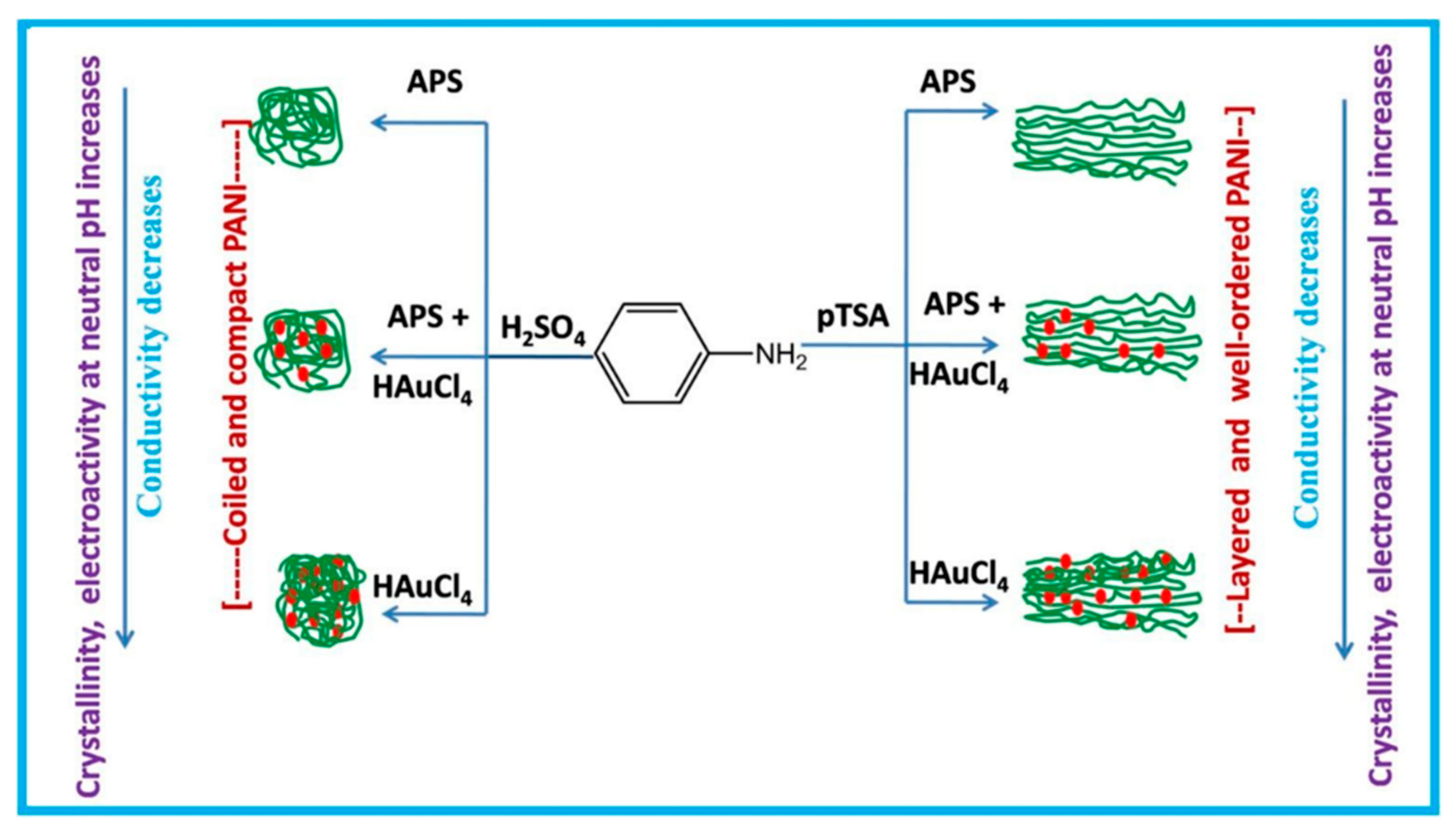

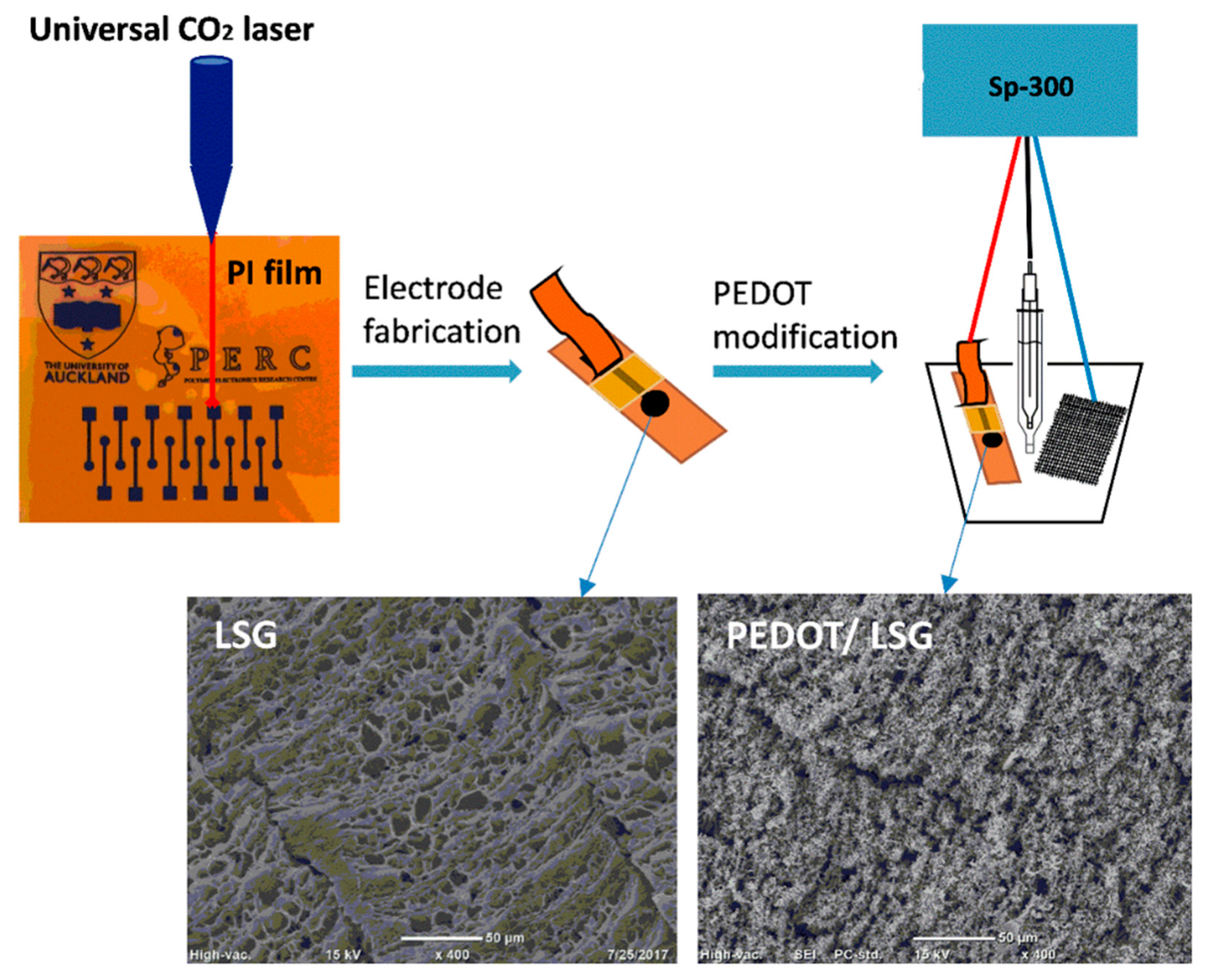
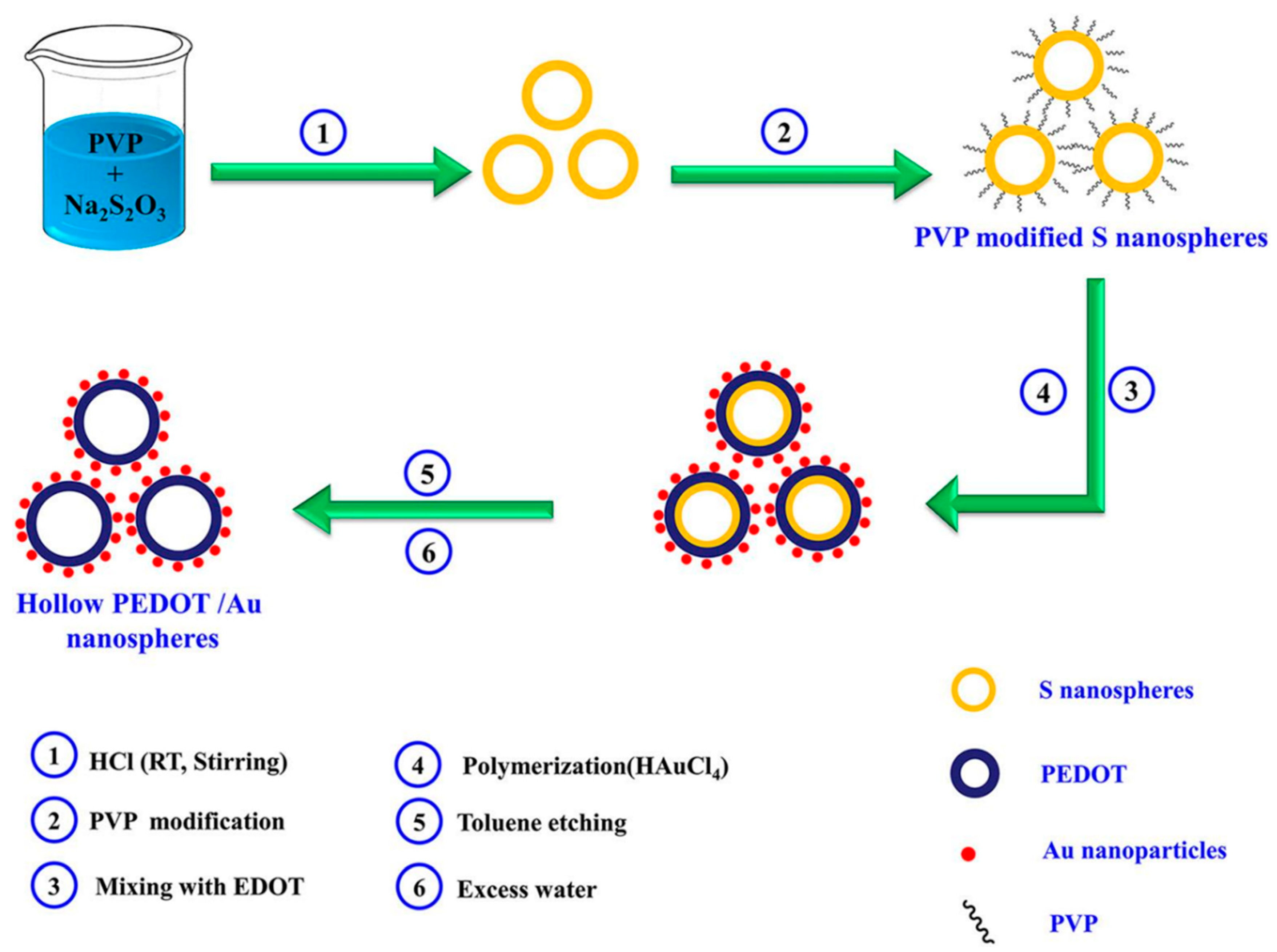
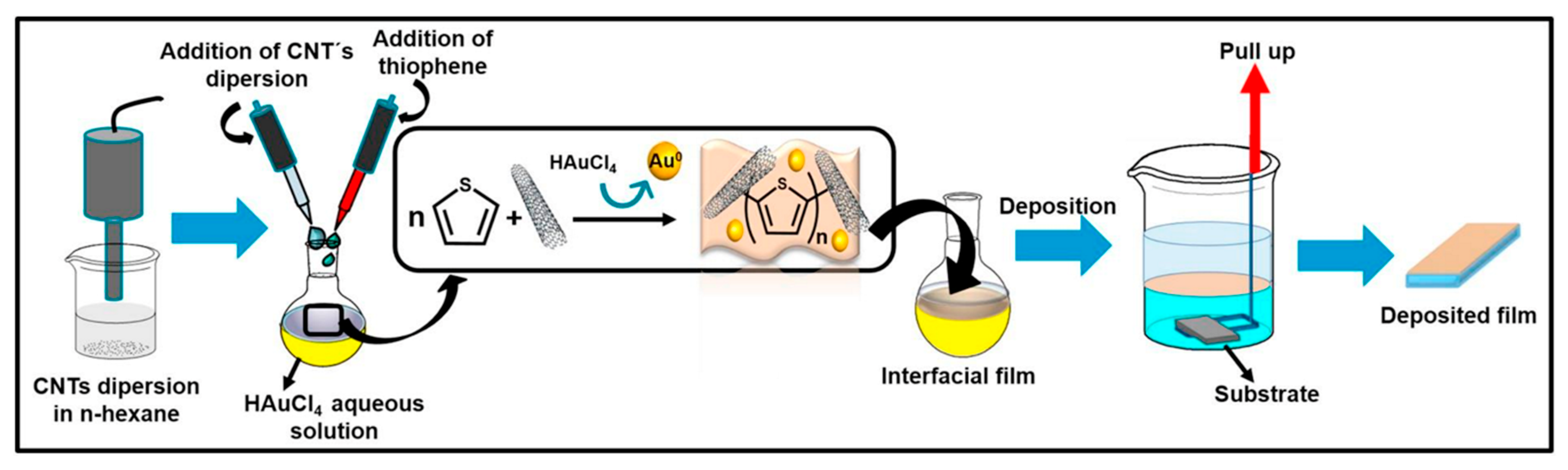

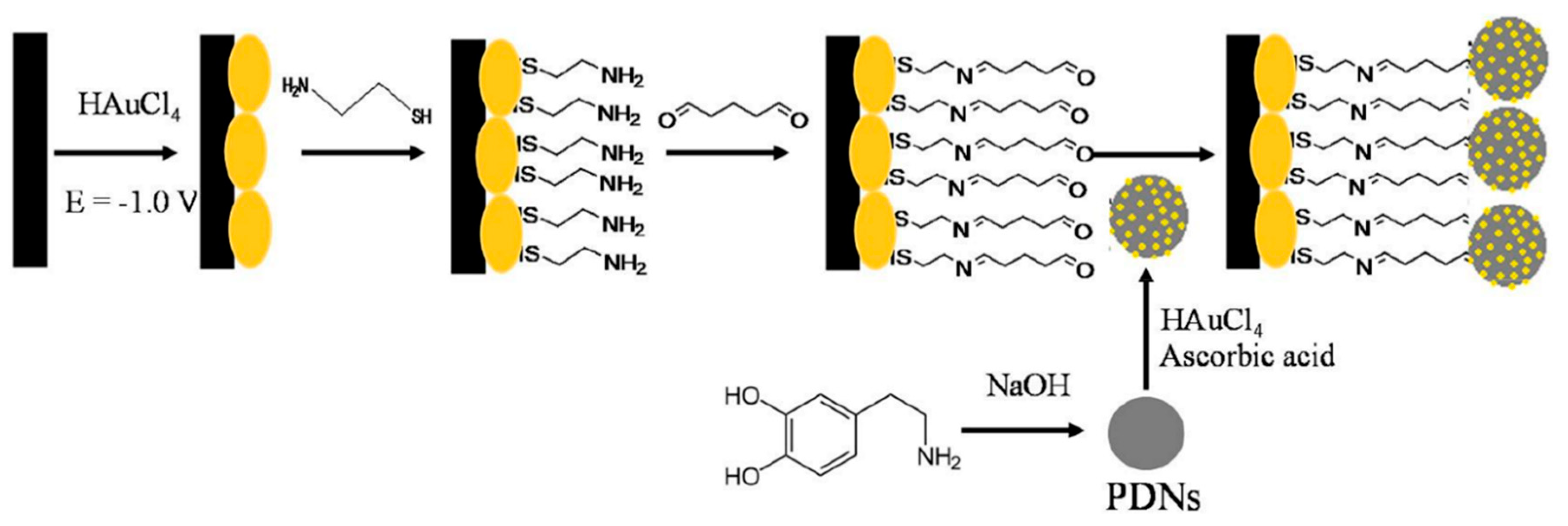

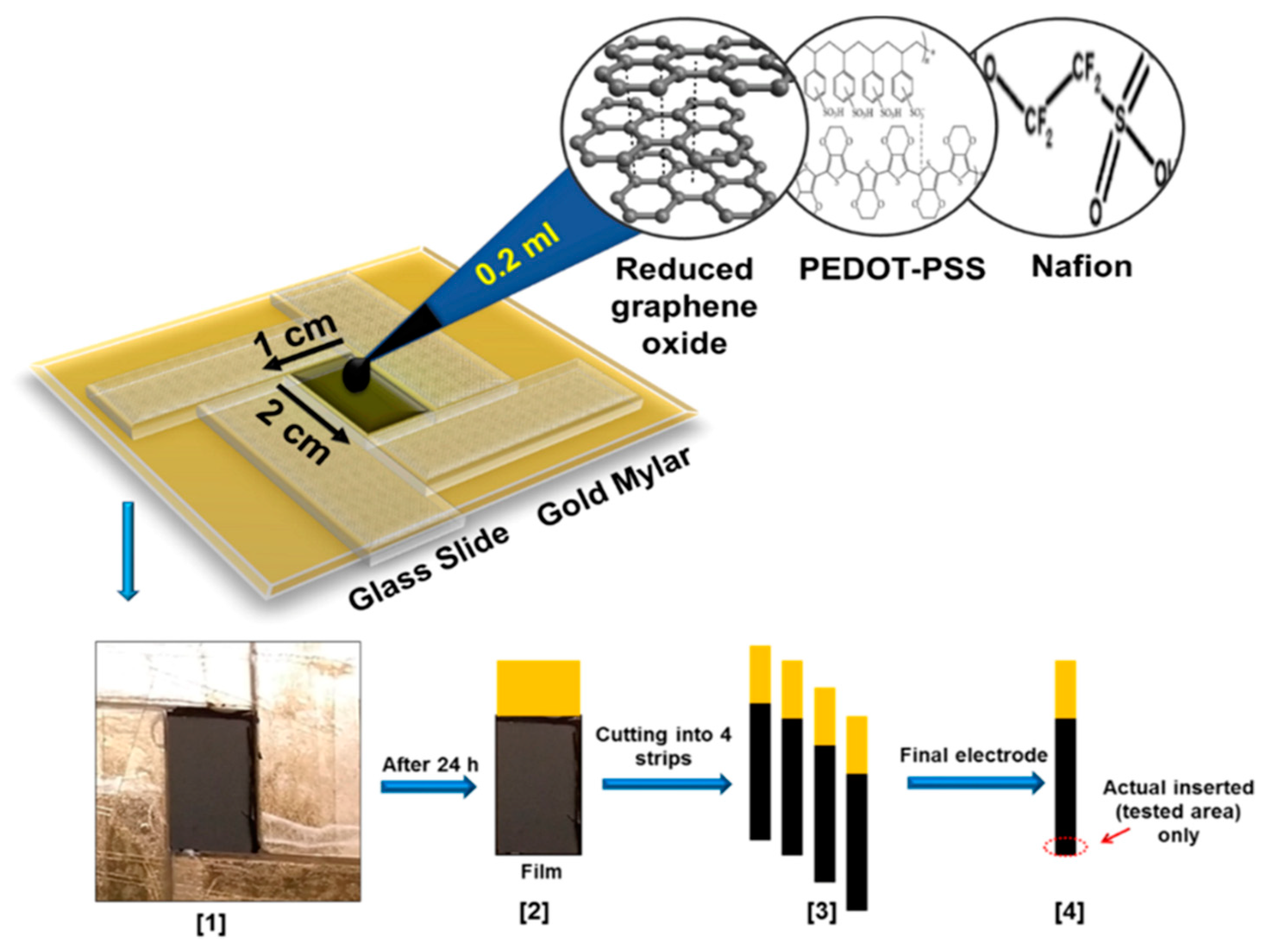
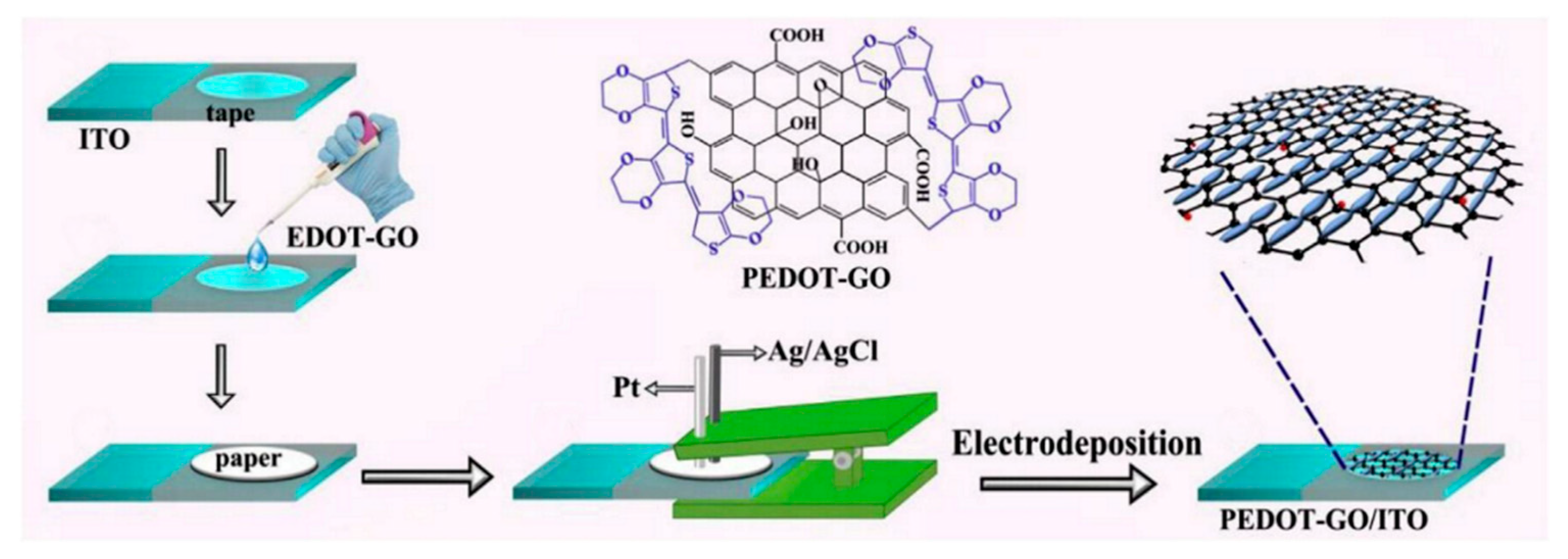
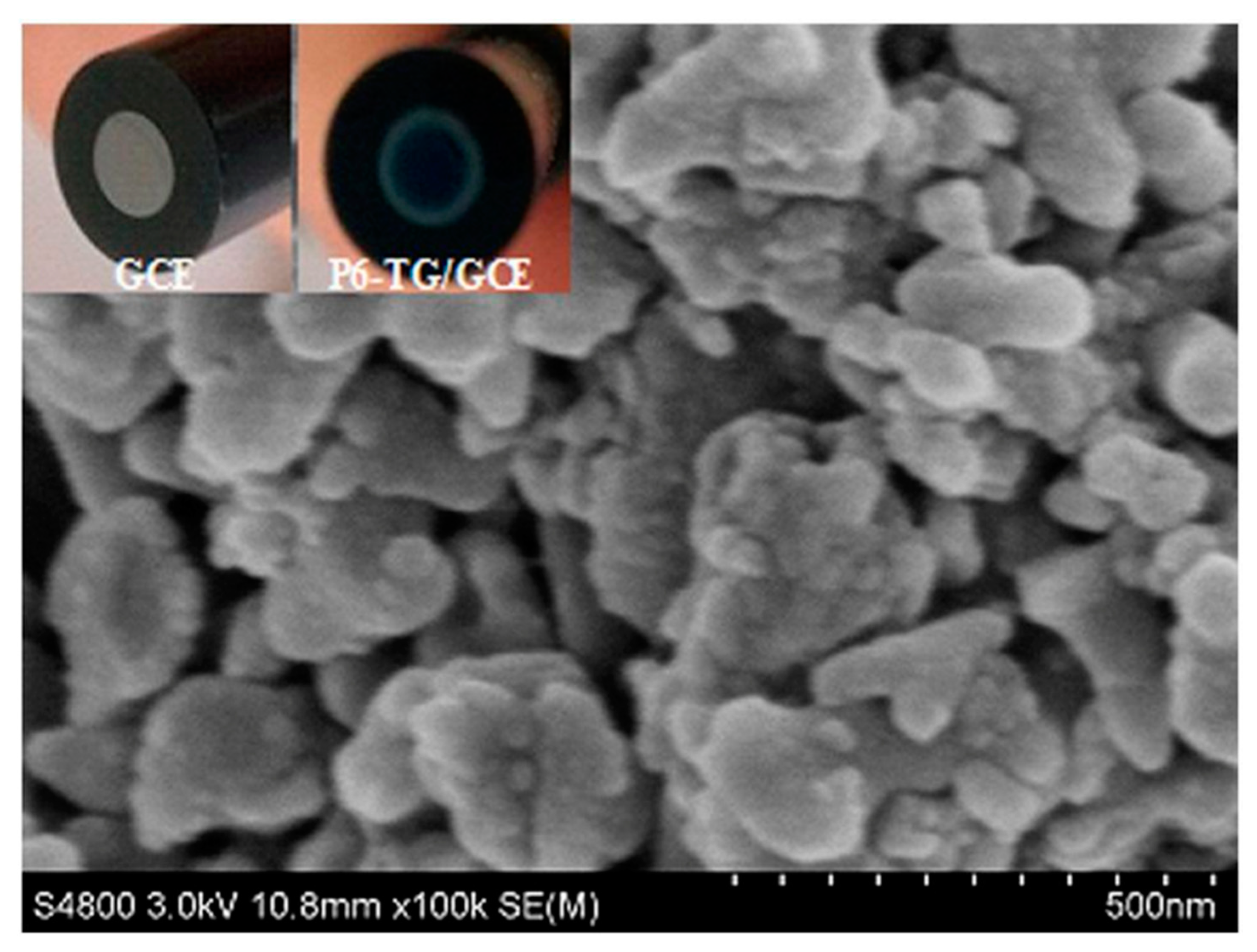
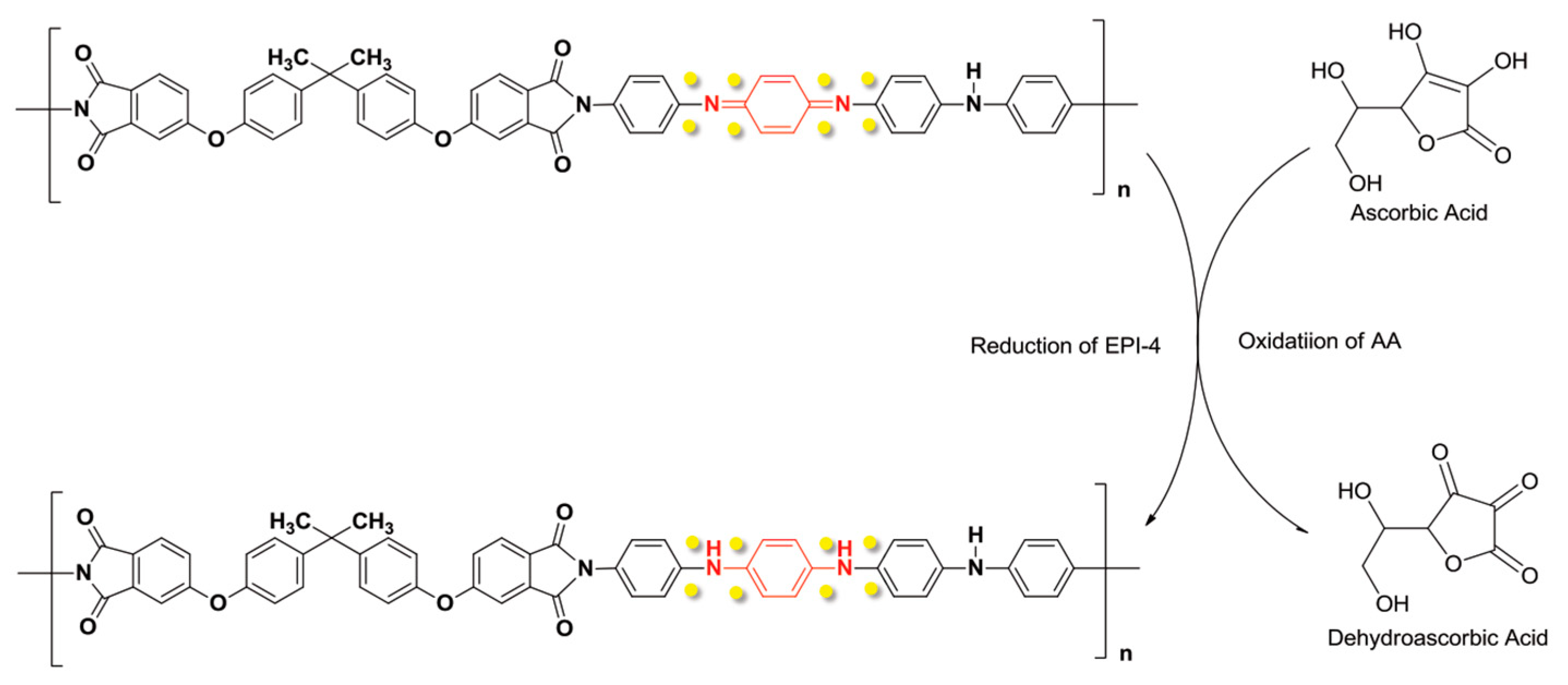
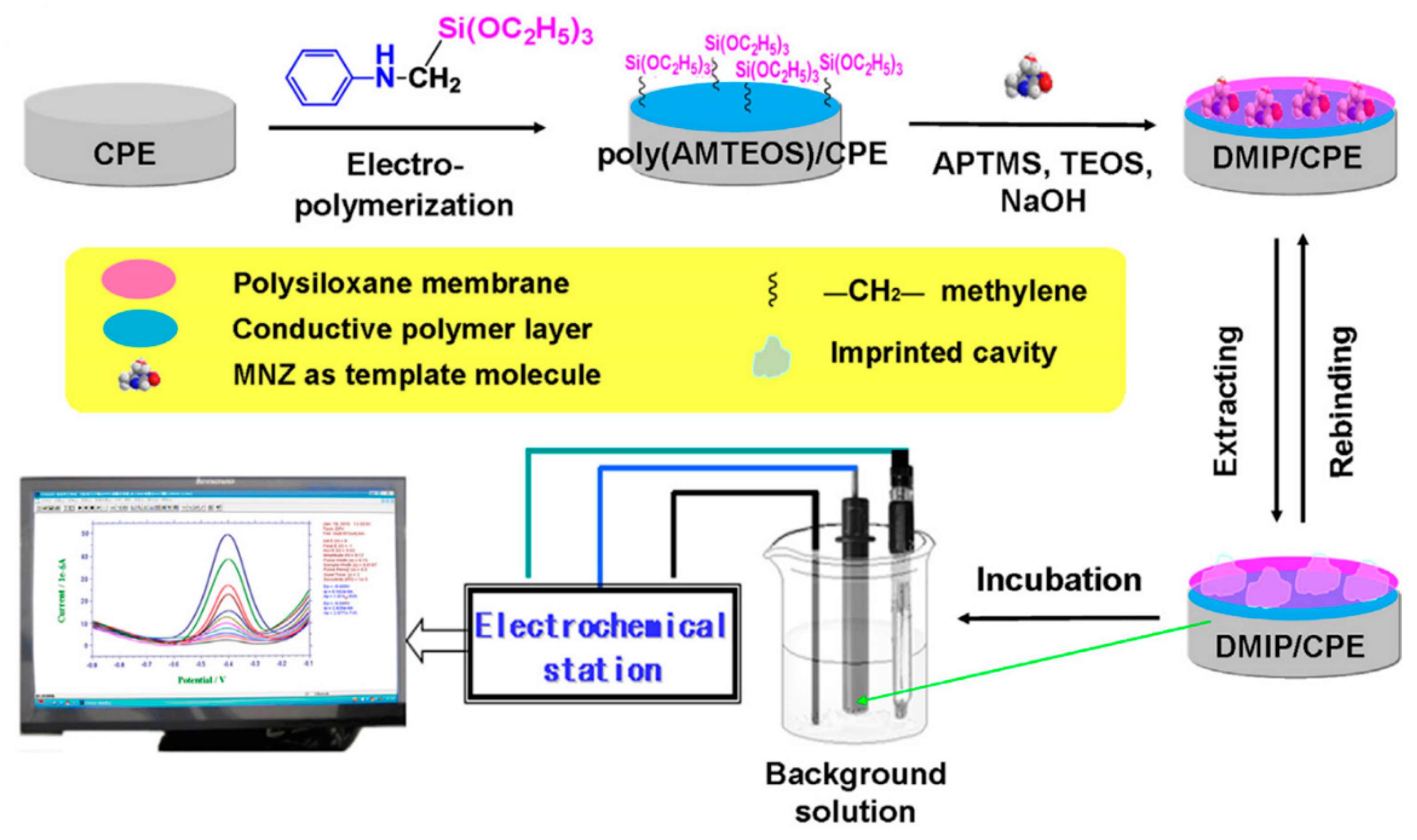

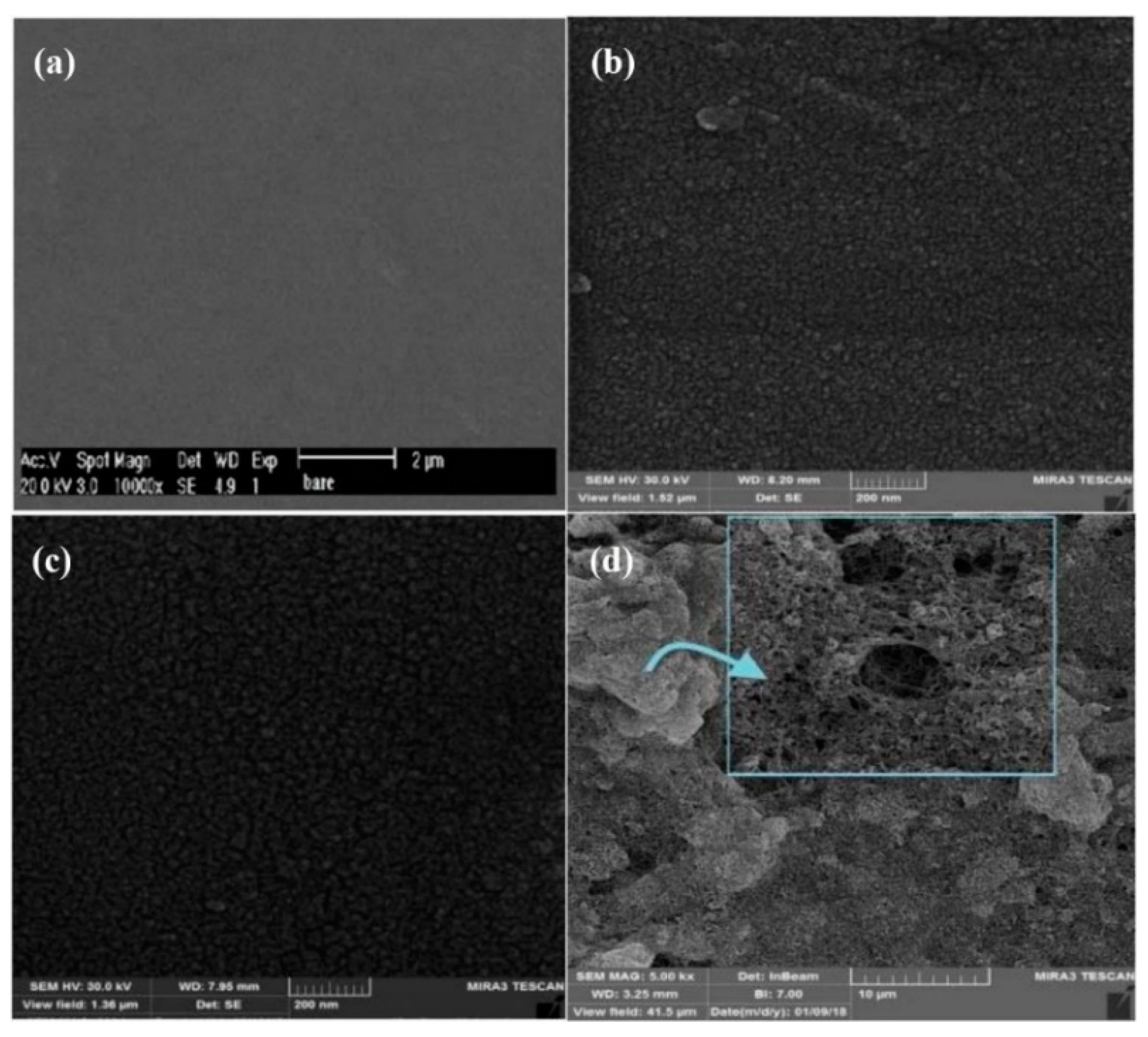
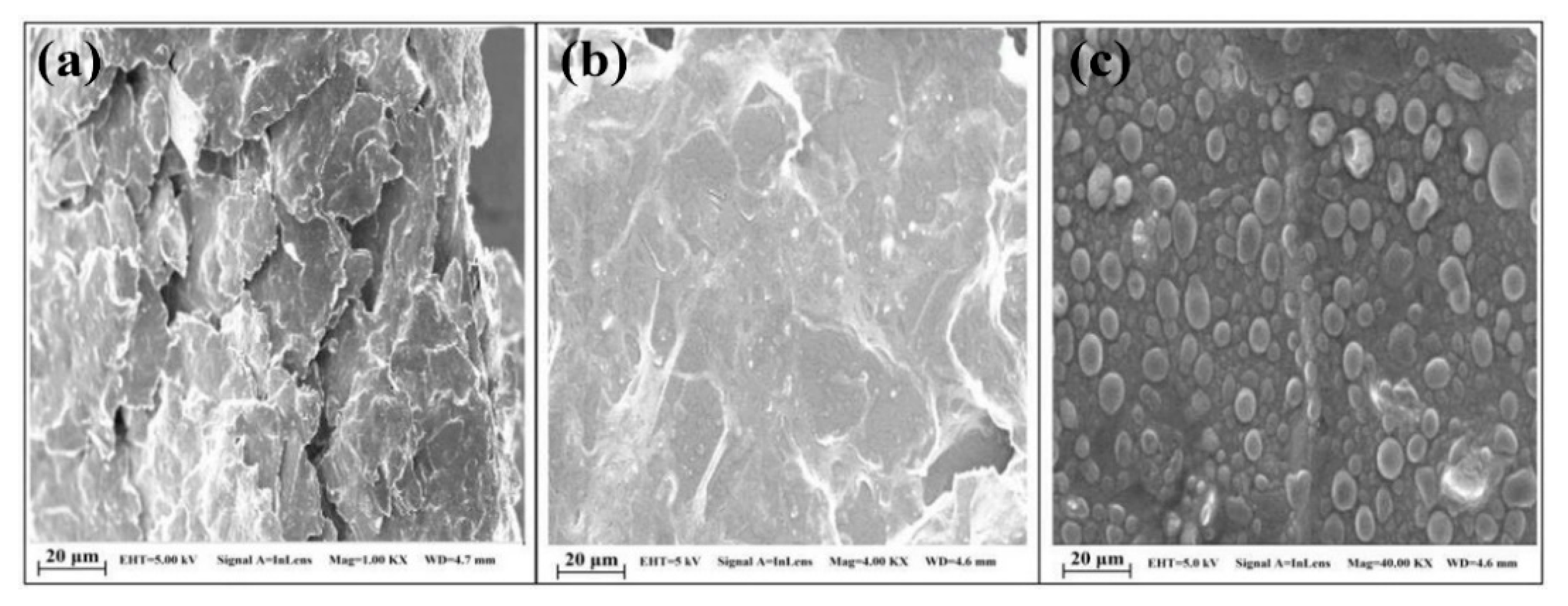
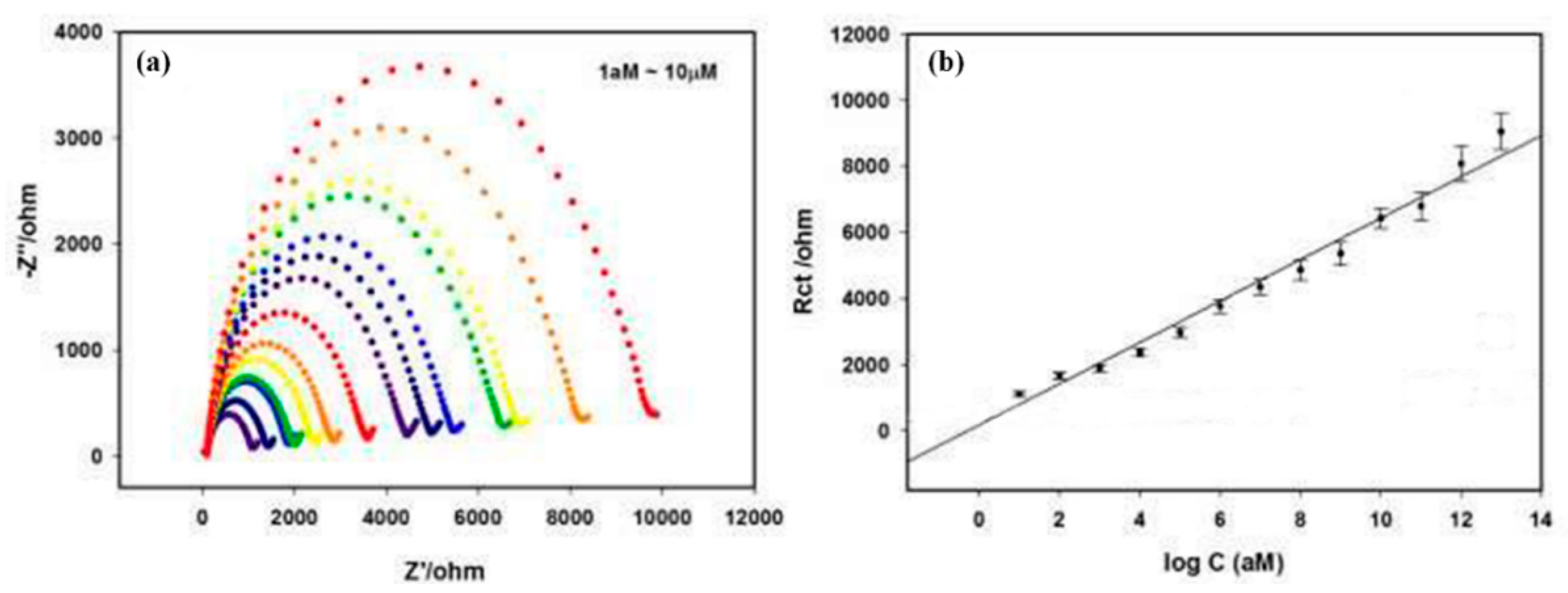

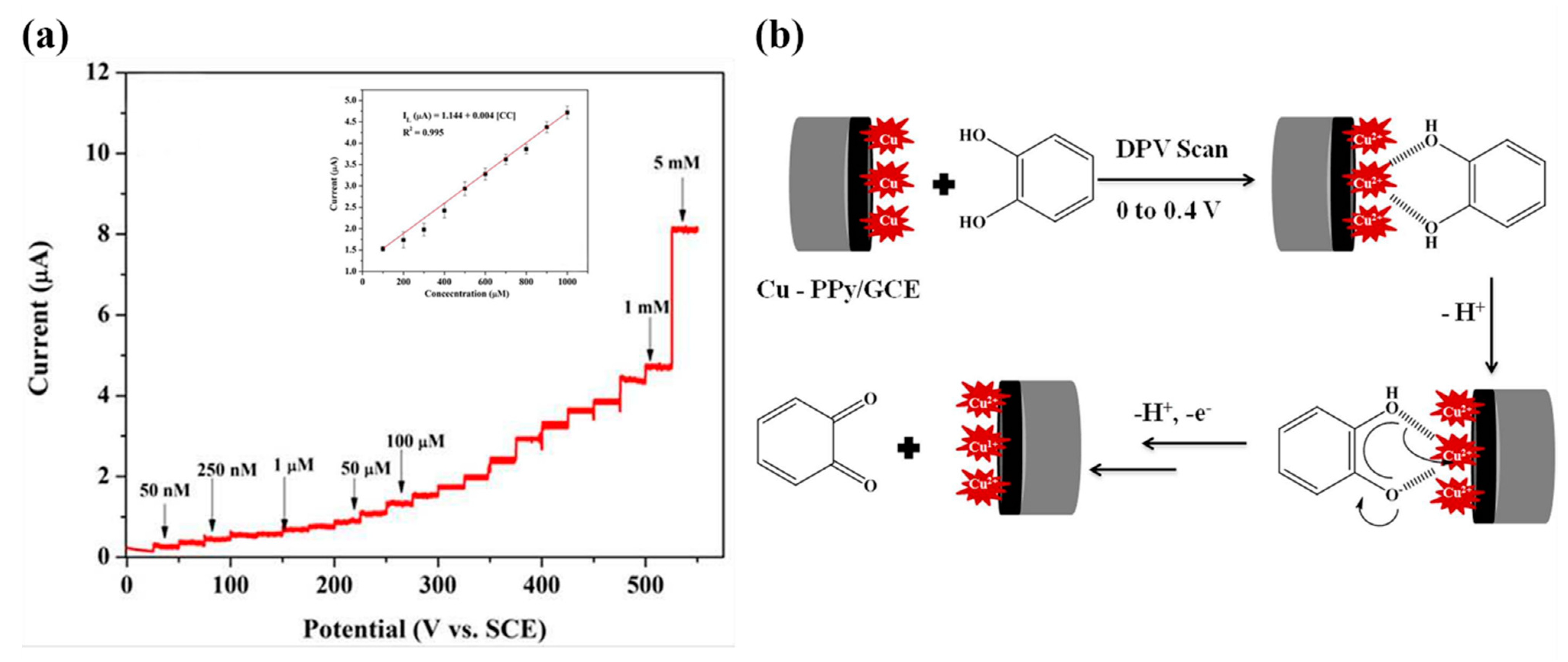
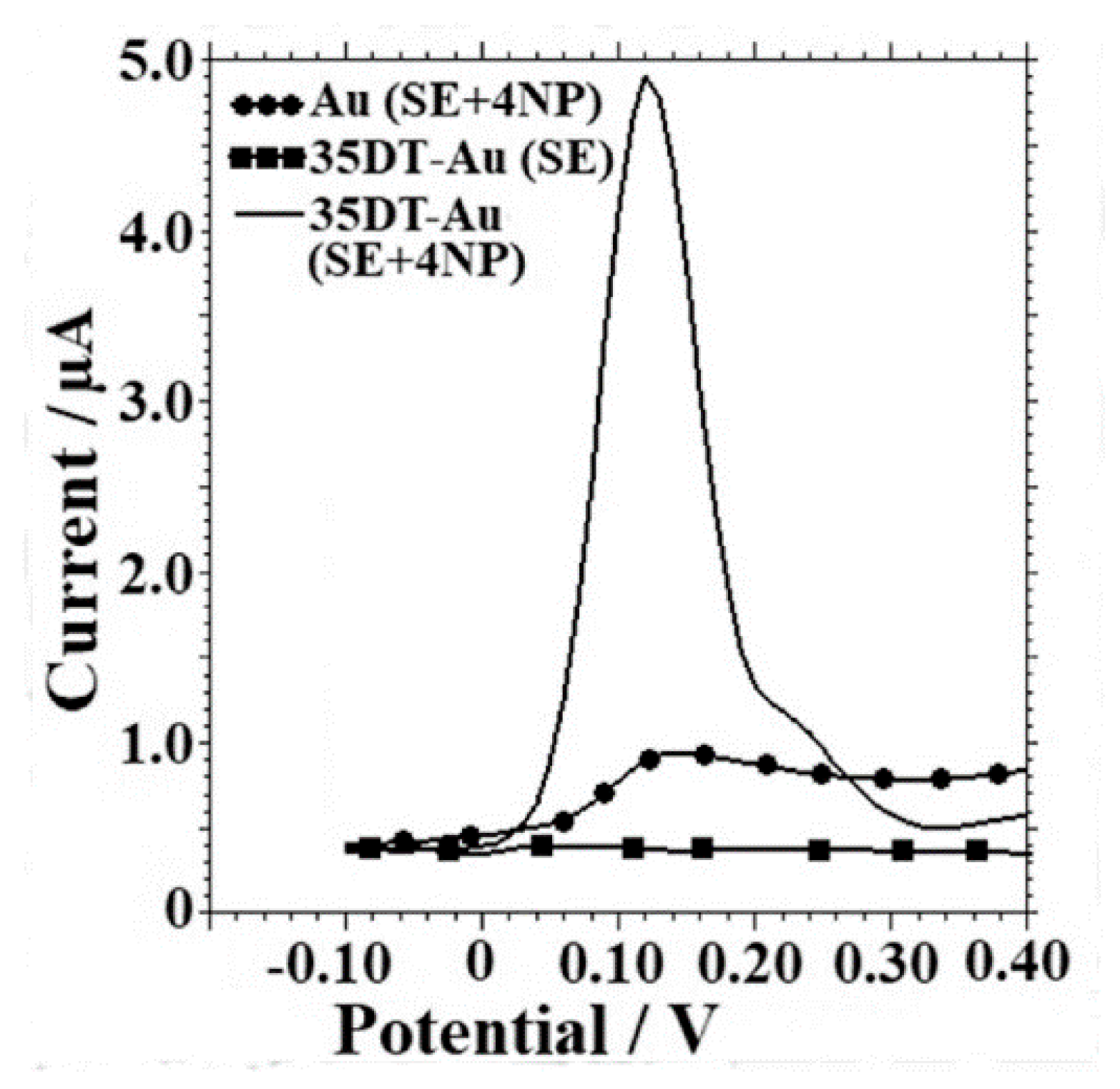
| Electrode Architecture | Conducting Polymer | Synthesis Method | Analytes | Detection Technique | LOD (µM) | Linear Range (µM) | Ref. |
|---|---|---|---|---|---|---|---|
| Dopamine | |||||||
| (PPY)-Ag | Polypyrrole | Self-assembled/liquid phase | DA | LSV | 0.00005 | 0.00005 to 0.003 | [93] |
| AuNP-GP-PEDOT:PSS/GCE | PEDOT:PSS | Self-assembled/liquid phase | DA | DPV | 0.0001 | 0.001 to 300 | [119] |
| Au-PDNs | Polydopamine | Self-assembled/liquid phase | DA, UA, AA, tryptophan | DPV | 0.0001 | 1 to 160 | [128] |
| OPPy/SDS-CNT | Polypyrrole | CA | DA | DPV | 0.000136 | 0.005 to 0.010 | [92] |
| GN/PoP | Poly (o-phenylenediamine) | CV | DA | SWV | 0.00016 | 0.001 to 150 | [103] |
| MWCNTs-COOH/Poly(TB)/GCE) | Poly (toluidine blue) | CV | DA | DPV | 0.00039 | 1 to 300 | [127] |
| ERGO-pEBT/AuNPs | Poly (eriochrome black T) | CV | DA, UA, AA | DPV | 0.009 | 0.5 to 20 | [126] |
| CD-f-PEDOT: PSS | PEDOT:PSS | Spin coating technique | DA, catechol | DPV | 0.009596 | 0.05 to 200 | [118] |
| Poly-β-CD(f-MWCNTs)/PANI | Polyaniline | CV | DA | DPV | 0.0164 | 2 to 24 | [99] |
| PrGO/MnO2 | Poly (3,4-ethylenedioxythiophene) | CV | DA, UA, AA | DPV | 0.02 | 0.03 to 45 | [116] |
| MWCNTs/CeO2-PEDOT | Poly (3,4-ethylenedioxythiophene) | Self-assembled/liquid phase | DA | DPV | 0.03 | 0.1 to 10 | [115] |
| AuNPs/PANI-co-PoAN/GO | Poly (aniline-co-o-anisidine) | Self-assembled/liquid phase | DA | SWV | 0.0334 | 5 to 100 | [101] |
| pHQ/AuNPs/NF | Poly (hydroquinone) | CV | DA | DPV | 0.0419 | 0.1 to 10 | [125] |
| GCE/PGBHA-afGQDs-MnO2 | Poly (glyoxal-bis(2-hydro- xyanil)) | CV | DA | DPV | 0.05 | 0.1 to 100 | [124] |
| rGo/Pd@PPy NP | Polypyrrole | Self-assembled/liquid phase | DA, UA, AA | DPV | 0.056 | 1000 to 15,000 | [89] |
| POMA-Au | Poly (o-methoxyaniline) | Self-assembled/liquid phase | DA, Folic acid | DPV | 0.062 | 10 to 300 | [100] |
| PEDOT/Au | Poly (3,4-ethylenedioxythiophene) | Self-assembled/liquid phase | DA, UA | DPV | 0.07 | 0.15 to 330 | [114] |
| CuTRZMoO4@PPy-n | Polypyrrole | Self-assembled/liquid phase | DA | DPV | 0.08 | 1 to 100 | [83] |
| OPPy/ERGO | Polypyrrole | CV | DA | DPV | 0.2 | 0.4 to 517 | [88] |
| Poly-AHMP | Poly-4-Amino-6-hydroxy-2-mercaptopyrimidine | CV | DA, Acetominphen | DPV | 0.2480 | 2.5 to 25 | [104] |
| PEDOT/LSA | Poly (3,4-ethylenedioxythiophene) | CV | DA | DPV | 0.26 | 0 to 5 | [113] |
| p-ProH/GCE) | Poly (procaterol hydrochloride) | CV | DA, UA | SWV | 0.3 | 1 to 100 | [123] |
| PEDOT/IL/GCE | Poly (3,4-ethylenedioxythiophene) | CV | DA | CV | 0.33 | 0.2 to 328 | [117] |
| PEDOT-LSG | Poly (3,4-ethylenedioxythiophene) | CA | DA | DPV | 0.33 | 1 to 150 | [112] |
| PT/Au/CNT | Polythiophene | Self-assembled/liquid phase | DA | DPV | 0.69 | 1 to 10 | [120] |
| PPy/C#SiO2 | Polypyrrole | Self-assembled/liquid phase | DA | DPV | 0.76 | 1 to 100 | [91] |
| PPy-SβCD | Polypyrrole | CA | DA | CA | 1 | Not reported | [87] |
| Poly phenol red/GCE | Poly phenol red | CV | DA, Acetaminophen | DPV | 1.6 | 20 to 160 | [122] |
| PANI-Au | Polyaniline | Self-assembled/liquid phase | DA | DPV | 5.25 | 7 to 100 | [98] |
| PS/MCPE | Poly (sudan III) | CV | DA | DPV | 9.3 | 10 to 90 | [121] |
| MIPs/ZNTs/FTO glass | Polypyrrole | CV | DA | DPV | Not reported | 0.02 to 5 | [90] |
| Epinephrine | |||||||
| EB-Ppy-BSA/GCE | Polypyrrole | Self assemble | EP, tyrosine | SWV | 0.0074 | 0.1 to 400 | [129] |
| (FA)/AuNP/GCE | poly-fuchsine acid | CV | EP, AA, UA | DPV | 0.01 | 0.5 to 792.7 | [135] |
| mpg-C3N4/PANI/CdO | Polyaniline | CA | EP, PR, CFX, mefenamic acid | DPV | 0.011 | 0.05 to 80 | [131] |
| PAPBA(MIPs)/MWCNTs | Poly (3- aminophenylboronic acid | CV | EP | DPV | 0.035 | 0.2 to 800 | [133] |
| Au/ZnO/Ppy/RGO | Polypyrrole | CA | EP, AA, UA | DPV | 0.058 | 0.6 to 500 | [130] |
| MIP/AuNP | Poly (3-Thiophene boronic acid) | CV | EP, tyrosine | DPV | 0.076 | 0.09 to 100 | [134] |
| MWCNT-PANI-TiO2 | Polyaniline | Self assemble | EP, tyrosine | DPV | 0.16 | 4.9 to 76.9 | [132] |
| PBCB/graphene/GCE | Poly (brilliant cresyl blue) | CV | EP | CV | 0.24 | 1 to 1000 | [136] |
| Serotonin | |||||||
| p(P3CA)/PGE | Poly (pyrrole-3-carboxylic acid) | CV | SER | AdSDPV | 0.0025 | 0.01 to 1 | [137] |
| AuNPs@rGO/pTBA Pd(C2H4N2S2)2) | Poly 2,2:5,2-terthiophene-3-(p- benzoic acid) | CV | SER, DA | SWV | 0.0025 | 0.02 to 20 | [142] |
| GR/p-AHNSA/SPCs | Poly 4-amino-3-hydroxy1- naphthalenesulfonic acid | CV | SER, DA | SWV | 0.003 | 0.05 to 150 | [139] |
| MWCNTs–CS–poly ( p-ABSA)/GCE | Poly (p-amino benzene sulfonic acid) | CV | SER | DPV | 0.08 | 0.1 to 100 | [138] |
| Fe3O4–MWCNT–poly (BCG | Poly (bromocresol green | CV | SER | DPV | 0.08 | 0.5 to 100 | [140] |
| rGO−PEDOT/PSS | PEDOT:PSS | Self assemble | SER | DPV | 0.1 | 1 to 10 | [141] |
| Uric Acid | |||||||
| Ox-PEDOT-nf/PGE | Poly (3,4 ethylenedioxythiophene) | CV | UA | DPV | 0.0013 | 0.01 to 20 | [147] |
| MIP/RGO | 2-amino-5-mercapto-1, 3, 4-thiadiazole | CV | UA and tyrosine | DPV | 0.0032 | 0.01 to 100 | [149] |
| PSA/ERCG/GCE | Poly (sulfosalicylic acid) | CV | UA and isoniazid | DPV | 0.012 | 0.02 to 15 | [150] |
| α-Fe2O3/PAn NTs | polyaniline | Self-assembled/liquid phase | UA | DPV | 0.038 | 0.01 to 5 | [145] |
| 6-TG/GCE | 6-thioguanine | CV | DA, UA, XA and HXA | DPV | 0.06 | 2 to 1600 | [152] |
| AuNPs/poly-TrB/GCE | Au-nanoparticles/poly-Trypan Blue | CV | UA, cysteine and tyrosine | DPV | 0.07 | 1 to 550 | [151] |
| POMANS-MWCNT/GPE | Polyortho-methoxyaniline | Self-assembled/liquid phase | UA and folic acid | LSV | 0.157 | 0.6 to 52 | [146] |
| PEDOT/GO/ITO | Poly (3,4 ethylenedioxythiophene) | Self-assembled/liquid phase | UA | DPV | 0.75 | 2 to 1000 | [148] |
| p-TPP/PPy/GO | polypyrrole | Self-assembled/liquid phase | UA | DPV | 1.15 | 5 to 200 | [144] |
| Ascorbic Acid | |||||||
| Graphite/PAMAN-CNT/p( Neutral red) | Neutral Red | CV | AA | CA | 0.053 | 0.2 -2500 | [156] |
| GCE/NiNP/CNT/PANI | PANI | CV | AA | DPV | 0.1 | 1.0 to 450 | [154] |
| GCE/PGBHA | Poly (glyoxal-bis(2-hydroxyanil)) | CV | AA | DPV | 0.26 | 1 to 8 | [159] |
| GCE/PPy@Celluloce | PPy | Homogeneous synthesis | AA | DPV | 0.75 | 10 to 50 | [153] |
| GCE/CNT-CA/PEDOT | PEDOT | CA | AA | CA | 4.2 | 0.1 to 20 mM | [158] |
| CPE/polyamic acid-AuNP | Polyamic acid derivates | Homogeneous synthesis | AA | CA | 18.5 | 10 to 1000 mM | [155] |
| GCE/PEDOT | PEDOT | CV | AA | CV | 45 | 30 to 500 mM | [157] |
| Glucose | |||||||
| GC/PEDOT-CNT-Cu2ONP | PEDOT | Homogeneous synthesis | Glucose | CA | 0.040 | 0.495 to 374 mM | [187] |
| GC/PEDOT-GO/CuNP | PEDOT | CV | Glucose | CA | 0.047 | 0.1 to 1300 | [186] |
| Pt/PANI-MMT/GS-GOx | PANI | CV | Glucose | CA | 0.1 | 10 to 1940 | [167] |
| Au/MWCNT/Pdplate/ GOx-PAB-PdNP/CS | Poly (3-anileneboronic acid) | CV, CA | Glucose | CA | 0.1 | 2 to 4500 | [168] |
| GC/PANI-NiONP | PANI | Homogeneus preparation | Glucose | CA | 0.19 | Up to 100 | [179] |
| SPCE/AuNP/pTBA-MICP | Poly (tertiophene), | CV | Glucose | Potentiometric | 0.19 | 0.32 to 1000 | [185] |
| GC/pPD/CuNP | Poly (o-phenylenediamine) | CV | Glucose | Ca | 0.25 | 5.0 to 1600 | [179] |
| Graphite/pPy/Ni(OH)2NP | pPy | CA | Glucose | CA | 0.3 | 1 to 4860 mM | [175] |
| FTO/AnB/AuNP | Poly (aniline blue) | CV | Glucose | CA | 0.4 | 0 to 50 | [178] |
| GC/PEDOT.PSS/NiNP | PEDOT-PSS | controlled potential coulometry | Glucose | CA | 0.69 | 2.5 to 1115 | [184] |
| GC/PEDOT-GO/NiNP | PEDOT | CV | Glucose | CA | 0.8 | 1 to 5100 | [183] |
| Pt/PANI-GRA/GS-GOx | PANI | CV | Glucose | CA | 2.8 | 10 to 1480 | [166] |
| GC/pPy-AgNP | pPy | Homogeneos polymerization | Glucose | CA | 3.6 | 25 to 2500 | [174] |
| GE/CNT-COOH-pAT/AuNP | Poly (2-aminothiophenol) | chemical polymerization | Glucose | LSV | 3.7 | 100 to 30,000 | [177] |
| PGE/PAMAN/pMB-GHD | Poly (methylene blue) | CV | Glucose | flow injection analysis | 4.0 | 0.001 to 1.0 | [173] |
| GC/pEDOT-PBA | Poly (EDOT-PBA) | CV | Glucose | EIS | 5.0 | 100 to 50,000 | [182] |
| GC/PANI-PVP-AuNP/ GOx-Nafion | PANI-PVP | CV | Glucose | CA | 10.0 | 0.05 to 2.25 | [165] |
| ITO/pPD/AgNP | Poly (o-phenylenediamine) | CV | Glucose | CA | 12.0 | 150 to 13,000 | [188] |
| ITO/PP3C-GO/GOx | Poly (pyrrole-3-carboxilic acid) | CV | Glucose | CA | 50.0 | 1 to mM | [163] |
| GC/PANI/AuNP | PANI | Drop-cast of solution polymer | Glucose | EIS | 100.0 | 300 to 1000 | [176] |
| G/p(EDOT-PdBPI)n-(HKCN)m-GOx | PEDOT | CV | Glucose | CA | 180.0 | 250 to 2500 | [170] |
| PGE/PEDOT.PSS-CuONP | PEDOT.PSS | Homogeneous synthesis and drop-cast | Glucose | CA | 230.0 | Up to 10 mM | [181] |
| Pt/PEDOT-PAA-GOx | PEDOT | CA | Glucose | CA | 290.0 | 960 to 3000 | [171] |
| Pt/PEDOT-BSA/AuNP-GOx | PEDOT | CV | Glucose | CV, LSV, CA | Not reported | 0.416 to 50 mM | [169] |
| Pt/PANI/GOx/PU/E-PU | PANI | CA | Glucose | CA | Not reported | 0–20 mM | [164] |
| GC/PHMeDOT | Poly (hydroxymethyl-3,4-ethylendioxythiophene) | CA | Glucose | CA | Not reported d | 1–9 mM | [180] |
| Hydrogen Peroxide | |||||||
| Nafion/HRP/ATh-γ-PGA/GE | ATh-γ-PGA | CV | H2O2 | DPV | 0.0000030 | 0.00001–0.010 | [197] |
| HRP/AuNPs/rGO/PEDOT:PSS/SPGE | PEDOT:PSS | CV | H2O2 | CA | 0.08 | 5–400 | [196] |
| Ag/PMB/GS/GCE | PMB | CV | H2O2 | CA | 0.15 | 0.5–1112 | [204] |
| PEDOT/PBNPs/GCE | PEDOT | CA | H2O2 | CA | 0.16 | 0.5–839 | [201] |
| PEDOT/PBNPs/Pt | PEDOT | SV | H2O2 | CA | 1.4 | 5–1000 | [202] |
| PPy3C-PPy/MPrPt/BDD | PPy3C-PPy | CV | H2O2 | CA | 2.0 | 5–49,000 | [205] |
| SBP/poly (EGDE-AA-ANI)/GCE | PANI | radical polymerization | H2O2 | CA | 2.2 | 5.0–50 | [199] |
| HRP/PAN-PNMThH | PAN-PNMThH | CV | H2O2 | CA | 3.2 | 5–60,000 | [198] |
| poly2AB/AuNPs/PGE | poly2AB | CV | H2O2 | CA | 36.7 | 60–100,000 | [203] |
| Electrode Architecture | Conducting Polymer | Synthesis Method | Analytes | Detection Technique | LOD (µM) | Linear Range (µM) | Ref. |
|---|---|---|---|---|---|---|---|
| Diverse Pharmaceuticals | |||||||
| Poly [(3, 6-diamino-9-ethylcarbazole)]/GCE | Poly [(3, 6-diamino-9-ethylcarbazole)] | CV | E2 | EIS | 0.36 aM | 1 aM to 10 μM | [223] |
| MnO2-Sb2O3/PANI//FTO | PANI | CV | ASA | DPV | 0.0002 | 0.0012–0.22868 | [241] |
| PPy/sol-gel/SiO2@AuNPs MIP/Au electrode | PPy | CV | ASA | SWV | 0.0002 | 0.001–0.01 | [242] |
| PPy/PB/GCE | PPy | CV | PR | DPV | 0.00053 | 0.001–100 | [228] |
| 3D-HPG/PTH/GCE | PTH | CV | MNZ | DPV | 0.001 | 0.05–70 | [209] |
| rGO/PPR/GCE | PPR | CV | CFX | DPV | 0.002 | 0.002–0.05 | [219] |
| Cu2+-PANI-Nano-ZSM-5/GCE | PANI | CV | PR | DPV | 0.008 | 0.015–800 | [237] |
| CS-MWCNTs+TiO2 NPs/PCC/nanoporous GCE | PCC | CV | Acyclovir | DPASV | 0.01 | 0.03–1 | [214] |
| PEBT/GCE | PEBT | CV | Acyclovir | DPV | 0.012 | 0.03–0.3 | [213] |
| P3MT/RGO/GCE | P3MT | CV | PR | DPV | 0.025 | 0.2–2.5 | [232] |
| PLum/f-MWCNTs/GCE | PLum | CV | PR | DPV | 0.025 | 0.04–32.2 | [233] |
| PEDOT:PSSLi/GCE | PEDOT | CA | PR | DPV | 0.05 | 0.14–400 | [234] |
| PEDOT:PSSLi:MWCNT/GCE | PEDOT | CA | PR | AdSDPV | 0.08 | 1.5–500 | [234] |
| PNB/GCE | PNB | CV | PR | DPV | 0.08 | 0.2–16.2 | [231] |
| DMIP/CPE | Poly(AMTEOS) | CV | MNZ | DPV | 0.091 | 0.4–200 | [207] |
| HRP/Pol/Pt | Pol | CA | E2 | DPV | 0.105 | 0.1–200 | [222] |
| MIP/AuNPs/GCE | PME | CV | MNZ | DPV | 0.12 | 0.5–1000 | [208] |
| SDS/PEB/CPE | PEB | CV | CFX | DPV | 0.183 | 50–90 | [218] |
| Poly(naphthol green B)/CPE | Poly(naphthol green B) | CV | PR | CV | 1.6 | 20–70 | [230] |
| Poly(rhodamine B)/CPE | Poly(rhodamine B) | CV | PR | CV | 2.2 | 20–90 | [229] |
| MWCNT/PANI/AuE | PANI | CV | PR | CA | 2.9 | 0.5–630 | [235] |
| Hydrazine | |||||||
| GCE/PDA@GO | Poly dopmanie | Homogeneous polymerization | Hidrazine | SWV | 0.01 | 0.03 to 100 | [262] |
| SPE/PDDA@[Cu(CN6)] | Poly (diallydimethylamonium chloride) | Commercial polymer | Hidrazine | CA | 0.01 | 0.03 to 570 | [257] |
| GCE/3D-PEDOT/CuxO | PEDOT | CA | Hidrazine | CA | 0.2 | 0.5 to 600 | [260] |
| GCE/PEDOT/ZnO | PEDOT | Chemical polymerization | Hidrazine | CA | 0.207 | 0.5 to 48 | [259] |
| GCE/PALS | Alizarin red S | CV | Hidrazine | CA | 0.28 | 1 to 600 | [261] |
| GCE/PPy-LS | Ppy | Galvanostatic | Hidrazine | CA | 1.6 | 1–80 | [253] |
| Paper/PEDOT/ZnO/Nf | PEDOT PSS | commercial polymer | Hidrazine | CA | 5.0 | 10 to 500 | [258] |
| FTO/PANI-gC3N4/AgNP | PANI | CV | Hidrazine | CV | 300.0 | 5–300 mM | [254] |
| CPE/PRA@NiFe2O4NP | Poly (rhodamine) | Chemical oxidation with KMnO4 | Hidrazine | CA, CV | Not reported | 1–50 mM | [256] |
| GCE/pyrolized PANI/CuNP/Nf | PANI | Chemical polymerization | Hidrazine | CV | Not reported | 10 to 100 mM | [255] |
| Nitrites | |||||||
| AuNP/ PEDOT/PMo9V3/PEI/GCE | PEDOT | CV | nitrite | CA | 0.001 | 0.0025–1430 | [271] |
| CoNS/GO/PPy/GCE | PPy | CV | nitrite | CA | 0.015 | 1.0–3200 | [273] |
| PdNPs/poly (1,5-DAN)/MWCNTs/GCE | Poly (1,5 DAN) | CV | nitrite | CA | 0.08 | 0.25–100 | [272] |
| nHAp/PEDOT/GCE | PEDOT | CA | nitrite | CA | 0.083 | 0.25–1050 | [269] |
| CQDs/PEDOT/GCE | PEDOT | CA | nitrite | CA | 0.088 | 0.5–1110 | [268] |
| Au/PEDOT-SH/PEDOT/GCE | PEDOT-SH/PEDOT | CV | nitrite | CA | 51.0 | 150–1000 | [270] |
| Phenolic Compounds | |||||||
| f-SWCNTs/PEDOTM/GCE | PEDOTM | CA | catechin | CV | 0.013 | 0.039–40.84 | [284] |
| Poly (NG B)/CPE | Poly (NG B) | CV | HQ | DPV | 0.01 | 0.1–110 | [286] |
| CC | CV | 0.19 | 0.20–90 | ||||
| HQ | CV | 0.20 | 0.20–90 | ||||
| Cu-PPy/GCE | PPy | CA | CC | CA | 0.010 | 0.05–1000 | [287] |
| DPV | 1.17 | 10–1750 | |||||
| 1D PEDOT-Gr/Ta | PEDOT | CV | HQ | DPV | 0.06 | 5–250 | [285] |
| CC | DPV | 0.08 | 0.4–350 | ||||
| RC | DPV | 0.16 | 6–2000 | ||||
| Nitroaromatic Compounds | |||||||
| ENPPy/SDS/GCE | ENPPy | CV | p-NP | SWV | 0.0001 | 0.0001–100 | [299] |
| Poly(35DT)/GE | Poly(35DT) | CV | 4-NP | DPV | 0.09 | 0.24–130.6 | [298] |
| Poly (p-ABSA)/GrE | Poly (p-ABSA) | CV | o-NP | SDV | 0.28 | 0.3–800 | [296] |
| p-NP | SDV | 0.3 | 0.3–700 | ||||
| m-NP | SDV | 0.5 | 0.3–700 | ||||
| PAR/GCE | PAR | CV | NF | DPV | 0.33 | 3.0–50 | [295] |
| NIT | DPV | 0.73 | 10.0–40 | ||||
| FL | DPV | 1.56 | 50–140 | ||||
| PME/MWCNT*/SPCE | PME | CV | NFZ | DPV | 0.006 | 0.05–2.0 | [297] |
| FZD | DPV | 0.007 | 0.05–2.0 | ||||
| NFT | DPV | 0.012 | 0.05–2.0 | ||||
| FTD | DPV | 0.014 | 0.05–5.0 | ||||
| AuNp/P(o-PDA-co-ANI)/GCE) | P(o-PDA-co-ANI) | CV | DNT | CV | 7.03 | 11–220 | [294] |
| TNT | CV | 9.25 | 11–176 | ||||
| Tetryl | CV | 13.23 | 14–348 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terán-Alcocer, Á.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. Electrochemical Sensors Based on Conducting Polymers for the Aqueous Detection of Biologically Relevant Molecules. Nanomaterials 2021, 11, 252. https://doi.org/10.3390/nano11010252
Terán-Alcocer Á, Bravo-Plascencia F, Cevallos-Morillo C, Palma-Cando A. Electrochemical Sensors Based on Conducting Polymers for the Aqueous Detection of Biologically Relevant Molecules. Nanomaterials. 2021; 11(1):252. https://doi.org/10.3390/nano11010252
Chicago/Turabian StyleTerán-Alcocer, Álvaro, Francisco Bravo-Plascencia, Carlos Cevallos-Morillo, and Alex Palma-Cando. 2021. "Electrochemical Sensors Based on Conducting Polymers for the Aqueous Detection of Biologically Relevant Molecules" Nanomaterials 11, no. 1: 252. https://doi.org/10.3390/nano11010252
APA StyleTerán-Alcocer, Á., Bravo-Plascencia, F., Cevallos-Morillo, C., & Palma-Cando, A. (2021). Electrochemical Sensors Based on Conducting Polymers for the Aqueous Detection of Biologically Relevant Molecules. Nanomaterials, 11(1), 252. https://doi.org/10.3390/nano11010252






