Effects of Catalyst Pretreatment on Carbon Nanotube Synthesis from Methane Using Thin Stainless-Steel Foil as Catalyst by Chemical Vapor Deposition Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Stainless-Steel Foil
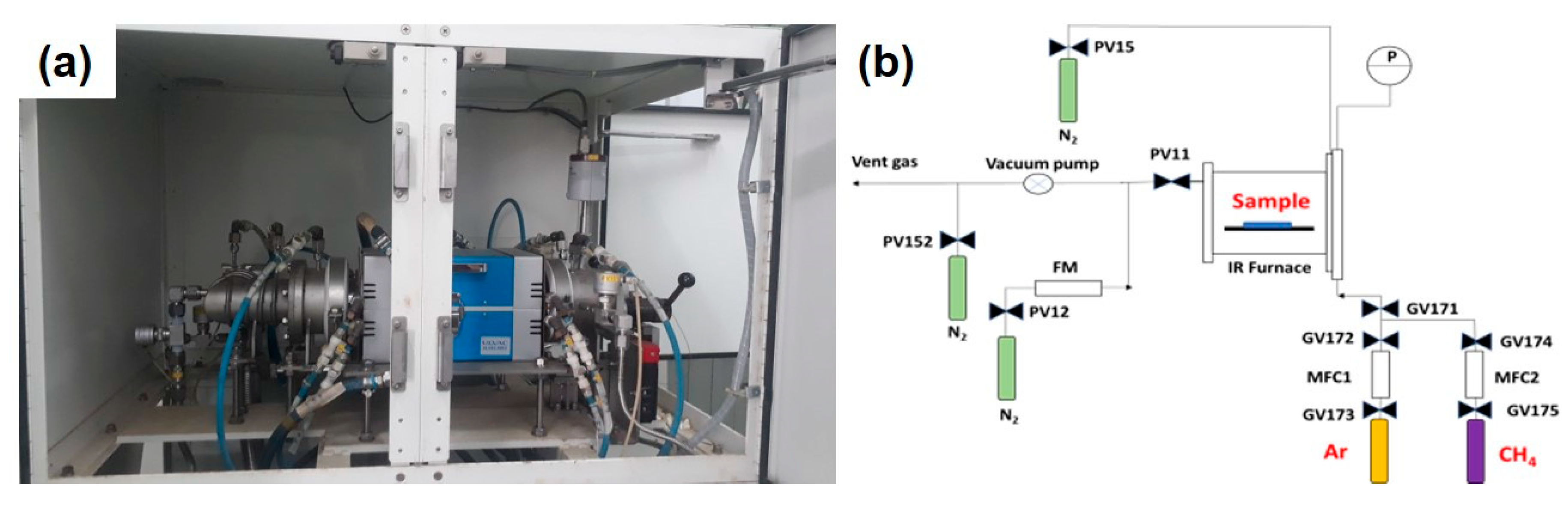
2.2. Catalyst Pretreatment and CNT Synthesis
2.3. Catalyst and Product Characterization
3. Results and Discussion
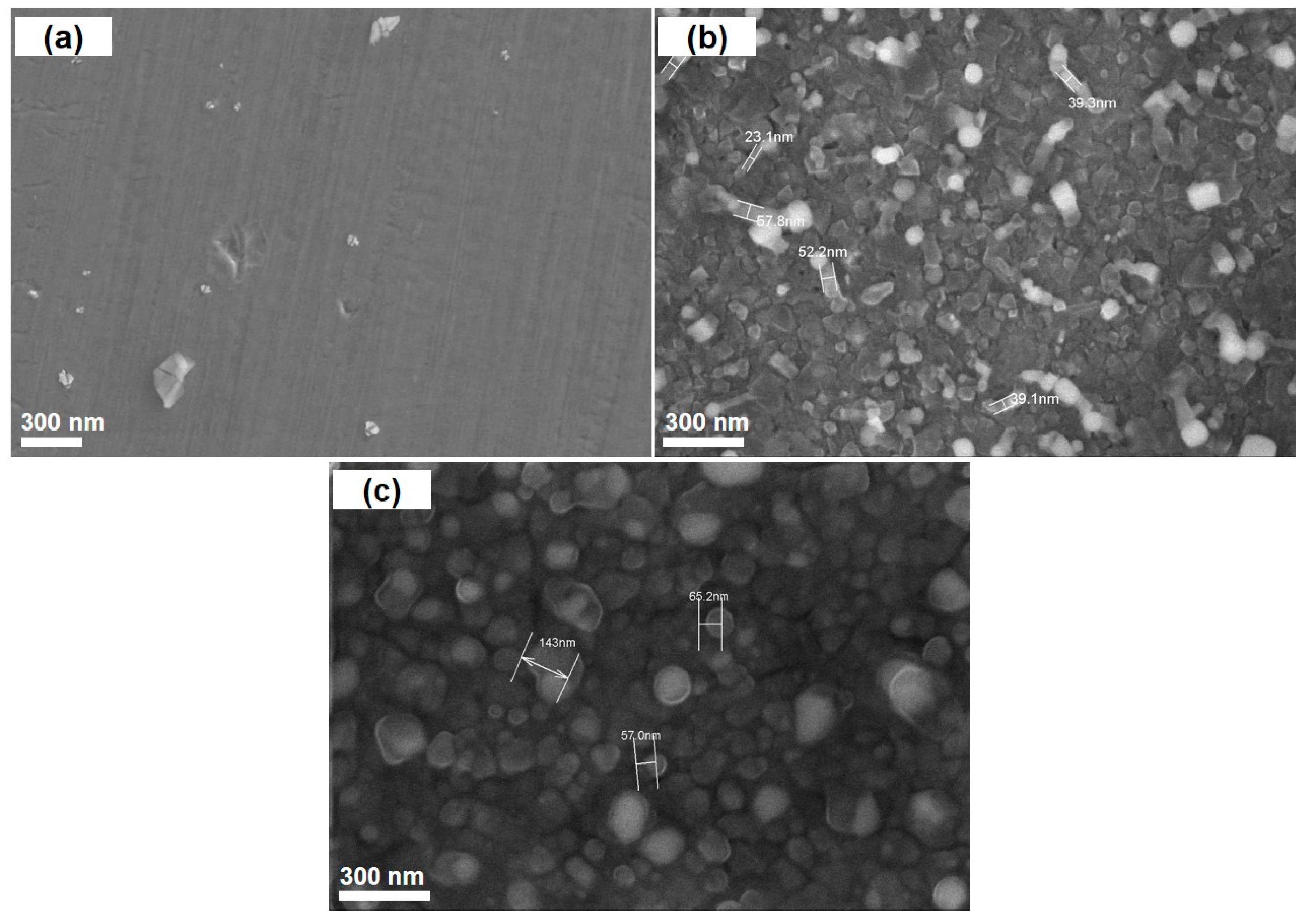
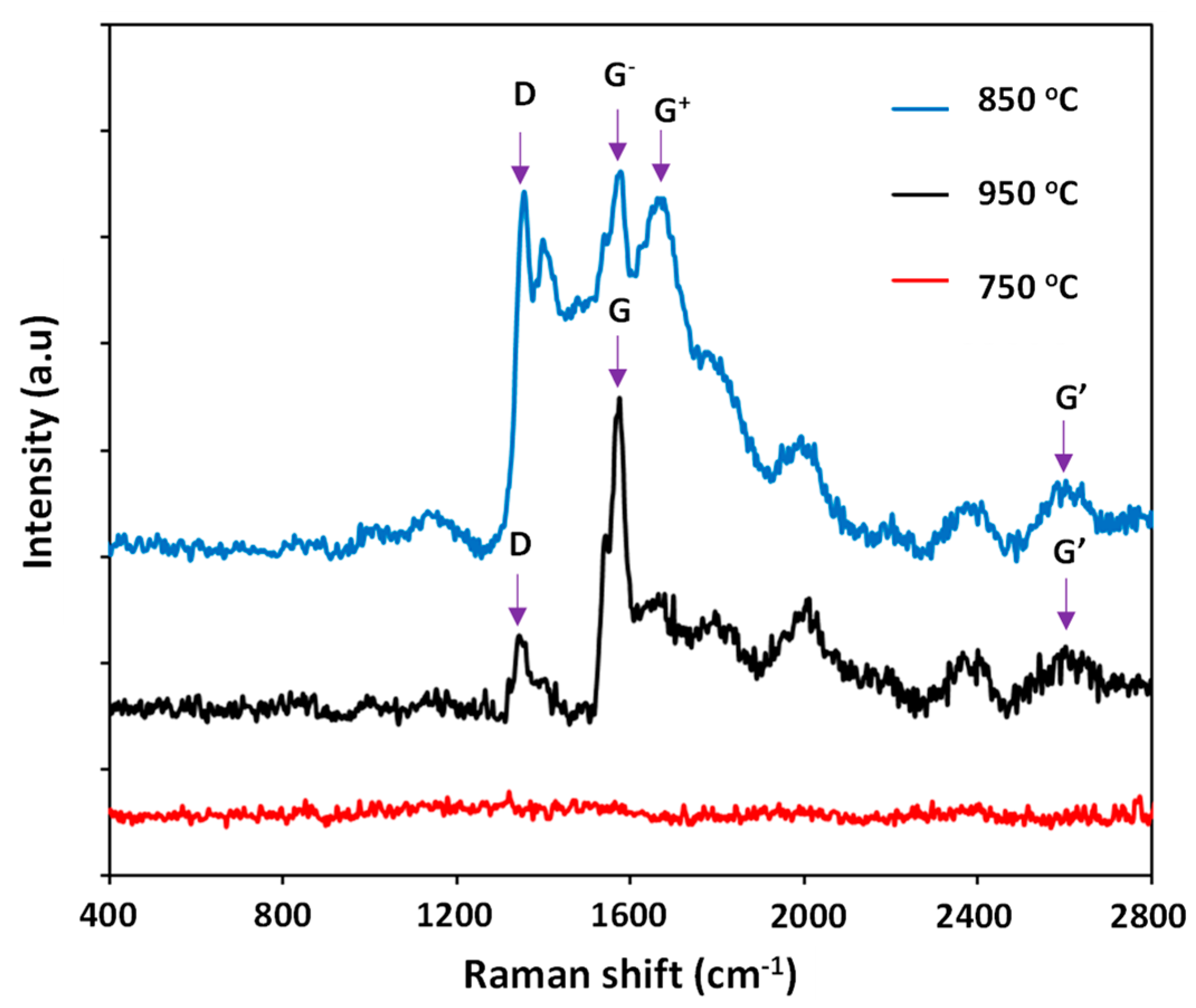
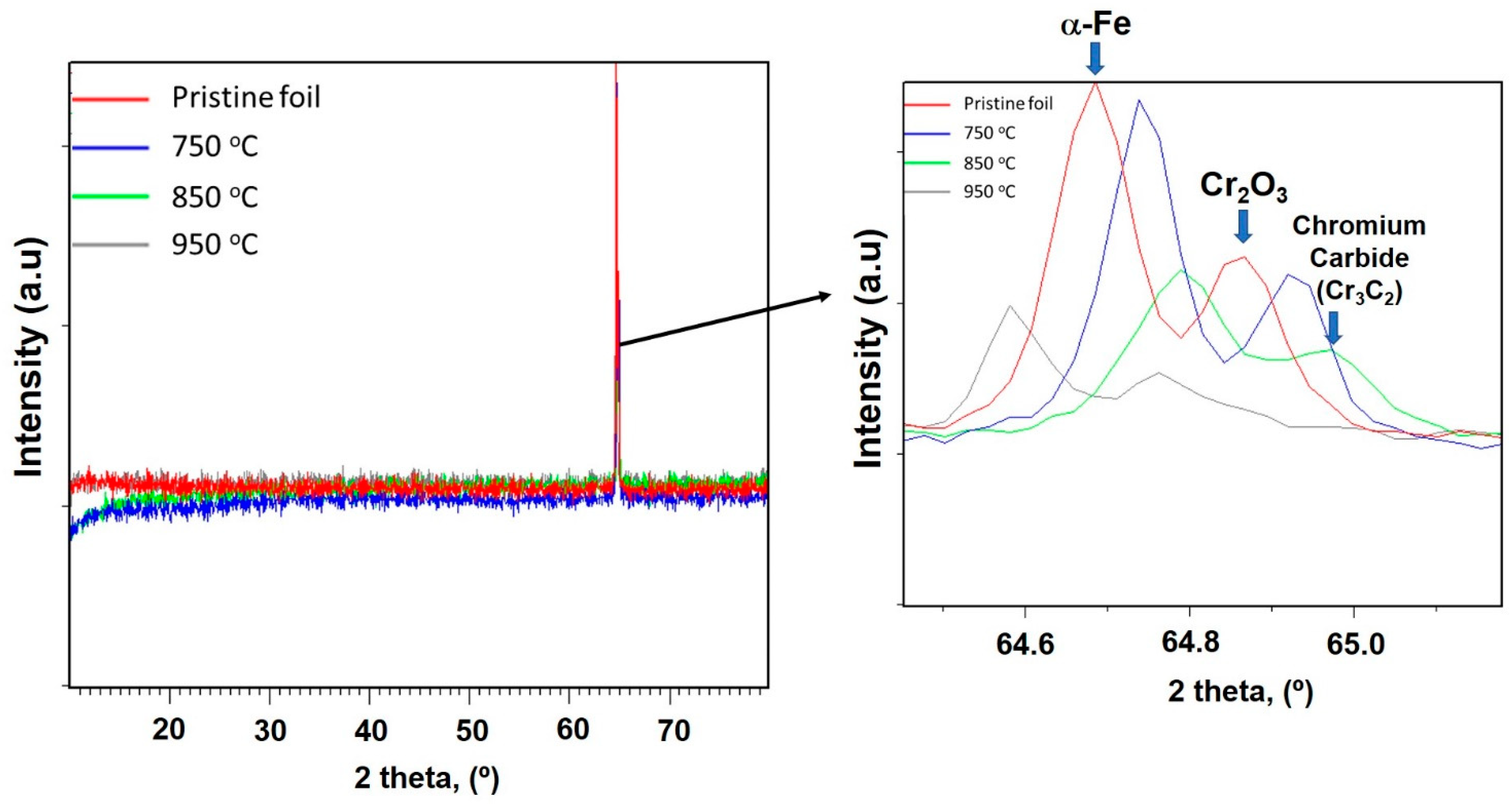
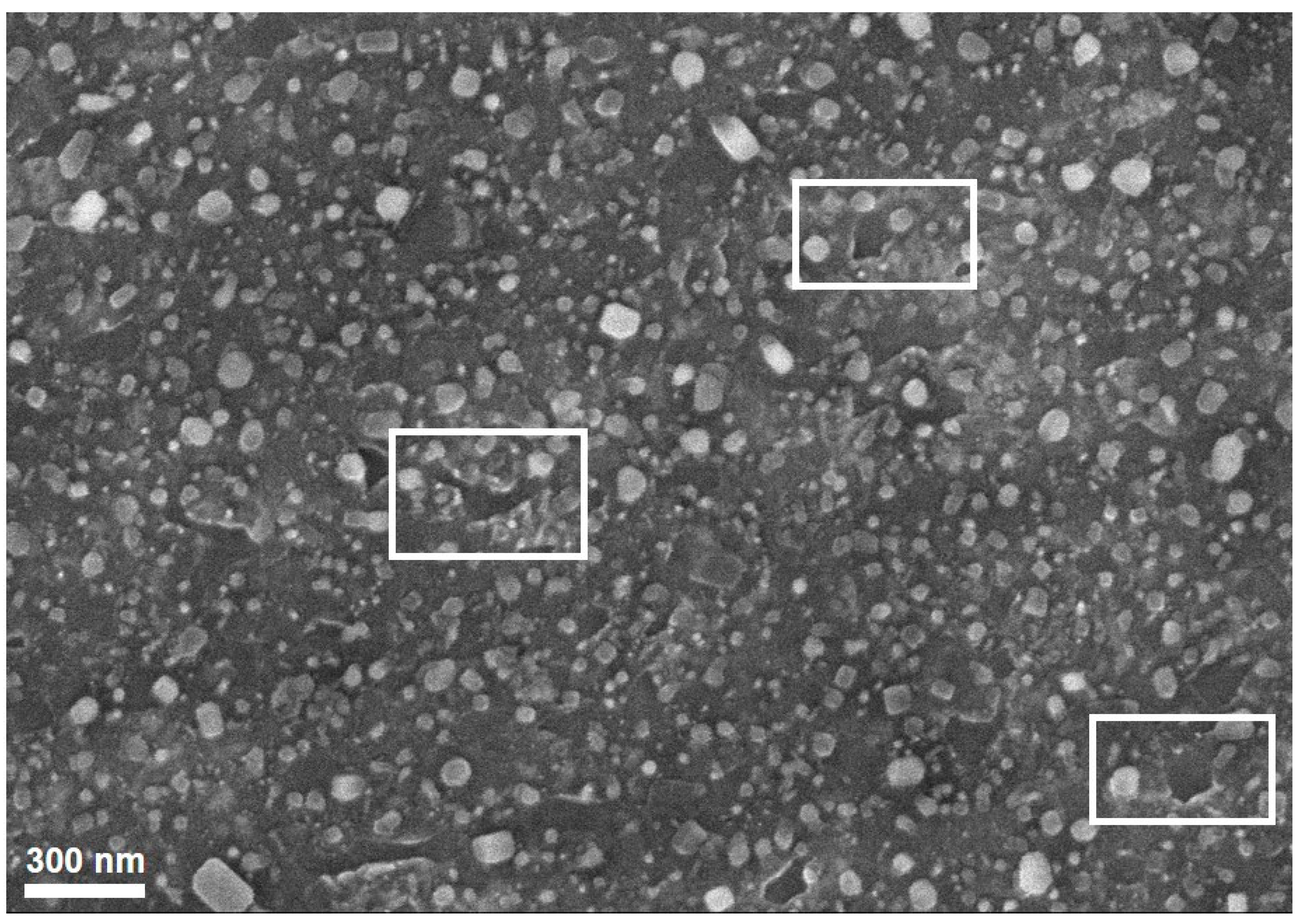
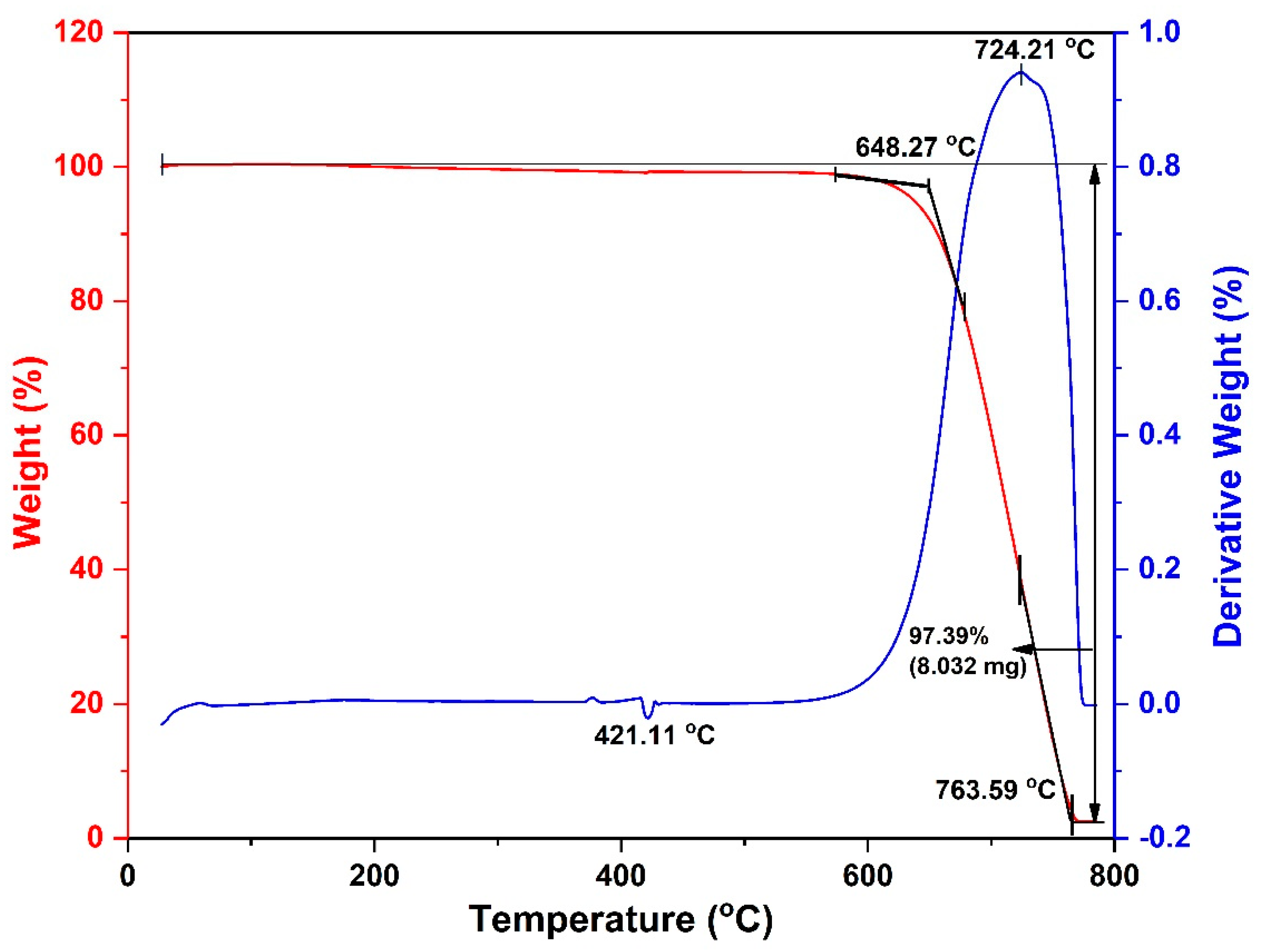
3.1. Effects of Catalyst Pretreatment Temperature on CNT Growth
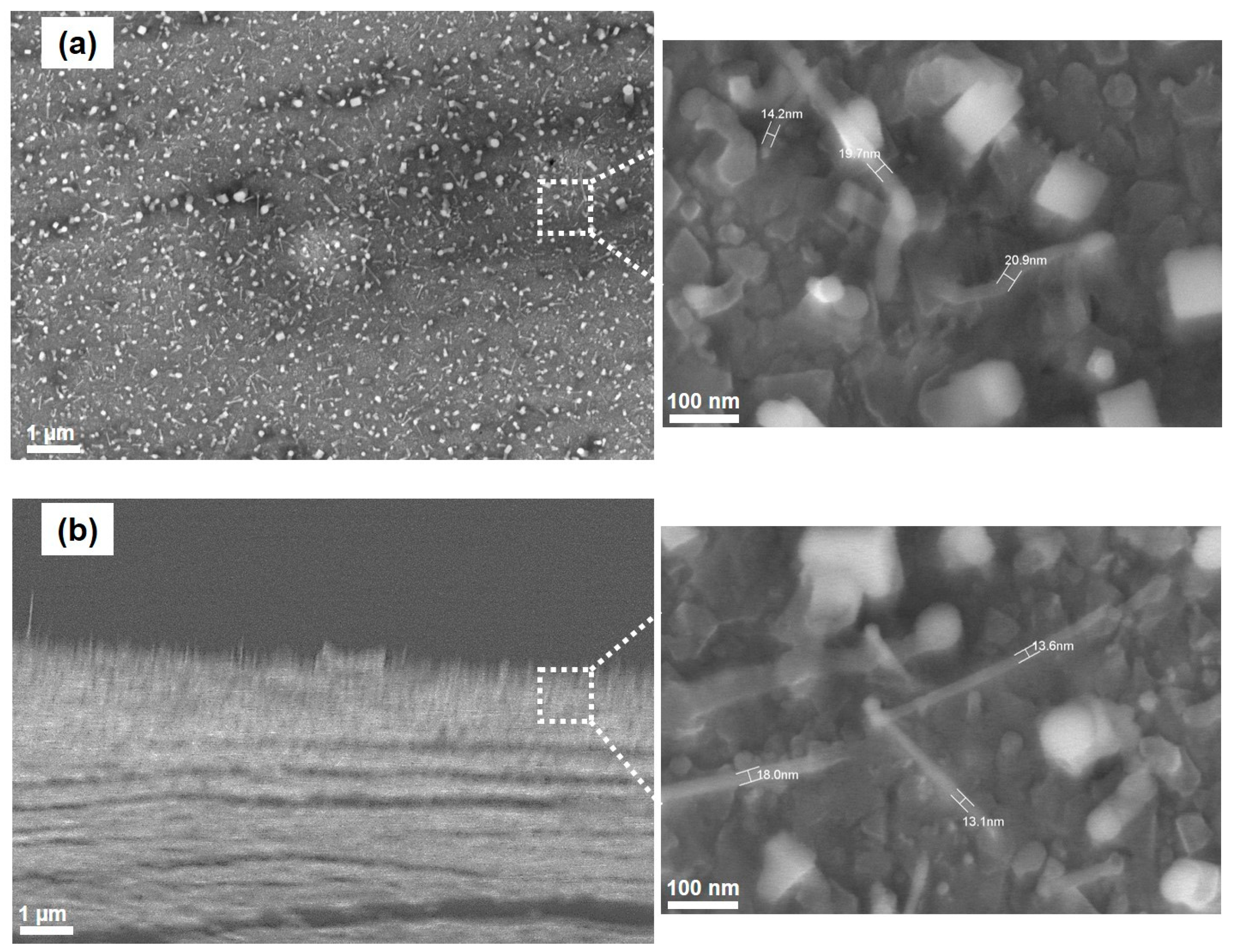
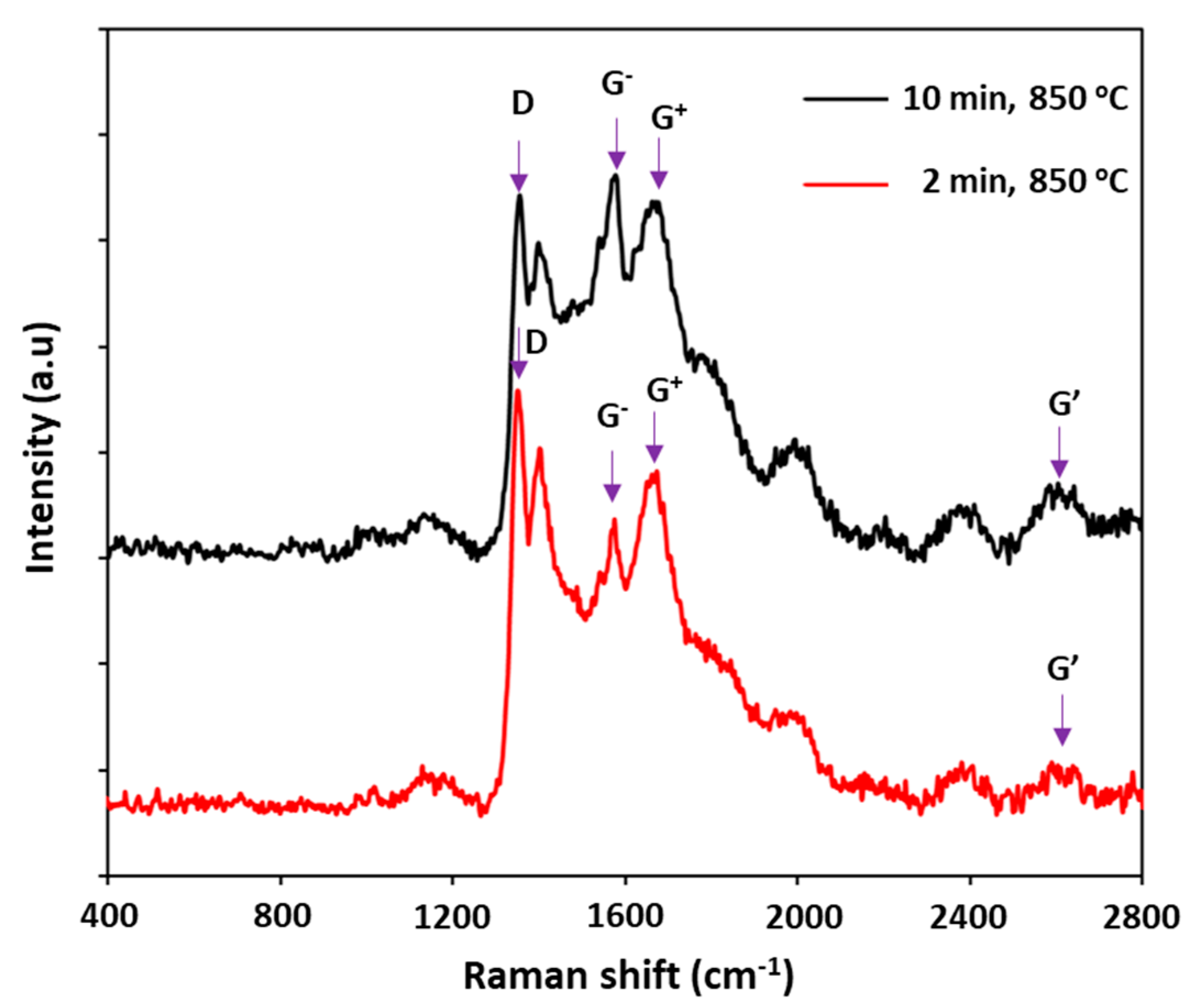
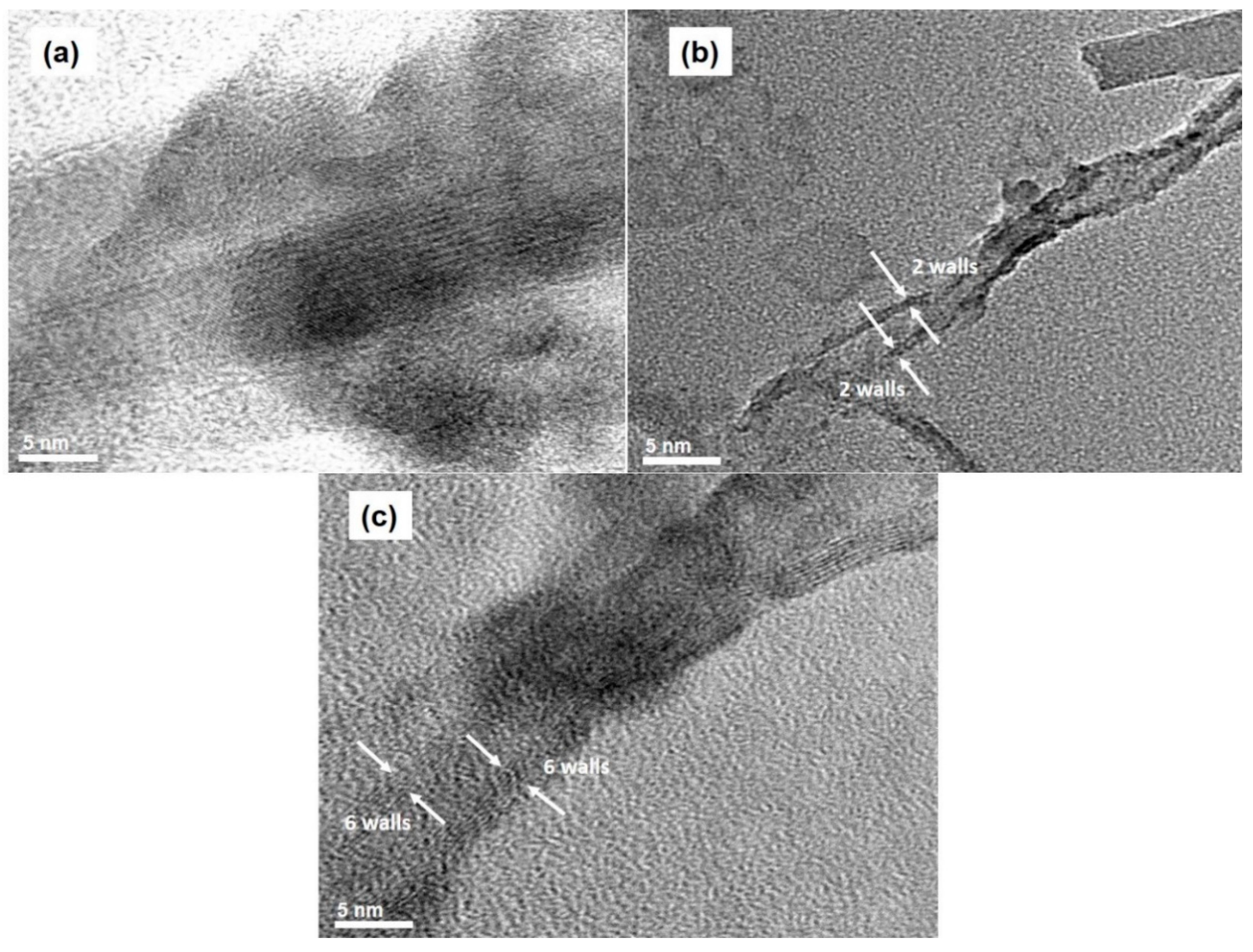
3.2. Effects of Catalyst Pretreatment Time on CNT Growth
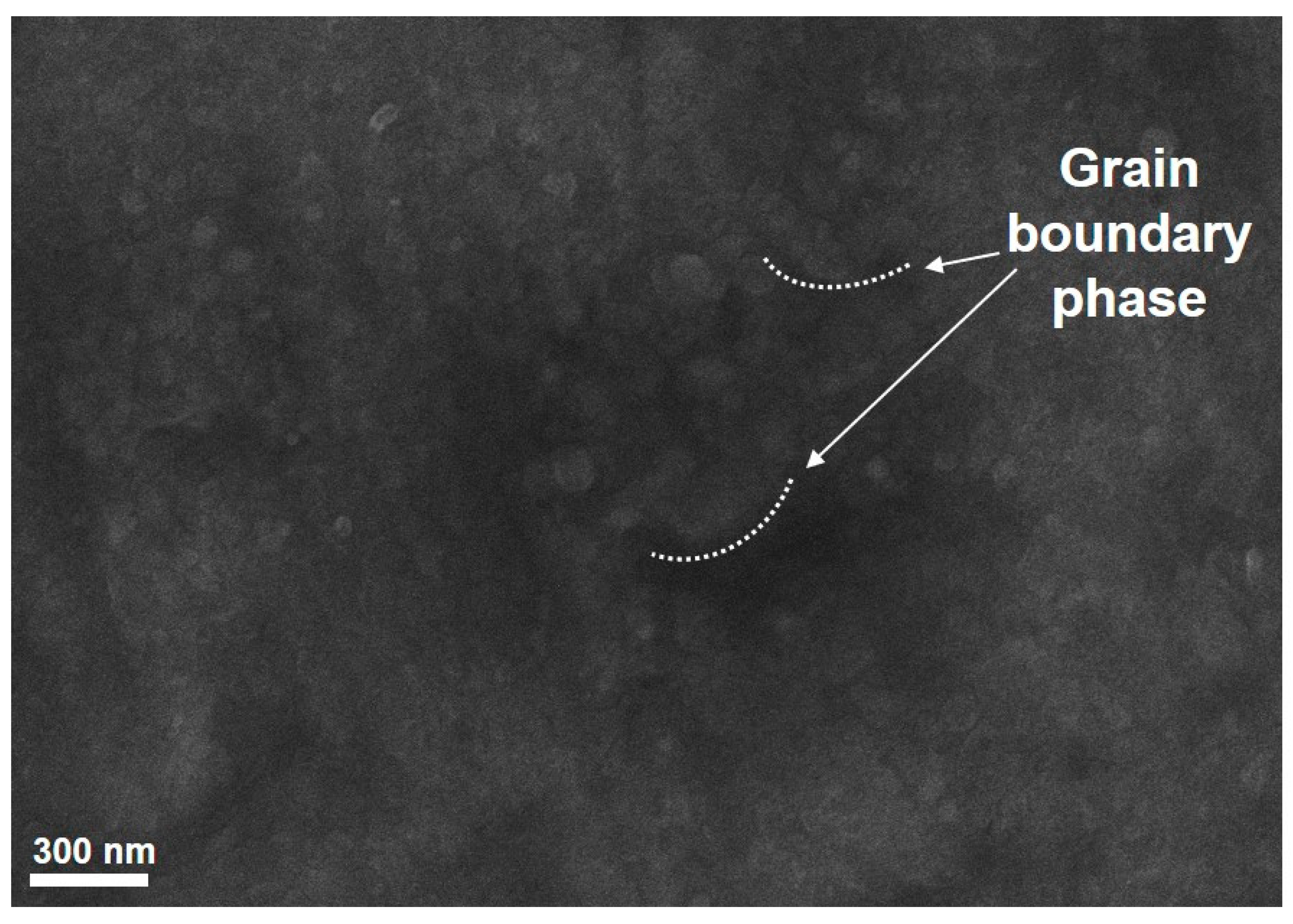
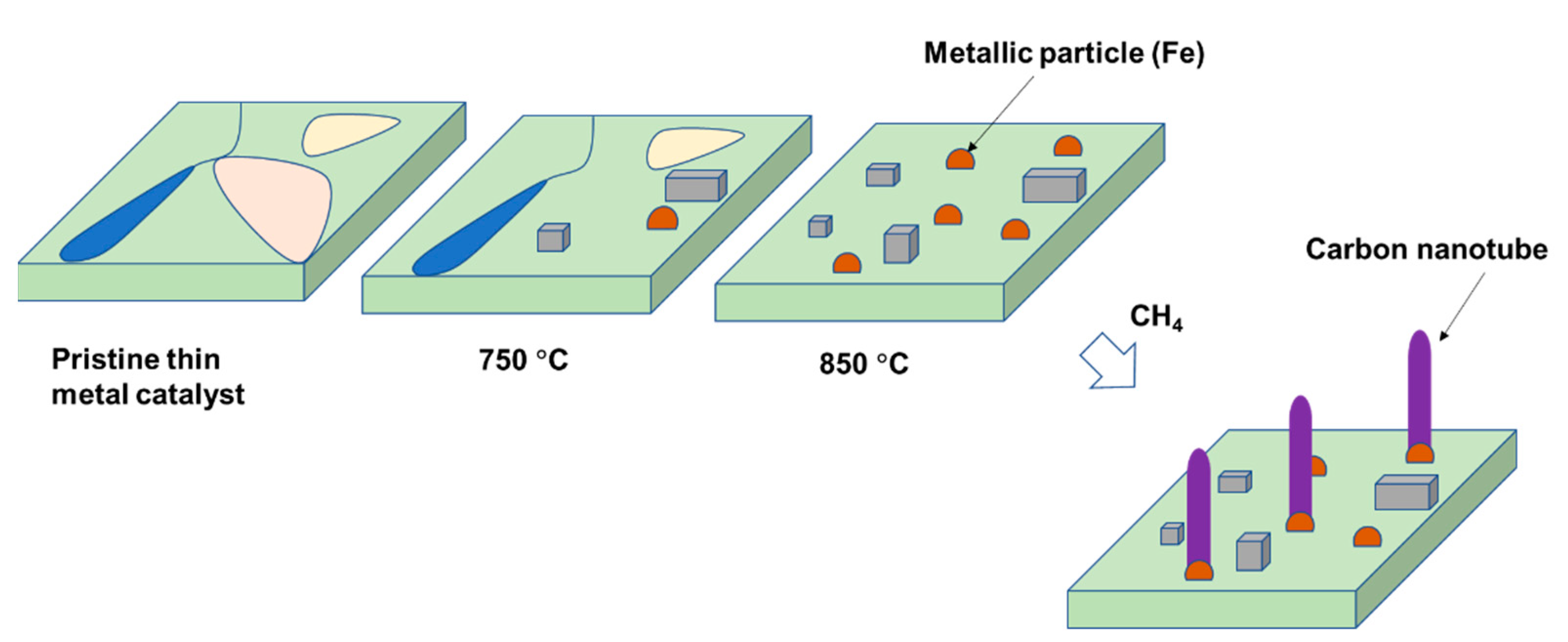
3.3. Proposing the Mechanism of Active Sites Formation on the Stainless-Steel Foil for CNT Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landi, B.J.; Ganter, M.J.; Cress, C.D.; DiLeo, R.A.; Raffaelle, R.P. Carbon nanotubes for lithium ion batteries. Energy Environ. Sci. 2009, 2, 638–654. [Google Scholar] [CrossRef]
- Lee, S.W.; Yabuuchi, N.; Gallant, B.M.; Chen, S.; Kim, B.-S.; Hammond, P.T.; Shao-Horn, Y. High-power lithium batteries from functionalized carbon-nanotube electrodes. Nat. Nanotechnol. 2010, 5, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Moy, D.; Niu, C.; Tennent, H.; Hoch, R. Lubricants Containing Carbon Nanotubes. U.S. Patent 6,828,282, 7 December 2004. [Google Scholar]
- Cornelio, J.A.C.; Cuervo, P.A.; Hoyos-Palacio, L.M.; Lara-Romero, J.; Toro, A. Tribological properties of carbon nanotubes as lubricant additive in oil and water for a wheel–rail system. J. Mater. Res. Technol. 2016, 5, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Tang, W.; Liu, X.; Huang, Z. Synthesis of ionic liquid decorated muti-walled carbon nanotubes as the favorable water-based lubricant additives. Appl. Phys. A 2017, 123, 680. [Google Scholar] [CrossRef]
- Postma, H.W.; Sellmeijer, A.; Dekker, C. Manipulation and imaging of individual single-walled carbon nanotubes with an atomic force microscope. Adv. Mater. 2000, 12, 1299–1302. [Google Scholar] [CrossRef]
- Stevens, R.M.; Frederick, N.A.; Smith, B.L.; Morse, D.E.; Stucky, G.D.; Hansma, P.K. Carbon nanotubes as probes for atomic force microscopy. Nanotechnology 2000, 11, 1. [Google Scholar] [CrossRef]
- Volodin, A.; Ahlskog, M.; Seynaeve, E.; Van Haesendonck, C.; Fonseca, A.; Nagy, J. Imaging the elastic properties of coiled carbon nanotubes with atomic force microscopy. Phys. Rev. Lett. 2000, 84, 3342. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, X.; Park, K.; Song, J.; Hong, J.; Zhou, L.; Mai, Y.-W.; Huang, H.; Goodenough, J.B. Hollow carbon-nanotube/carbon-nanofiber hybrid anodes for Li-ion batteries. J. Am. Chem. Soc. 2013, 135, 16280–16283. [Google Scholar] [CrossRef]
- Jeon, H.; Park, J.; Shon, M. Corrosion protection by epoxy coating containing multi-walled carbon nanotubes. J. Ind. Eng. Chem. 2013, 19, 849–853. [Google Scholar] [CrossRef]
- Pieters, B. Nanocomposites in automotive: Research activities and business realities: Automotive. JEC Compos. 2007, 30–31. [Google Scholar]
- Isaacs, J.A.; Tanwani, A.; Healy, M.; Dahlben, L. Economic assessment of single-walled carbon nanotube processes. J. Nanopart. Res. 2010, 12, 551–562. [Google Scholar] [CrossRef]
- Fagan-Murphy, A.; Kataria, S.; Patel, B.A. Electrochemical performance of multi-walled carbon nanotube composite electrodes is enhanced with larger diameters and reduced specific surface area. J. Solid State Electrochem. 2016, 20, 785–792. [Google Scholar] [CrossRef]
- Dong, Z.; Li, B.; Cui, C.; Qian, W.; Jin, Y.; Wei, F. Catalytic methane technology for carbon nanotubes and graphene. React. Chem. Eng. 2020, 5, 991–1004. [Google Scholar] [CrossRef]
- Qian, W.; Liu, T.; Wang, Z.; Yu, H.; Li, Z.; Wei, F.; Luo, G. Effect of adding nickel to iron–alumina catalysts on the morphology of as-grown carbon nanotubes. Carbon 2003, 41, 2487–2493. [Google Scholar] [CrossRef]
- Han, I.T.; Kim, B.K.; Kim, H.J.; Yang, M.; Jin, Y.W.; Jung, S.; Lee, N.; Kim, S.K.; Kim, J.M. Effect of Al and catalyst thicknesses on the growth of carbon nanotubes and application to gated field emitter arrays. Chem. Phys. Lett. 2004, 400, 139–144. [Google Scholar] [CrossRef]
- Killian, J.L.; Zuckerman, N.B.; Niemann, D.L.; Ribaya, B.P.; Rahman, M.; Espinosa, R.; Meyyappan, M.; Nguyen, C.V. Field emission properties of carbon nanotube pillar arrays. J. Appl. Phys. 2008, 103, 064312. [Google Scholar] [CrossRef]
- Shaikhutdinov, S.K.; Avdeeva, L.; Goncharova, O.; Kochubey, D.; Novgorodov, B. Coprecipitated Ni-Al and Ni-Cu-Al Catalysts for Methane Decomposition and Carbon Deposition. 1. Genesis of Calcined and Reduced Catalysts. Appl. Catal. A Gen. 1995, 126, 125–139. [Google Scholar] [CrossRef]
- Muradov, N. Hydrogen via methane decomposition: An application for decarbonization of fossil fuels. Int. J. Hydrogen Energy 2001, 26, 1165–1175. [Google Scholar] [CrossRef]
- Yahyazadeh, A.; Khoshandam, B. Carbon nanotube synthesis via the catalytic chemical vapor deposition of methane in the presence of iron, molybdenum, and iron–molybdenum alloy thin layer catalysts. Results Phys. 2017, 7, 3826–3837. [Google Scholar] [CrossRef]
- Lepro, X.; Lima, M.D.; Baughman, R.H. Spinnable carbon nanotube forests grown on thin, flexible metallic substrates. Carbon 2010, 48, 3621–3627. [Google Scholar] [CrossRef]
- Van Chuc, N.; Dung, N.D.; Hong, P.N.; Quang, L.; Khoi, P.H.; Minh, P.N. Synthesis of carbon nanotubes on steel foils. J. Korean Phys. Soc. 2008, 52, 1368. [Google Scholar] [CrossRef]
- Saito, R.; Hofmann, M.; Dresselhaus, G.; Jorio, A.; Dresselhaus, M. Raman spectroscopy of graphene and carbon nanotubes. Adv. Phys. 2011, 60, 413–550. [Google Scholar] [CrossRef]
- Usachov, D.Y.; Davydov, V.Y.; Levitskii, V.S.; Shevelev, V.O.; Marchenko, D.; Senkovskiy, B.V.; Vilkov, O.Y.; Rybkin, A.G.; Yashina, L.V.; Chulkov, E.V. Raman spectroscopy of lattice-matched graphene on strongly interacting metal surfaces. ACS Nano 2017, 11, 6336–6345. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Chemical vapor deposition of carbon nanotubes: A review on growth mechanism and mass production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, S.M.; Cesano, F.; Scarano, D.; Edvinsson, T. Resonance Raman and IR spectroscopy of aligned carbon nanotube arrays with extremely narrow diameters prepared with molecular catalysts on steel substrates. Phys. Chem. Chem. Phys. 2017, 19, 30667–30674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, R.; Modak, A.; Schechter, A. A Comparative Study of Plasma-Treated Oxygen-Doped Single-Walled and Multiwalled Carbon Nanotubes as Electrocatalyst for Efficient Oxygen Reduction Reaction. ACS Sustain. Chem. Eng. 2019, 7, 11396–11406. [Google Scholar] [CrossRef]
- Hennrich, F.; Krupke, R.; Lebedkin, S.; Arnold, K.; Fischer, R.; Resasco, D.E.; Kappes, M.M. Raman spectroscopy of individual single-walled carbon nanotubes from various sources. J. Phys. Chem. B 2005, 109, 10567–10573. [Google Scholar] [CrossRef]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Dorantes-Rosales, H.J.; Lopez-Hirata, V.M.; Gonzalez-Velazquez, J.L.; Cayetano-Castro, N.; Saucedo-Muñoz, M.L. Precipitation Process in Fe-Ni-Al-based Alloys. Superalloys 2015, 77. [Google Scholar] [CrossRef] [Green Version]
- Duc Vu Quyen, N.; Quang Khieu, D.; Tuyen, T.N.; Xuan Tin, D.; Thi Hoang Diem, B. Carbon nanotubes: Synthesis via chemical vapour deposition without hydrogen, surface modification, and application. J. Chem. 2019, 2019, 4260153. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Yabuuchi, K.; Kimura, A.; Ukai, S.; Oono, N.; Kaito, T.; Torimaru, T.; Hayashi, S. Effect of Cr/Al contents on the 475ºC age-hardening in oxide dispersion strengthened ferritic steels. Nucl. Mater. Energy 2016, 9, 610–615. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Saito, K.; Pan, C.; Williams, F.; Gordon, A. Nanotubes from methane flames. Chem. Phys. Lett. 2001, 340, 237–241. [Google Scholar] [CrossRef]
- Talapatra, S.; Kar, S.; Pal, S.; Vajtai, R.; Ci, L.; Victor, P.; Shaijumon, M.; Kaur, S.; Nalamasu, O.; Ajayan, P. Direct growth of aligned carbon nanotubes on bulk metals. Nat. Nanotechnol. 2006, 1, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Kokarneswaran, M.; Selvaraj, P.; Ashokan, T.; Perumal, S.; Sellappan, P.; Murugan, K.D.; Ramalingam, S.; Mohan, N.; Chandrasekaran, V. Discovery of carbon nanotubes in sixth century BC potteries from Keeladi, India. Sci. Rep. 2020, 10, 1–6. [Google Scholar] [CrossRef]
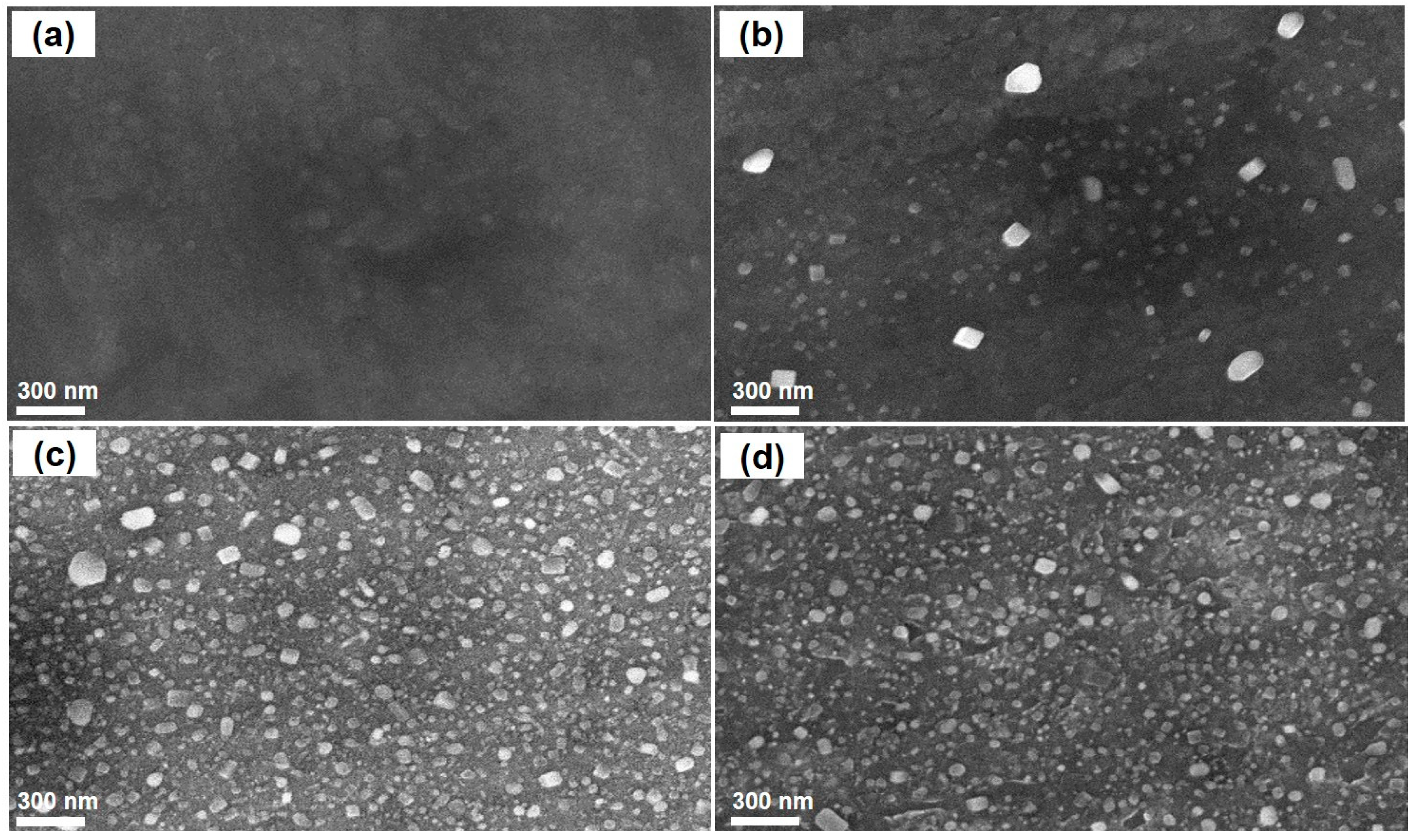


| Element | Composition, % Atom |
|---|---|
| C | 8.5 |
| O | 16.2 |
| Al | 9.7 |
| Cr | 15.1 |
| Fe | 50.5 |
| Elements | Atom.% after Pretreatment at Temperature of | Atom.% of Stainless-Steel Foil (From Table 1) | ||
|---|---|---|---|---|
| 750 °C | 850 °C | 950 °C | ||
| C | 9.9 | 6.1 | 8.3 | 8.5 |
| O | 11.8 | 10.6 | 11 | 16.2 |
| Al | 10.9 | 11.1 | 11.9 | 9.7 |
| Cr | 15.2 | 17.3 | 16.0 | 15.1 |
| Fe | 52.2 | 54.9 | 52.8 | 50.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huynh, T.M.; Nguyen, S.; Nguyen, N.T.K.; Nguyen, H.M.; Do, N.U.P.; Nguyen, D.C.; Nguyen, L.H.; Nguyen, C.V. Effects of Catalyst Pretreatment on Carbon Nanotube Synthesis from Methane Using Thin Stainless-Steel Foil as Catalyst by Chemical Vapor Deposition Method. Nanomaterials 2021, 11, 50. https://doi.org/10.3390/nano11010050
Huynh TM, Nguyen S, Nguyen NTK, Nguyen HM, Do NUP, Nguyen DC, Nguyen LH, Nguyen CV. Effects of Catalyst Pretreatment on Carbon Nanotube Synthesis from Methane Using Thin Stainless-Steel Foil as Catalyst by Chemical Vapor Deposition Method. Nanomaterials. 2021; 11(1):50. https://doi.org/10.3390/nano11010050
Chicago/Turabian StyleHuynh, Thuan Minh, Sura Nguyen, Ngan Thi Kim Nguyen, Huan Manh Nguyen, Noa Uy Pham Do, Danh Cong Nguyen, Luong Huu Nguyen, and Cattien V. Nguyen. 2021. "Effects of Catalyst Pretreatment on Carbon Nanotube Synthesis from Methane Using Thin Stainless-Steel Foil as Catalyst by Chemical Vapor Deposition Method" Nanomaterials 11, no. 1: 50. https://doi.org/10.3390/nano11010050




