A Novel Thermochemical Metal Halide Treatment for High-Performance Sb2Se3 Photocathodes
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of Sb2Se3 Layers
2.2. SbCl3 Treatment
2.3. Film Characterization
2.4. Structure Fabrication and Characterization
3. Result and Discussion
3.1. Morphological Studies
3.2. Compositional Analysis
3.3. Structural Analysis
3.4. Optical Data Analysis
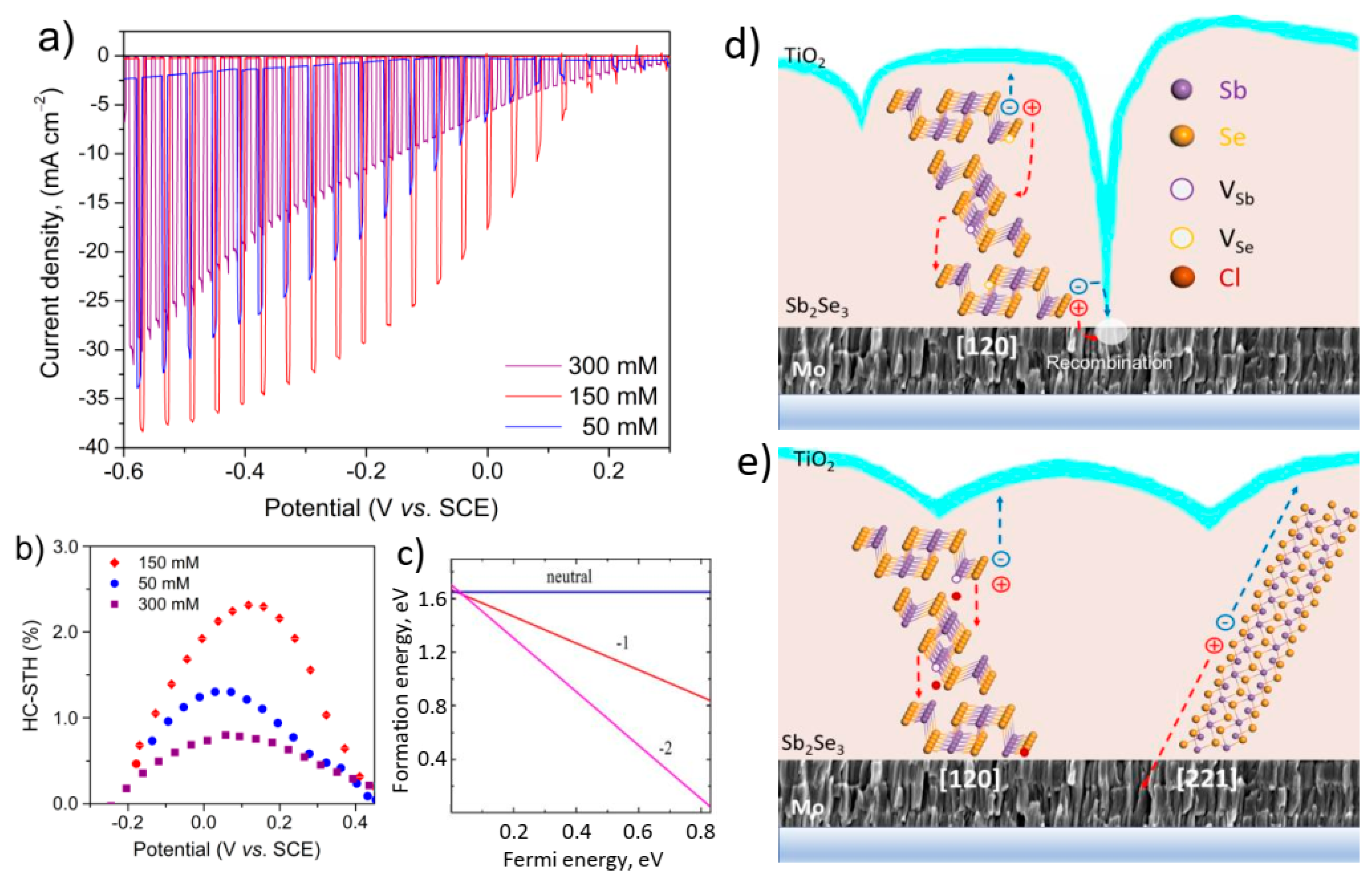
3.5. Photoelectrochemical (PEC) Analysis
3.6. PEC Performance of Treated Sb2Se3 Photocathodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poortmans, J.; Arkhipov, V. Thin Film Solar Cells: Fabrication, Characterization and Applications; Wiley: Chichester, UK, 2006. [Google Scholar]
- Solar Frontier Press Release Solar Frontier Achieves World Record Thin-Film Solar Cell Efficiency of 23.35%. Available online: http://www.solar-frontier.com/eng/news/2019/0117_press.html (accessed on 20 December 2020).
- Green, M.A.; Hishikawa, Y.; Warta, W. Solar Cell Efficiency Tables (Version 50). Prog. Photovolt. Res. Appl. 2017, 25, 668–676. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.H.; Richter, M.H.; May, M.M.; Ohlmann, J.; Lackner, D.; Dimroth, F.; Hannappel, T.; Atwater, H.A.; Lewerenz, H.J. Monolithic Photoelectrochemical Device for Direct Water Splitting with 19% Efficiency. ACS Energy Lett. 2018, 3, 1795–1800. [Google Scholar] [CrossRef]
- Polivtseva, S.; Acik, I.O.; Krunks, M.; Tõnsuaadu, K.; Mere, A. Thermoanalytical study of precursors for tin sulfide thin films deposited by chemical spray pyrolysis. J. Therm. Anal. Calorim. 2015, 121, 177–185. [Google Scholar] [CrossRef]
- Kois, J.; Polivtseva, S.; Bereznev, S. The cost-effective deposition of ultra-thin titanium (IV) oxide passivating layers for improving photoelectrochemical activity of SnS electrodes. Thin Solid Films 2019, 671, 152–156. [Google Scholar] [CrossRef]
- Li, Z.; Liang, X.; Li, G.; Liu, H.; Zhang, H.; Guo, J.; Chen, J.; Shen, K.; San, X.; Yu, W.; et al. 9.2%-efficient core-shell structured antimony selenide nanorod array solar cells. Nat. Commun. 2019, 10, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spalatu, N.; Hiie, J.; Kaupmees, R.; Volobujeva, O.; Krustok, J.; Oja-Acik, I.; Krunks, M. Post-deposition Processing of SnS Thin Films and Solar Cells: Prospective Strategy to Obtain Large, Sintered and Doped SnS Grains by Recrystallization in the Presence of a Metal Halide Flux. ACS Appl. Mater. Interfaces 2019, 11, 17539–17554. [Google Scholar] [CrossRef]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y.; Mitzi, D.B. Device Characteristics of CZTSSe Thin-Film Solar Cells with 12.6% Efficiency. Adv. Energy Mater. 2014, 4, 1301465. [Google Scholar] [CrossRef]
- Kois, J.; Polivtseva, S.; Mamedov, D.; Samieipour, A.; Karazhanov, S.Z. Visible Light-Assisted Instability of Kesterite Cu2ZnSnS4: What are the implications? Sol. Energy Mater. Sol. Cells 2020, 208, 110384. [Google Scholar] [CrossRef]
- Yao, T.; An, X.; Han, H.; Chen, J.Q.; Li, C. Photoelectrocatalytic Materials for Solar Water Splitting. Adv. Energy Mater. 2018, 8, 1800210. [Google Scholar] [CrossRef]
- Kim, J.H.; Hansora, D.; Sharma, P.; Jang, J.W.; Lee, J.S. Toward practical solar hydrogen production—An artificial photosynthetic leaf-to-farm challenge. Chem. Soc. Rev. 2019, 48, 1908–1971. [Google Scholar] [CrossRef]
- Hu, S.; Xiang, C.; Haussener, S.; Bergercd, A.D.; Lewis, N.S. An analysis of the optimal band gaps of light absorbers in integrated tandem photoelectrochemical water-splitting systems. Energy Environ. Sci. 2013, 6, 2984. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Woo, H.; Wu, P.; Hong, H.J.; Jung, W.G.; Kim, B.J.; Vanel, J.C.; Choi, J.W. Simple eco-friendly synthesis of the surfactant free SnS nanocrystal toward the photoelectrochemical cell application. Sci. Rep. 2017, 7, 16531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Oh, Y.; Kim, J.; Jeong, M.J.; Park, J.H.; Moon, J. Molecular Chemistry Controlled Hybrid-ink Derived Efficient Cu2ZnSnS4 Photocathodes for Photoelectrochemical Water Splitting. ACS Energy Lett. 2016, 1, 1127–1136. [Google Scholar] [CrossRef]
- Oh, Y.; Yang, W.; Kim, J.; Jeong, S.; Moon, J. Enhanced Photocurrent of Transparent CuFeO2 Photocathodes by Self-Light-Harvesting Architecture. ACS Appl. Mater. Interfaces 2017, 9, 14078–14087. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Xue, D.J.; Tang, J. Antimony selenide thin-film solar cells. Semicond. Sci. Technol. 2016, 31, 063001. [Google Scholar] [CrossRef]
- Wen, X.; Chen, C.; Lu, S.; Li, K.; Kondrotas, R.; Zhao, Y.; Chen, W.; Gao, L.; Wang, C.; Zhang, J.; et al. Vapor transport deposition of antimony selenide thin film solar cells with 7.6% efficiency. Nat. Commun. 2018, 9, 2179. [Google Scholar] [CrossRef]
- Guo, C.; Liang, X.; Liu, T.; Liu, Y.; Yang, L.; Lai, W.; Schropp, R.E.I.; Song, D.; Mai, Y.; Li, Z. 1D/3D Alloying Induced Phase Transition in light absorbers for highly efficient Sb2Se3 Solar Cells. Sol. RRL 2020, 4, 2000054. [Google Scholar] [CrossRef]
- Park, J.; Yang, W.; Tan, J.; Lee, H.; Yun, J.W.; Shim, S.G.; Park, Y.S.; Moon, J. Hierarchal Nanorod-Derived Bilayer Strategy to Enhance the Photocurrent Density of Sb2Se3 Photocathodes for Photoelectrochemical Water Splitting. ACS Energy Lett. 2020, 5, 136–145. [Google Scholar] [CrossRef]
- Zhou, H.; Feng, M.; Feng, M.; Gong, X.; Zhang, D.; Zhou, Y.; Chen, S. Gradient doping of sulfur in Sb2Se3 nanowire arrays as photoelectrochemical photocathode with a 2% half-cell solar-to-hydrogen conversion efficiency. Appl. Phys. Lett. 2020, 116, 113902. [Google Scholar] [CrossRef]
- Yang, W.; Kim, J.H.; Hutter, O.S.; Phillips, L.J.; Tan, J.; Park, J.; Lee, H.; Major, J.D.; Lee, J.S.; Moon, J. Benchmark performance of low-cost Sb2Se3 photocathodes for unassisted solar overall water splitting. Nat. Commun. 2020, 11, 861. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Yang, W.; Oh, Y.; Lee, H.; Lee, S.; Shin, H.; Kim, J.; Moon, J. Self-Oriented Sb2Se3 Nanoneedle Photocathodes for Water Splitting Obtained by Simple Spin-Coating Method. J. Mater. Chem. 2017, A5, 2180. [Google Scholar] [CrossRef]
- Sarah, M.; Nair, M.T.S.; Nair, P.K. Antimony selenide absorber thin films in all-chemically deposited solar cells. J. Electrochem. Soc. 2009, 156, H327–H332. [Google Scholar]
- Polivtseva, S.; Spalatu, N.; Abdalla, A.; Volobujeva, O.; Hiie, J.; Bereznev, S. Pulsed Laser Deposition of Zn(O,Se) Layers for Optoelectronic Application. ACS Appl. Energy Mater. 2018, 11, 6505–6512. [Google Scholar] [CrossRef]
- Spalatu, N.; Krunks, M.; Hiie, J. Structural and optoelectronic properties of CdCl2 activated CdTe thin films modified by multiple thermal annealing. Thin Solid Films 2017, 633, 106–111. [Google Scholar] [CrossRef]
- Wada, T.; Hashimoto, Y.; Nishiwaki, S.; Sato, T.; Hayashi, S.; Negami, T.; Miyake, H. High efficiency Cu(In,Ga)Se2 solar cells with modified CIGS surface. Sol. Energy Mater. Sol. Cells 2001, 67, 305–310. [Google Scholar] [CrossRef]
- Ramanathan, K.; Hasoon, F.S.; Smith, S.; Young, D.L.; Contreras, M.A.; Johnson, P.K.; Pudov, A.O.; Sites, J.R. Surface treatment of CuInGaSe2 thin films and its effect on the photovoltaic properties of solar cells. J. Phys. Chem. Solids 2003, 64, 1495–1498. [Google Scholar] [CrossRef]
- Ros, C.; Andreu, T.; Morante, J.R. Photoelectrochemical water splitting: A road from stable metal oxides to protected thin film solar cells. J. Mater. Chem. A 2020, 8, 10625–10669. [Google Scholar] [CrossRef]
- Xiaobo, H.; Jiahua, T.; Youyang, W.; Juanjuan, X.; Guoen, W.; Chuanjun, Z.; Shaoqiang, C.; Ziqiang, Z.; Junhao, C. 5.91%-efficient Sb2Se3 solar cells with a radio-frequency magnetron-sputtered CdS buffer layer. Appl. Mater. Today 2019, 16, 367–374. [Google Scholar]
- Tang, R.; Zheng, Z.H.; Su, Z.H.; Li, X.J.; Wei, Y.D.; Zhang, X.H.; Fu, Y.Q.; Luo, J.T.; Fan, P.; Liang, G.X. Highly efficient and stable planar heterojunction solar cell based on sputtered and post-selenized Sb2Se3 thin film. Nano Energy 2019, 64, 103929. [Google Scholar] [CrossRef]
- Hobson, T.D.C.; Phillips, L.J.; Hutter, O.S.; Shiel, H.; Swallow, J.E.N.; Savory, C.N.; Nayak, P.K.; Mariotti, S.; Das, B.; Bowen, L.; et al. Isotype Heterojunction Solar Cells Using n-Type Sb2Se3 Thin Films. Chem. Mater. 2020, 32, 2621–2630. [Google Scholar] [CrossRef] [Green Version]
- Steinmann, V.; Brandt, R.; Chakraborty, R.; Jaramillo, R.; Young, M.; Ofori-Okai, B.K.; Yang, C.; Polizzotti, A.; Nelson, K.A.; Gordon, R.G.; et al. The Impact of Sodium Contamination in Tin Sulfide Thin-Film Solar Cells. APL Mater. 2016, 4, 026103. [Google Scholar] [CrossRef] [Green Version]
- Vidal-Fuentes, P.; Guc, M.; Alcobe, X.; Jawhari, T.; Placidi, M.; Pérez-Rodríguez, A.; Saucedo, E.; Izquierdo Roca, V. Multiwavelength excitation Raman scattering study of Sb2Se3. compound: Fundamental vibrational properties and secondary phases detection. 2D Mater. 2019, 6, 045054. [Google Scholar] [CrossRef]
- Allen, J.P.; Carey, J.J.; Walsh, A.; Scanlon, D.O.; Watson, G.W. Electronic structures of antimony oxides. J. Phys. Chem. C 2013, 117, 14759–14769. [Google Scholar] [CrossRef]
- Cody, C.A.; Dicarlo, L.; Darlington, R.K. Vibrational and thermal study of antimony oxides. Inorg. Chem. 1979, 18, 1572–1576. [Google Scholar] [CrossRef]
- Guc, M.; Andrade-Arvizu, J.; Ahmet, I.Y.; Oliva, F.; Placidi, M.; Alcobe, X.; Saucedo, E.; Perez-Rodrıguez, A.; Johnson, A.L.; Izquierdo Roca, V. Structural and vibrational properties of α- and π-SnS polymorphs for photovoltaic applications. Acta Mater. 2020, 183, 1–10. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, I.; Katyal, S.C. Physical and optical properties of binary amorphous selenium-antimony thin films. J. Appl. Phys. 2009, 105, 053509. [Google Scholar] [CrossRef]
- Guo, L.; Shinde, P.S.; Ma, Y.; Li, L.; Pan, S.; Yan, F. Scalable Core–Shell MoS2/Sb2Se3 Nanorod Array Photocathodes for Enhanced Photoelectrochemical Water Splitting. Sol. RRL 2019, 4, 1900442. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, J.S.; Baek, S.K.; Yun, Y.D.; Cho, S.W.; Jung, S.H.; Cho, H.K. Electrochemical surface charge-inversion from semi-insulating Sb2Se3 photoanodes and abrupt photocurrent generation for water splitting. Energy Environ. Sci. 2018, 11, 2540–2549. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Li, C.; Chen, Q.; Zhen, Z.; Jiang, X.; Zhong, M.; Zhang, F.; Zhu, H. Scalable Low-Band-Gap Sb2Se3 Thin-Film Photocathodes for Efficient Visible−Near Infrared Solar Hydrogen Evolution. ACS Nano 2017, 11, 12753–12763. [Google Scholar] [CrossRef]
- Chen, C.; Li, K.; Chen, S.; Wang, L.; Lu, S.; Liu, Y.; Li, D.; Song, H.; Tang, J. Efficiency Improvement of Sb2Se3 Solar Cell via Grain Boundary Inversion. ACS Energy Lett. 2018, 3, 2335–2341. [Google Scholar] [CrossRef]




| Treatment | M (mM) | Elements (at.%) | Ra | Eg (eV) | D (nm) (±0.7) | Lattice Parameters (Å) (±0.001) | |||
|---|---|---|---|---|---|---|---|---|---|
| Sb | Se | (nm) | a | b | c | ||||
| pristine | 42.1 | 57.6 | 30 | 1.30 (1.21) | 73 | 11.591 | 11.729 | 3.901 | |
| SbCl3-glycerol | 50 | 40.8 | 59.2 | 33 | 1.27 | 83 | 11.612 | 11.760 | 3.973 |
| 150 | 40.1 | 59.9 | 21 | 1.34 | 95 | 11.594 | 11.733 | 3.907 | |
| 300 | 40.4 | 59.6 | 30 | 1.32 | 83 | 11.592 | 11.731 | 3.903 | |
| 450 | 42.0 | 57.9 | 38 | 1.32 (1.22) | 75 | 11.591 | 11.730 | 3.902 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polivtseva, S.; Adegite, J.O.; Kois, J.; Mamedov, D.; Karazhanov, S.Z.; Maricheva, J.; Volobujeva, O. A Novel Thermochemical Metal Halide Treatment for High-Performance Sb2Se3 Photocathodes. Nanomaterials 2021, 11, 52. https://doi.org/10.3390/nano11010052
Polivtseva S, Adegite JO, Kois J, Mamedov D, Karazhanov SZ, Maricheva J, Volobujeva O. A Novel Thermochemical Metal Halide Treatment for High-Performance Sb2Se3 Photocathodes. Nanomaterials. 2021; 11(1):52. https://doi.org/10.3390/nano11010052
Chicago/Turabian StylePolivtseva, Svetlana, Joseph Olanrewaju Adegite, Julia Kois, Damir Mamedov, Smagul Zh. Karazhanov, Jelena Maricheva, and Olga Volobujeva. 2021. "A Novel Thermochemical Metal Halide Treatment for High-Performance Sb2Se3 Photocathodes" Nanomaterials 11, no. 1: 52. https://doi.org/10.3390/nano11010052






