Design and Assessment of a Polyurethane Carrier Used for the Transmembrane Transfer of Acyclovir
Abstract
:1. Introduction
2. Materials and Methods
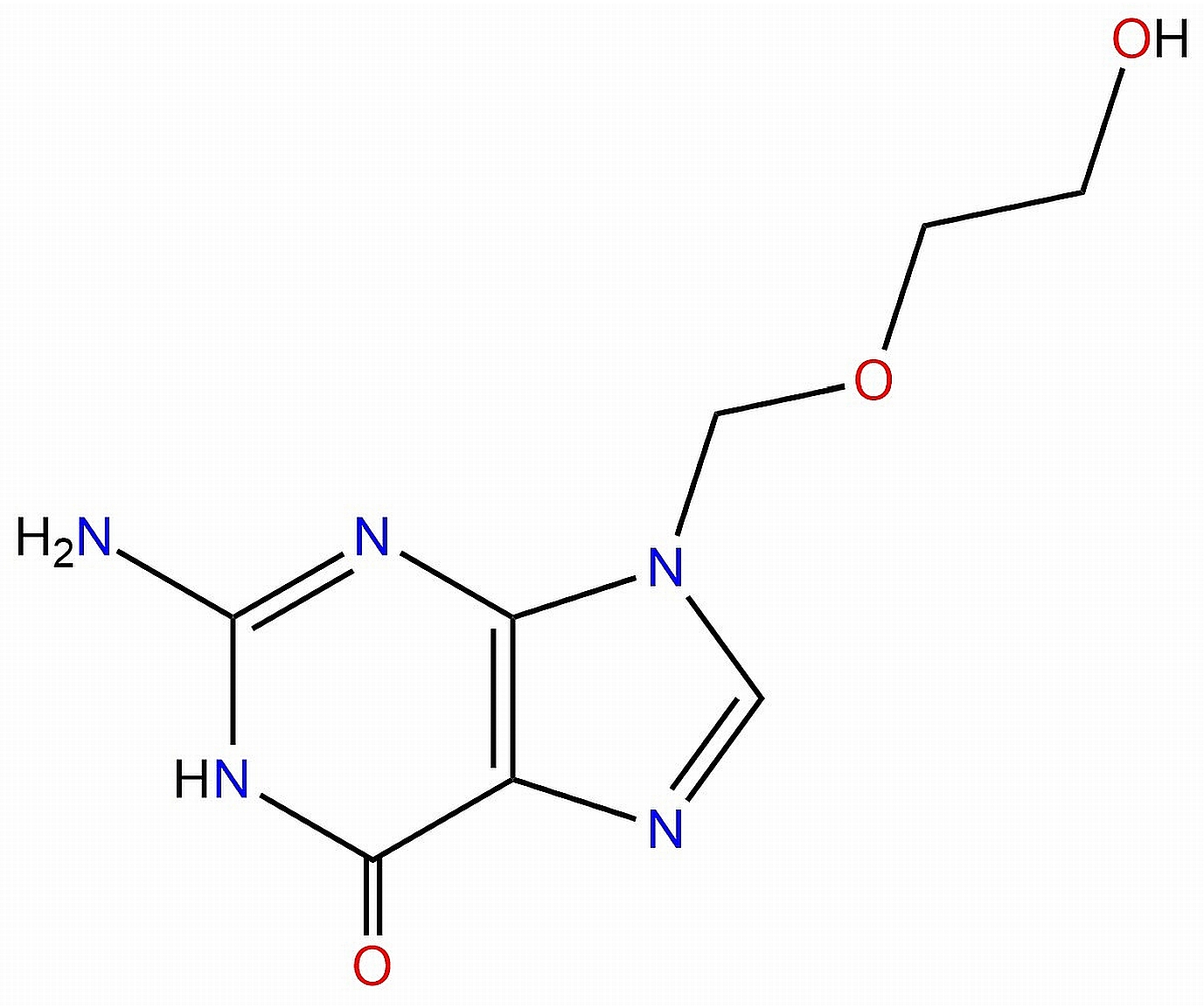
2.1. Reagents
2.2. Synthesis of the PU Particles
2.3. Drug Loading
2.4. Drug Release Profile
2.5. Dissolution Profile
2.6. The Penetrability Rate
2.7. Thermal Analysis
2.8. Zetasizer Tests
2.9. Scanning Electron Microscopy (SEM)
2.10. FT-IR Spectroscopy
2.11. Small-Angle Neutron Scattering (SANS)
2.12. Skin Irritation Evaluations
2.13. Statistics and Ethical Approval
3. Results
3.1. Drug Loading Efficacy
3.2. Drug Release Profile
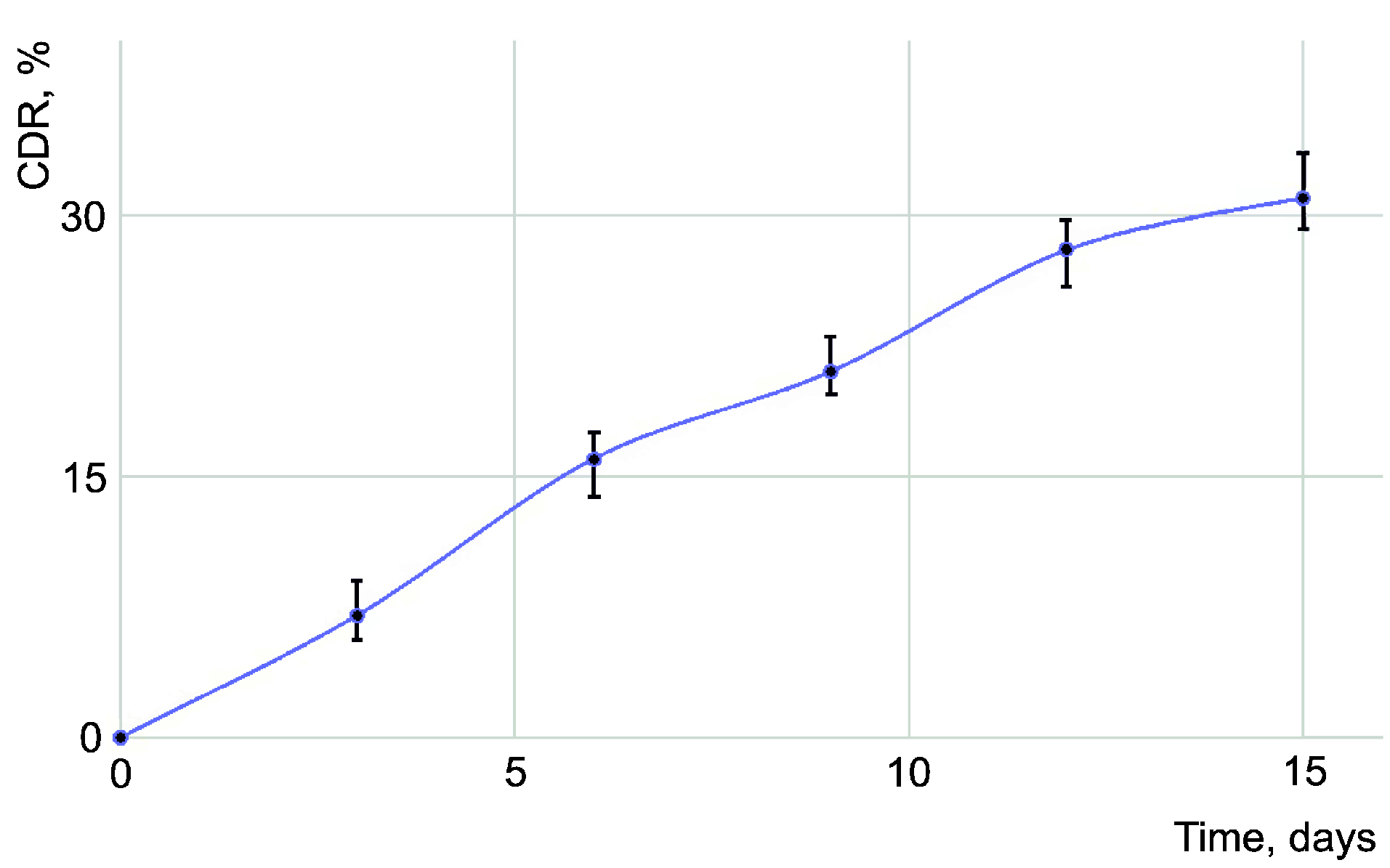
3.3. Dissolution Tests
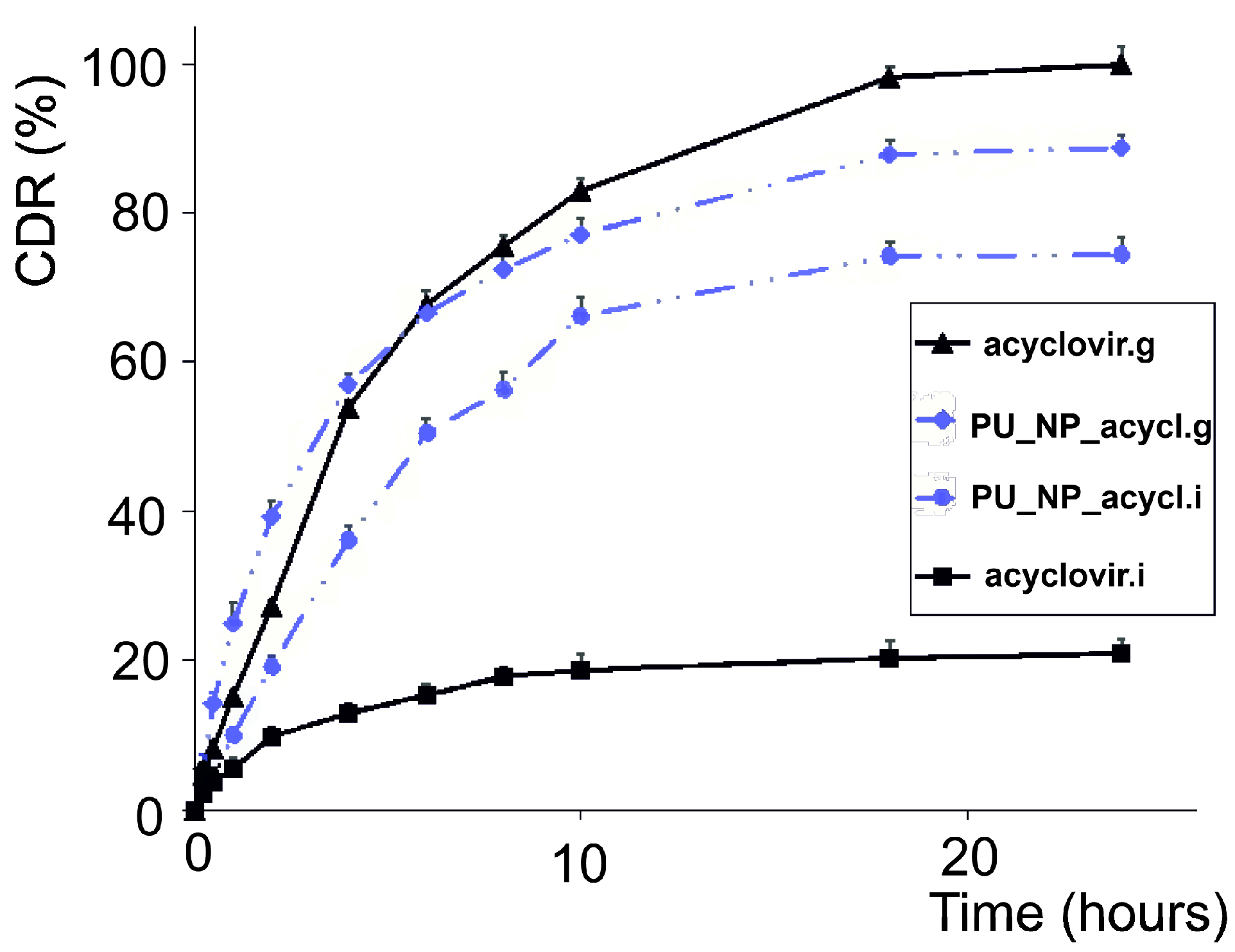
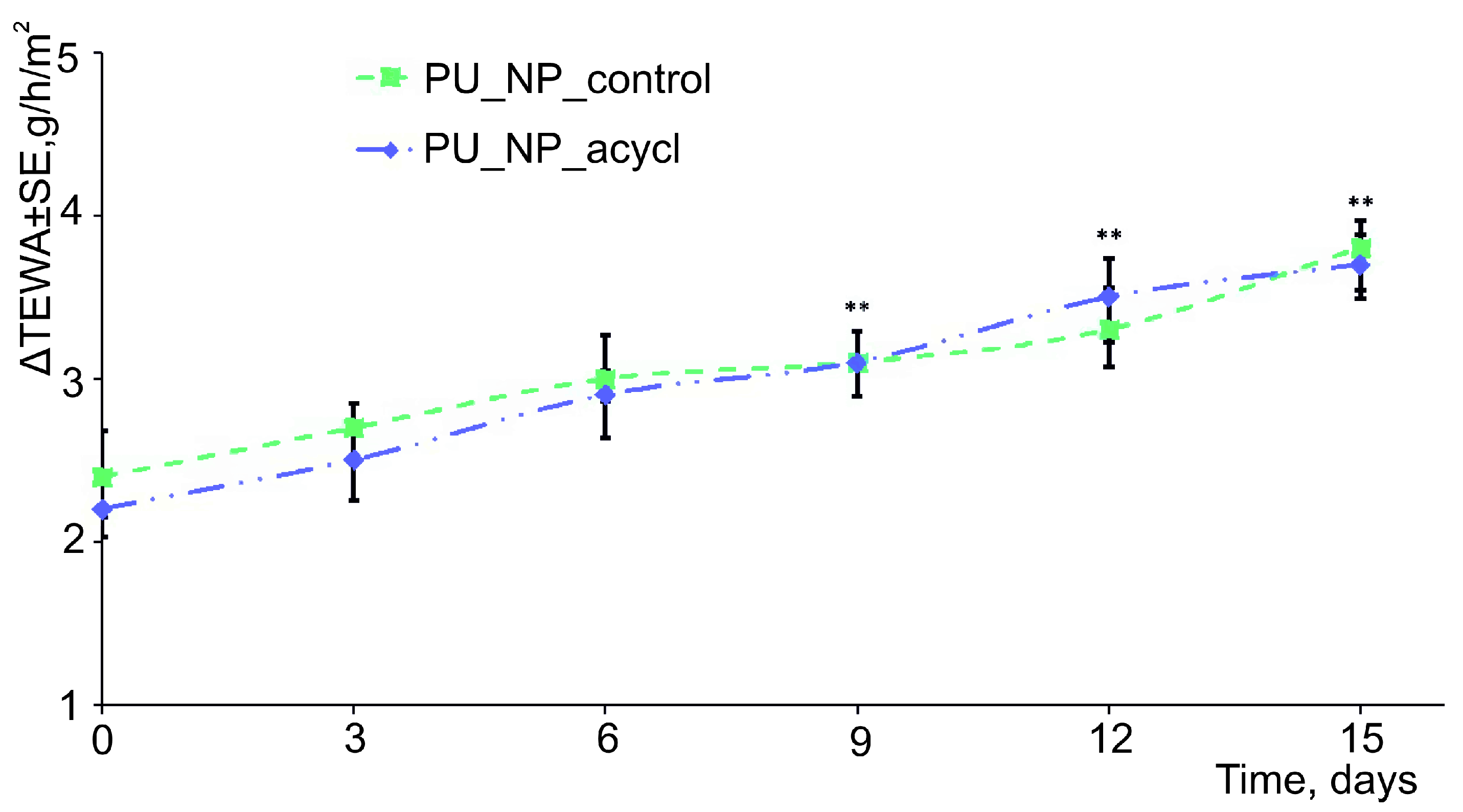
3.4. The Penetration of Membranes
3.5. Thermal Analysis
3.6. Zetasizer Tests
3.7. Scanning Electron Microscopy (SEM)
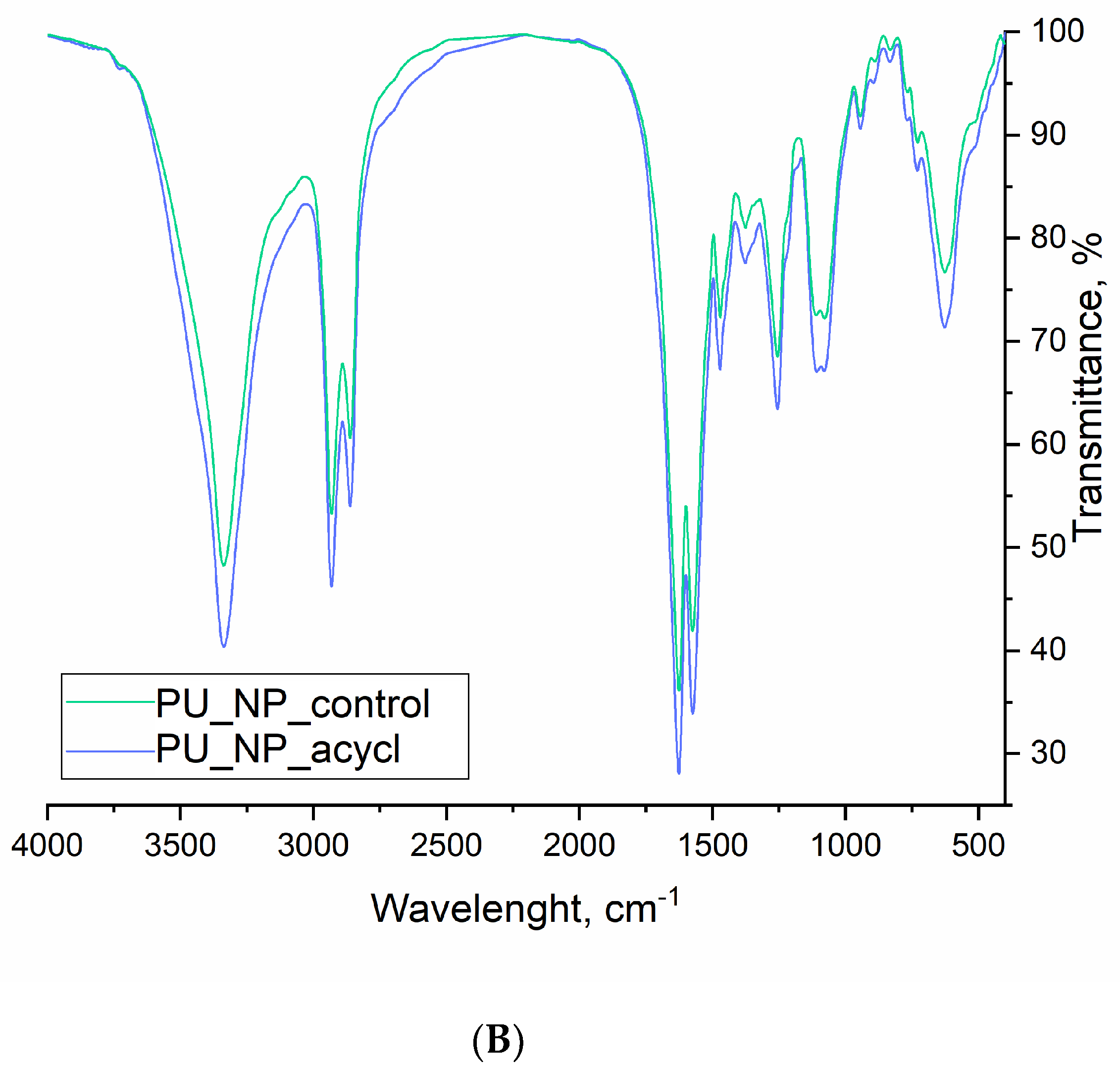
3.8. FT-IR Spectroscopy
3.9. SANS Analysis
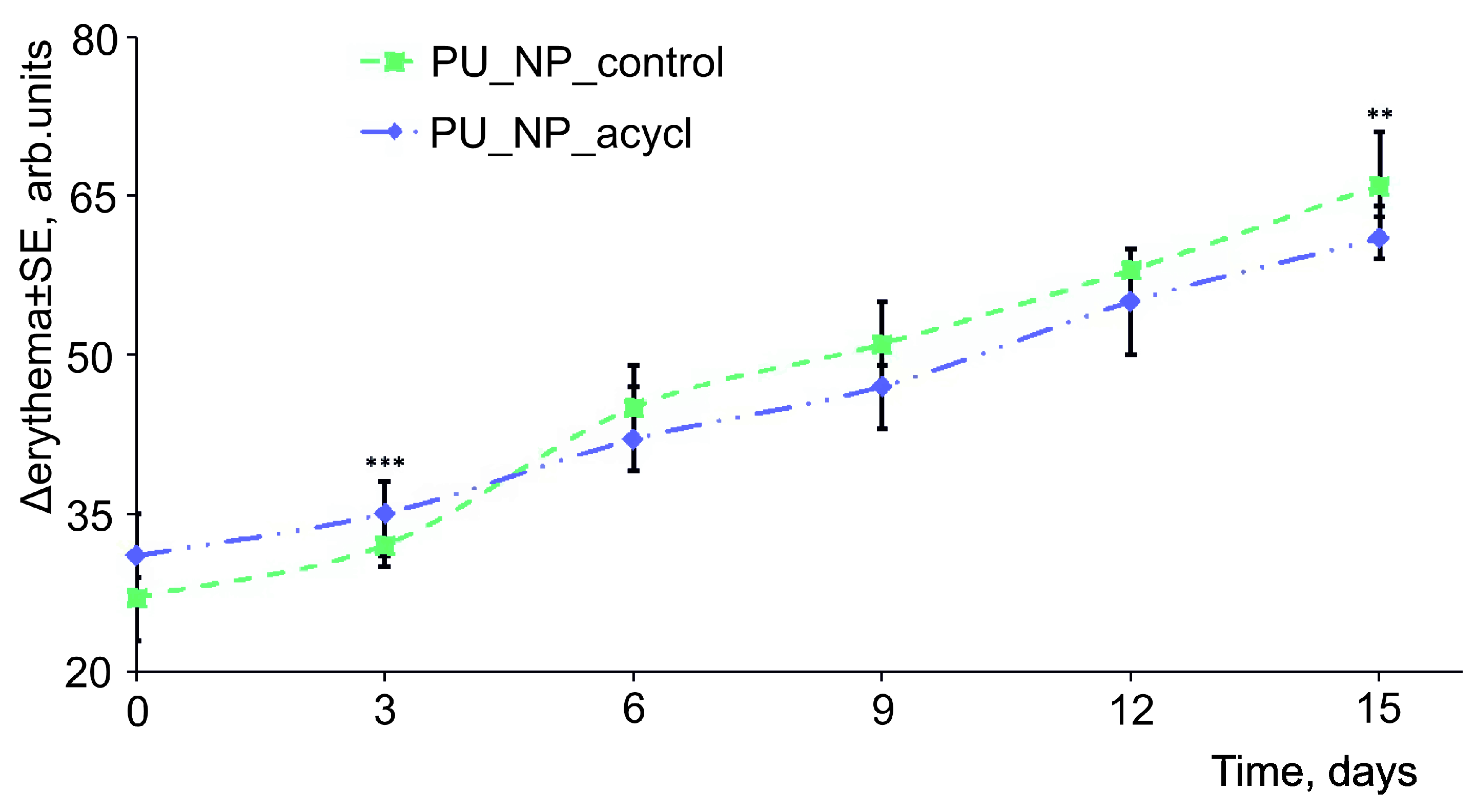
3.10. Skin Irritation Evaluations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kłysik, K.; Pietraszek, A.; Karewicz, A.; Nowakowska, M. Acyclovir in the Treatment of Herpes Viruses—A Review. Curr. Med. Chem. 2020, 27, 4118–4137. [Google Scholar] [CrossRef]
- De Bony, F.; Tod, M.; Bidault, R.; On, N.T.; Posner, J.; Rolan, P. Multiple interactions of cimetidine and probenecid with valaciclovir and its metabolite acyclovir. Antimicrob. Agents Chemother. 2002, 46, 458–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.J.; Jang, H.N.; Cho, H.S.; Bae, E.; Lee, T.W.; Chang, S.H.; Park, D.J. The incidence, risk factors, and clinical outcomes of acute kidney injury (staged using the RIFLE classification) associated with intravenous acyclovir administration. Ren. Fail. 2018, 40, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.; Main, J. Aciclovir neurotoxicity is an important side effect of therapy in patients with renal impairment. Clin. Med. 2009, 9, 630. [Google Scholar] [CrossRef] [PubMed]
- Guney, E.; Akcay, B.I.S.; Erdogan, G.; Unlu, C.; Bozkurt, K.; Onur, U. Systemic side effects of antiviral therapy in a patient with acute retinal necrosis. Ocul. Immunol. Inflamm. 2014, 22, 233–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoaib, M.; Bahadur, A.; Saeed, A.; Ur Rahman, M.S.; Naseer, M.M. Biocompatible, pH-responsive, and biodegradable polyurethanes as smart anti-cancer drug delivery carriers. React. Funct. Polym. 2018, 127, 153–160. [Google Scholar] [CrossRef]
- Shoaib, M.; Ur Rahman, M.S.; Saeed, A.; Naseer, M.M. Mesoporous bioactive glass-polyurethane nanocomposites as reservoirs for sustained drug delivery. Colloids Surf. B Biointerfaces. 2018, 172, 806–811. [Google Scholar] [CrossRef]
- Lembo, D.; Swaminathan, S.; Donalisio, M.; Civra, A.; Pastero, L.; Aquilano, D.; Vavia, P.; Trotta, F.; Cavalli, R. Encapsulation of Acyclovir in new carboxylated cyclodextrin-based nanosponges improves the agent’s antiviral efficacy. Int. J. Pharm. 2013, 443, 262–272. [Google Scholar] [CrossRef]
- Reolon, J.B.; Brustolin, M.; Accarini, T.; Viçozzi, G.P.; Sari, M.H.M.; Bender, E.A.; Haas, S.E.; Brum, M.C.S.; Gündel, A.; Colomé, L.M. Co-encapsulation of acyclovir and curcumin into microparticles improves the physicochemical characteristics and potentiates in vitro antiviral action: Influence of the polymeric composition. Eur. J. Pharm. Sci. 2019, 131, 167–176. [Google Scholar] [CrossRef]
- Hassan, H.; Adam, S.K.; Othman, F.; Shamsuddin, A.F.; Basir, R. Antiviral nanodelivery systems: Current trends in acyclovir administration. J. Nanomater. 2016, 2016, 4591634. [Google Scholar] [CrossRef] [Green Version]
- Cîtu, I.M.; Borcan, F.; Zambori, C.; Tița, B.; Păunescu, V.; Ardelean, S. Influence of crosslinking agent—chain extender ratio on the properties of hyperbranched polyurethane structures used as dendritic drug carrier. Rev. Chim. Bucharest. 2015, 66, 119–123. [Google Scholar]
- Ali, B.M.; Velavan, B.; Sudhandiran, G.; Sridevi, J.; Nasar, A.S. Radical dendrimers: Synthesis, anti-tumor activity and enhanced cytoprotective performance of TEMPO free radical functionalized polyurethane dendrimers. Eur. Polym. J. 2019, 122, 109354. [Google Scholar] [CrossRef]
- Kütting, M.; Roggenkamp, J.; Urban, U.; Schmitz-Rode, T.; Steinseifer, U. Polyurethane heart valves: Past, present and future. Expert Rev. Med. Devices 2011, 8, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.S.; White, K.A.; Wolcott, C.A.; Wang, A.Y.; Kuhn, R.W.; Taylor, J.E.; John, J.K. Development of a hybrid artificial pancreas with a dense polyurethane membrane. ASAIO J. 1993, 39, M261–M267. [Google Scholar]
- Bolcu, C.; Borcan, F. The Study of the Reactions of 2,4-dihydroxy-benzophenone with mono- and diisocyanates. Mater. Plast. 2005, 42, 35–39. [Google Scholar]
- Brockman, K.S.; Lai, B.F.L.; Kizhakkedathu, J.N.; Santerre, J.P. Hemocompatibility of Degrading Polymeric Biomaterials: Degradable Polar Hydrophobic Ionic Polyurethane versus Poly(lactic-co-glycolic) Acid. Biomacromolecules 2017, 18, 2296–2305. [Google Scholar] [CrossRef]
- van Minnen, B.; van Leeuwen, M.B.; Kors, G.; Zuidema, J.; van Kooten, T.G.; Bos, R.R. In vivo resorption of a biodegradable polyurethane foam, based on 1,4-butanediisocyanate: A three-year subcutaneous implantation study. J. Biomed. Mater. Res. A 2008, 85, 972–982. [Google Scholar] [CrossRef]
- Munteanu, M.F.; Ardelean, A.; Borcan, F.; Trifunschi, S.I.; Gligor, R.; Ardelean, S.A.; Coricovac, D.; Pinzaru, I.; Andrica, F.; Borcan, L.-C. Mistletoe and Garlic Extracts as Polyurethane Carriers—A Possible Remedy for Choroidal Melanoma. Curr. Drug Deliv. 2017, 14, 1178–1188. [Google Scholar] [CrossRef]
- Borcan, L.C.; Dudas, Z.; Len, A.; Fuzi, J.; Borcan, F.; Tomescu, M.C. Synthesis and characterization of a polyurethane carrier used for a prolonged transmembrane transfer of a chili pepper extract. Int. J. Nanomed. 2018, 13, 7155–7166. [Google Scholar] [CrossRef] [Green Version]
- Borcan, F.; Preda, M.; Borcan, L.C.; Pinzaru, I.; Florescu, S.; Sisu, E.; Poenaru, M. Comparative Characterization of Birch Bark Extracts Encapsulated Inside Polyurethane Microstructures. Mater. Plast. 2018, 55, 385–388. [Google Scholar] [CrossRef]
- Borcan, F.; Chirita-Emandi, A.; Andreescu, N.I.; Borcan, L.-C.; Albulescu, R.C.; Puiu, M.; Tomescu, M.C. Synthesis and preliminary characterization of polyurethane nanoparticles with ginger extract as a possible cardiovascular protector. Int. J. Nanomed. 2019, 14, 3691–3703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyurethane Market. Available online: https://www.marketsandmarkets.com/Market-Reports/polyurethane-market-151784541.html (accessed on 7 November 2020).
- Reddy, S.A.; Chakraborty, R.; Sen, S.; Parameshappa, B. Spectrophotometric determination and validation of Acyclovir. Arch. Appl. Sci. Res. 2011, 3, 328–332. [Google Scholar]
- Quan, H.; Park, Y.K.; Kim, S.K.; Heo, S.J.; Koak, J.Y.; Han, J.S.; Lee, J.H. Surface Characterization and Human Stem Cell Behaviors of Zirconia Implant Disks Biomimetic-Treated in Simulated Body Fluid. Int. J. Oral Maxillofac. Implant. 2016, 31, 928–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racoviceanu, R.; Trandafirescu, C.; Voicu, M.; Ghiulai, R.; Borcan, F.; Dehelean, C.; Watz, C.; Aigner, Z.; Ambrus, R.; Coricovac, D.E.; et al. Solid Polymeric Nanoparticles of Albendazole: Synthesis, Physico-Chemical Characterization and Biological Activity. Molecules 2020, 25, 5130. [Google Scholar] [CrossRef] [PubMed]
- Glatter, O.; Kratky, O. Small Angle X-ray Scattering; Academic Press: New York, NY, USA, 1982; pp. 17–52. [Google Scholar]
- Karolewicz, B.; Nartowski, K.; Pluta, J.; Górniak, A. Physicochemical characterization and dissolution studies of acyclovir solid dispersions with Pluronic F127 prepared by the kneading method. Acta Pharm. 2016, 66, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Boretos, J.W.; Pierce, W.S. Segmented polyurethanes: A polyester polymer. An initial evaluation for biomedical applications. J. Biomed. Mater. Res. 1968, 2, 121–130. [Google Scholar] [CrossRef]
- Pivec, T.; Smole, M.; Gašparič, P.; Kleinschek, K.S. Polyurethanes for medical use. Tekstilec 2017, 60, 182–197. [Google Scholar] [CrossRef]
- Rangasamy, M.; Ayyasamy, B.; Raju, S.; Gummadevelly, S.; Shaik, S. Formulation and in vitro evaluation of niosome encapsulated Acyclovir. J. Pharm. Res. 2008, 1, 163–166. [Google Scholar]
- Lima, T.; Feitosa, R.C.; Dos Santos-Silva, E.; Dos Santos-Silva, A.M.; Siqueira, E.; Machado, P.; Cornélio, A.M.; do Egito, E.; Fernandes-Pedrosa, M.F.; Farias, K.; et al. Improving Encapsulation of Hydrophilic Chloroquine Diphosphate into Biodegradable Nanoparticles: A Promising Approach against Herpes Virus Simplex-1 Infection. Pharmaceutics 2018, 10, 255. [Google Scholar] [CrossRef] [Green Version]
- Pacifici, G.N. The Effects and Pharmacokinetics of Acyclovir in Neonates. Int. J. Pediatr. 2016, 4, 4099–4115. [Google Scholar] [CrossRef]
- Meadows, K.C.; Dressman, J.B. Mechanism of acyclovir uptake in rat jejunum. Pharm. Res. 1990, 7, 299–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Sharma, R.; Jain, D.K. Nanotechnology Based Approaches for Enhancing Oral Bioavailability of Poorly Water Soluble Antihypertensive Drugs. Scientifica 2016, 2016, 8525679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Antiviral and Antineoplastic Drugs, and Other Pharmaceutical Agents. Available online: https://www.ncbi.nlm.nih.gov/books/NBK401433/ (accessed on 17 December 2020).
- Owusu, F.W.A.; Boakye-Gyasi, M.E.; Mante, P.K.; Ekuadzi, E.; Ofori-Kwakye, K.; Woode, E. Formulation and evaluation of sustained release matrix tablets of capparis erythrocarpos roots extract to improve patient compliance in management of arthritis. Sci. Afr. 2019, 6, e00172. [Google Scholar] [CrossRef]
- Soni, P.; Solanki, D. Formulation and Evaluation of Sustained Release Matrix Tablet of Antiviral Drug by Natural polysaccharide. Int. J. Chemtech Res. 2018, 11, 323–328. [Google Scholar] [CrossRef]
- Shin, S.; Kim, T.H.; Jeong, S.W.; Chung, S.E.; Lee, D.Y.; Kim, D.-H.; Shin, B.S. Development of a gastroretentive delivery system for acyclovir by 3D printing technology and its in vivo pharmacokinetic evaluation in Beagle dogs. PLoS ONE 2019, 14, e0216875. [Google Scholar] [CrossRef]
- Stulzer, H.K.; Lacerda, L.; Tagliari, M.P.; Silva, M.A.S.; Fávere, V.T.; Laranjeira, M.C.M. Synthesis and characterization of cross-linked malonylchitosan microspheres for controlled release of acyclovir. Carb. Polym. 2008, 73, 490–497. [Google Scholar] [CrossRef]
- Angle, M.R.; Wang, A.; Thomas, A.; Schaefer, A.T.; Melosh, N.A. Penetration of cell membranes and synthetic lipid bilayers by nanoprobes. Biophys. J. 2014, 107, 2091–2100. [Google Scholar] [CrossRef] [Green Version]
- Marini, V.G.; Martelli, S.M.; Zornio, C.F.; Caon, T.; Simões, C.M.O.; Micke, G.A.; de Oliveira, M.A.L.; Machado, V.G.; Soldi, V. Biodegradable nanoparticles obtained from zein as a drug delivery system for terpinen-4-ol. Quim. Nova 2014, 37, 839–843. [Google Scholar] [CrossRef]
- Somdee, P.; Lassu-Kuknyo, T.; Konya, C.; Szabó, T.; Marossy, K. Thermal analysis of polyurethane elastomers matrix with different chain extender contents for thermal conductive application. J. Therm. Anal. Calorim. 2019, 138, 1003–1010. [Google Scholar] [CrossRef] [Green Version]
- Day, N.S.; Majumdar, S.; Rao, M.E.B. Multiparticulate Drug Delivery Systems for Controlled Release. Trop. J. Pharm. Res. 2008, 7, 1067–1075. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heghes, A.; Șoica, C.M.; Ardelean, S.; Ambrus, R.; Muntean, D.; Gălușcan, A.; Dragoș, D.; Ionescu, D.; Borcan, F. Influence of emulsifiers on the characteristics of polyurethane structures used as drug carrier. Chem. Cent. J. 2013, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Borcan, F.; Len, A.; Bordejevic, D.A.; Dudas, Z.; Tomescu, M.C. Obtaining and characterization of a polydisperse system used as a transmembrane carrier for isosorbide derivatives. Front. Chem. 2020, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, D.; Mahmoudi, N.; Li, P.; Ma, K.; Doutch, J.; Foglia, F.; Heenan, R.K.; Barlow, D.; Lawrence, M.J. Revealing the Hidden Details of Nanostructure in a Pharmaceutical Cream. Sci. Rep. 2020, 10, 4082. [Google Scholar] [CrossRef]
- Kéri, M.; Forgács, A.; Papp, V.; Bányai, I.; Veres, P.; Len, A.; Dudás, Z.; Fábián, I.; Kalmár, J. Gelatin content governs hydration induced structural changes in silica-gelatin hybrid aerogels—Implications in drug delivery. Acta Biomat. 2020, 105, 131–145. [Google Scholar] [CrossRef]
- Ahuja, G.; Pathak, K. Porous carriers for controlled/modulated drug delivery. Indian J. Pharm. Sci. 2009, 71, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Kose, O.; Erkekoglu, P.; Sabuncuoglu, S.; Kocer-Gumusel, B. Evaluation of skin irritation potentials of different cosmetic products in Turkish market by reconstructed human epidermis model. Regul. Toxicol. Pharmacol. 2018, 98, 268–273. [Google Scholar] [CrossRef]
- Oestmann, E.; Lavrijsen, A.P.M.; Hermans, J.; Ponec, M. Skin barrier function in healthy volunteers as assessed by transepidermal water loss and vascular response to hexyl nicotinate: Intra- and inter-individual variability. Br. J. Dermatol. 1993, 128, 130–136. [Google Scholar] [CrossRef]
- Coleman, K.P.; Grailer, T.P.; McNamara, L.R.; Rollins, B.L.; Christiano, N.J.; Kandárová, H.; De Jonge, W.H. Preparation of irritant polymer samples for an in vitro round robin study. Toxicol. Vitr. 2018, 50, 401–406. [Google Scholar] [CrossRef]
- Ciorba, D.; Tataru, A.; Avram, A.; Almasi, A.; Moldovan, M. Influence of environmental chloroform concentrations on biophysics skin parameters. Farmacia 2015, 63, 313–317. [Google Scholar]






| Sample | Size, nm (Signal Intensity) | PDI 1 | Zeta Potential, mV |
|---|---|---|---|
| PU_NP_acycl | 78 ± 4 (100%) | 0.2 | +29.1 ± 3.4 |
| PU_NP_control | 91 ± 9 (82%) 214 ± 11 (18%) | 0.5 | +26.9 ± 2.1 |
| Wavenumber, cm−1 | Functional Group | Presence in Sample | |
|---|---|---|---|
| Acyclovir | PU_NP_acycl/PU_NP_control | ||
| 3520–3440, b | Dimeric O-H stretch32 | yes | no |
| 3340–3335, s | N-H stretch10,32 | no | yes |
| 3300–3180, b | O-H stretch32 | yes | no |
| 2930–2660, s | Methyl C-H asym/sym stretch32 | no | yes |
| 2860–2710, b | Methoxy C-H stretch32 | yes | no |
| 1710–1630, b | Ring C=O stretch10 | yes | no |
| 1625–1575, s | Urethane N-H deformation10 | no | yes |
| 1535–1470, s | Methylene C-H bend32 N-H deformation10 | yes | yes |
| 1390–1350, b | Aromatic C-N stretch32 | yes | no |
| 1255 + 940–730 w, s | C-O- stretch32 | no | yes |
| 1220–1180, s | Tertiary amine C-N stretch32 | yes | no |
| 1110–1075, b | Alkyl ether C-O-C stretch32 | yes | yes |
| 770–625, s | Skeletal C-C vibrations32 | yes | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borcan, F.; Len, A.; Dehelean, C.A.; Dudás, Z.; Ghiulai, R.; Iftode, A.; Racoviceanu, R.; Soica, C.M. Design and Assessment of a Polyurethane Carrier Used for the Transmembrane Transfer of Acyclovir. Nanomaterials 2021, 11, 51. https://doi.org/10.3390/nano11010051
Borcan F, Len A, Dehelean CA, Dudás Z, Ghiulai R, Iftode A, Racoviceanu R, Soica CM. Design and Assessment of a Polyurethane Carrier Used for the Transmembrane Transfer of Acyclovir. Nanomaterials. 2021; 11(1):51. https://doi.org/10.3390/nano11010051
Chicago/Turabian StyleBorcan, Florin, Adél Len, Cristina A. Dehelean, Zoltán Dudás, Roxana Ghiulai, Andrada Iftode, Roxana Racoviceanu, and Codruta M. Soica. 2021. "Design and Assessment of a Polyurethane Carrier Used for the Transmembrane Transfer of Acyclovir" Nanomaterials 11, no. 1: 51. https://doi.org/10.3390/nano11010051




.jpg)

