Interfacial Design on Graphene–Hematite Heterostructures for Enhancing Adsorption and Diffusion towards Superior Lithium Storage
Abstract
:1. Introduction
2. Calculation Methodology
2.1. Computing Parameters
2.2. Heterostructures Construction
3. Results and Discussion
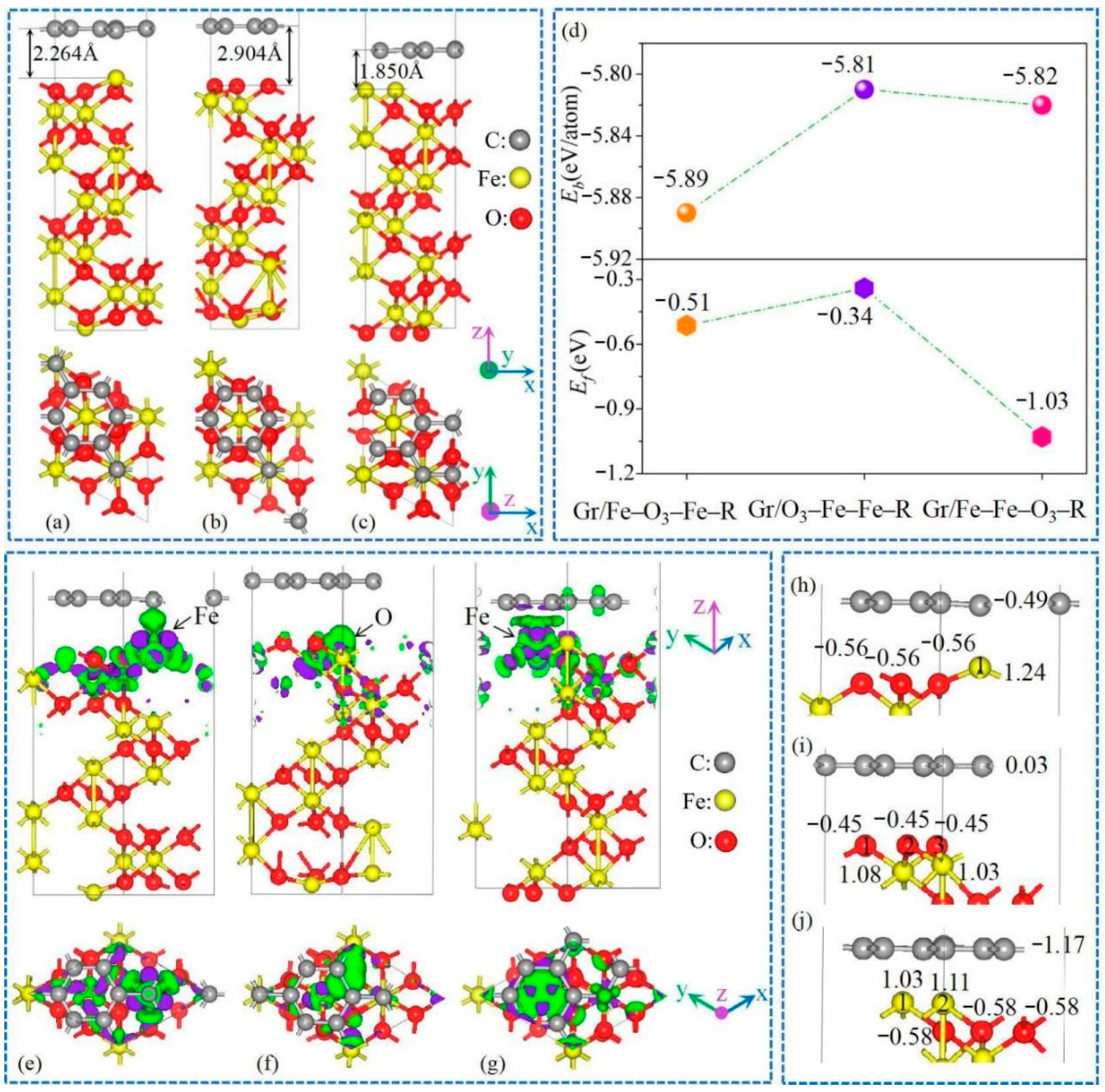
3.1. Geometric Structures
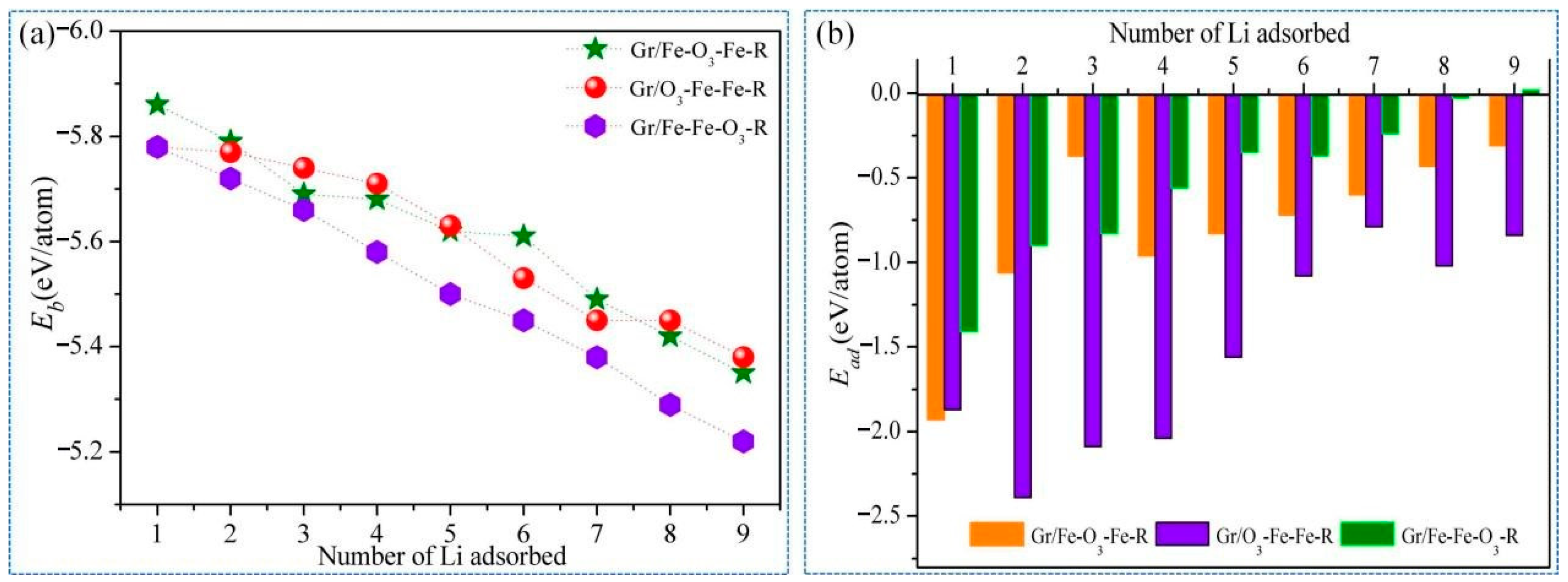
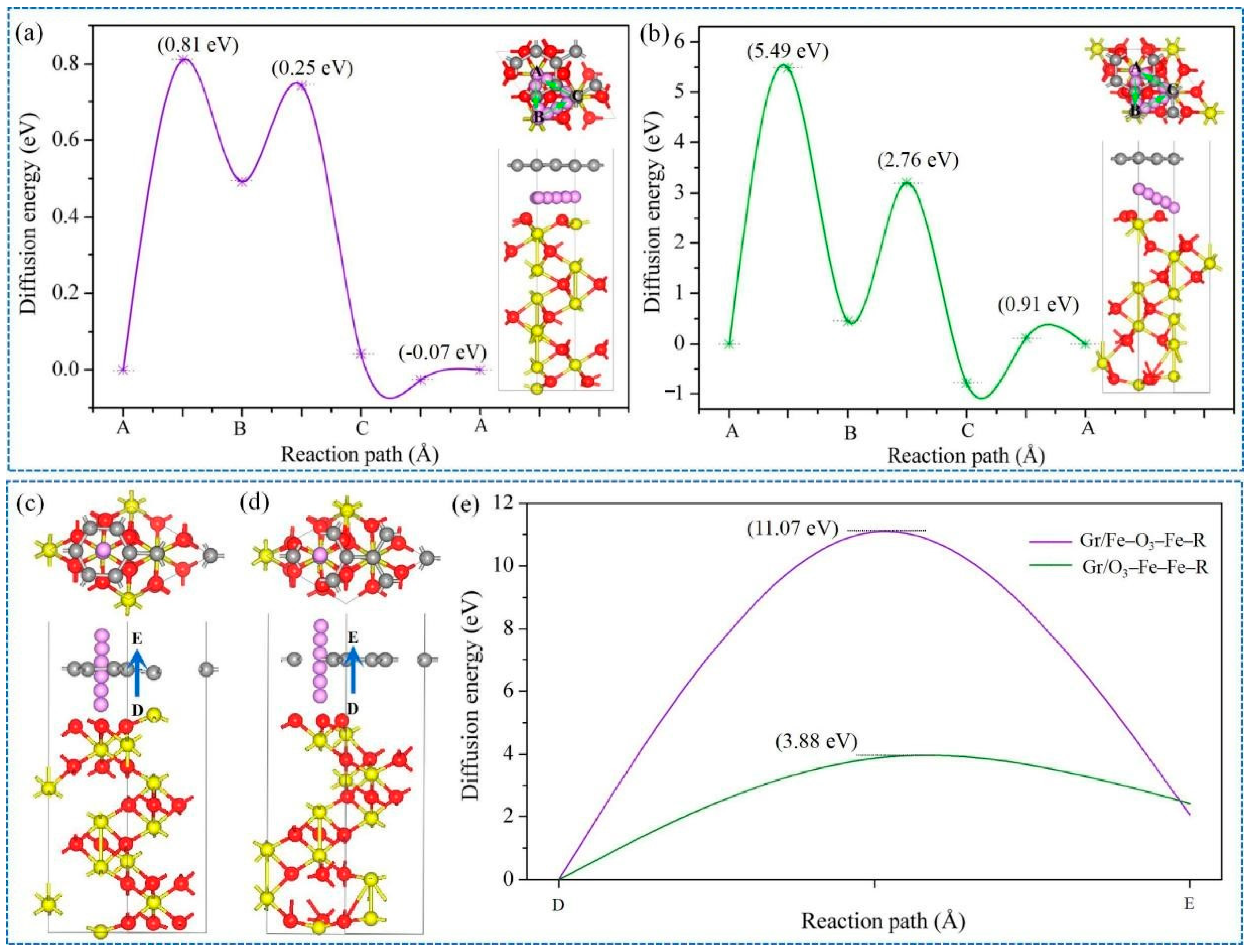
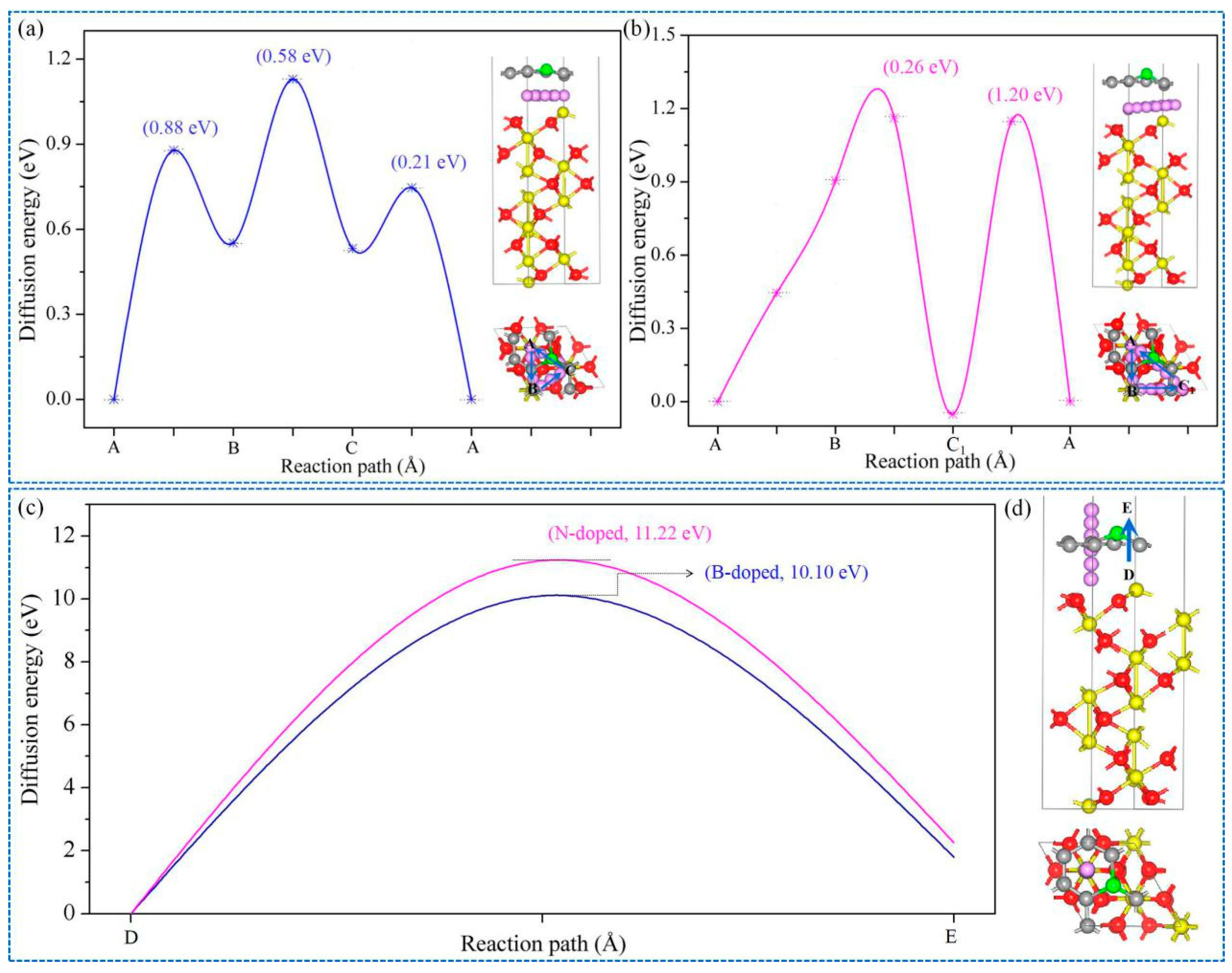
3.2. Lithium Adsorption and Diffusion Behaviours
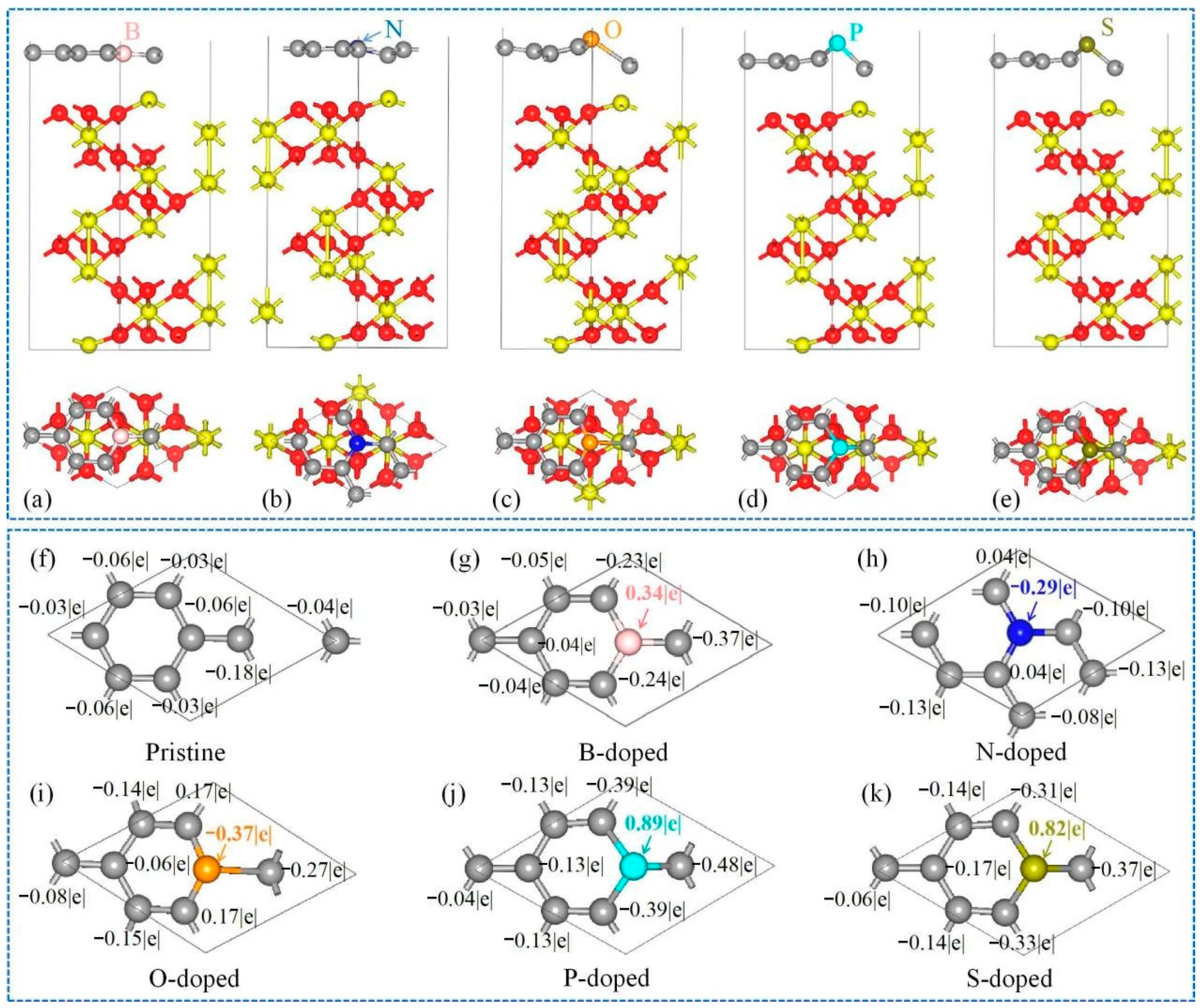
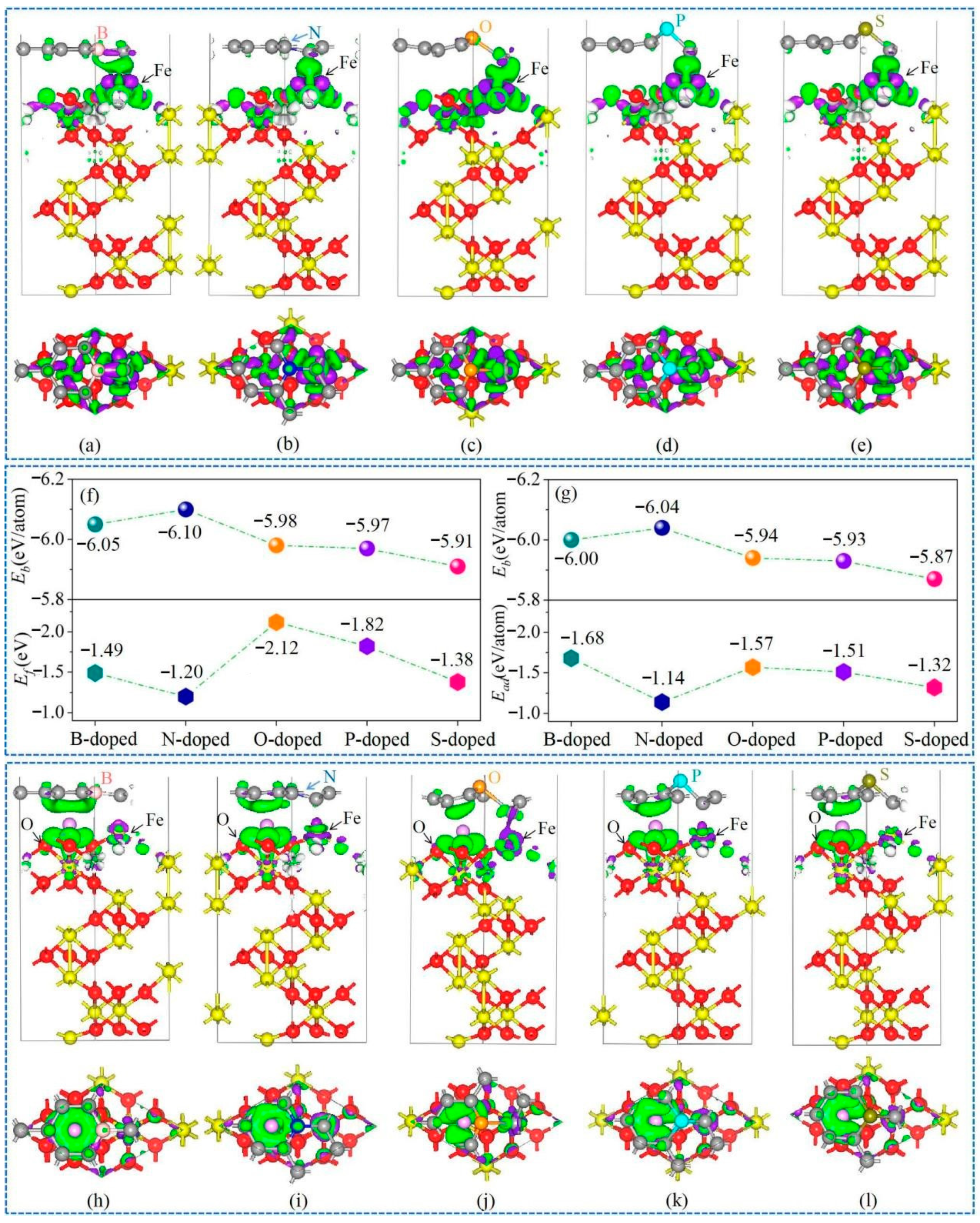
3.3. Interfacial Optimization by Using Heteroatoms Doped Gr
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Xu, L.; Li, W.; Gou, X. α-Fe2O3 nanotubes in gas sensor and lithium-ion battery applications. Adv. Mater. 2005, 17, 582–586. [Google Scholar] [CrossRef]
- Wu, C.Z.; Yin, P.; Zhu, X.; Ouyang, C.Z.; Xie, Y. Synthesis of hematite (α-Fe2O3) nanorods: diameter-size and shape effects on their applications in magnetism, lithium ion battery, and gas sensors. J. Phys. Chem. B 2006, 110, 17806–17812. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Horvat, J.; Kim, H.S.; Kim, W.S.; Wang, G.X. Synthesis of mesoporous α-Fe2O3 nanostructures for highly sensitive gas sensors and high capacity anode materials in lithium ion batteries. J. Phys. Chem. C 2010, 114, 18753–18761. [Google Scholar] [CrossRef]
- Xu, X.; Cao, R.; Jeong, S.; Cho, J. Spindle-like mesoporous α-Fe2O3 anode material prepared from MOF template for high-rate lithium batteries. Nano. Lett. 2012, 12, 4988–4991. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Z.; Zhu, P.N.; Reddy, M.V.; Chowdari, B.V.R.; Ramakrishna, S. Maghemite nanoparticles on electrospun CNFs template as prospective lithium-ion battery anode. ACS Appl. Mater. Interfaces 2014, 6, 1951–1958. [Google Scholar] [CrossRef]
- Guo, W.X.; Sun, W.W.; Lv, L.P.; Kong, S.F.; Wang, Y. Microwave-assisted morphology evolution of Fe-based metal-organic frameworks and their derived Fe2O3 nanostructures for Li-ion storage. ACS Nano 2017, 11, 4198–4205. [Google Scholar] [CrossRef]
- Teng, X.; Qin, Y.; Wang, X.; Li, H.; Shang, X.; Fan, S.; Li, Q.; Xu, J.; Cao, D.; Li, S. A nanocrystalline Fe2O3 film anode prepared by pulsed laser deposition for lithium-ion batteries. Nanoscale Res. Lett. 2018, 13, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Mei, J.; Liao, T.; Kou, L.; Sun, Z. Two-dimensional metal oxide nanomaterials for next-generation rechargeable batteries. Adv. Mater. 2017, 29, 1700176. [Google Scholar] [CrossRef]
- Zeng, S.Y.; Tang, K.B.; Li, T.W.; Liang, Z.H.; Wang, D.; Wang, Y.K.; Zhou, W.W. Hematite hollow spindles and microspheres: Selective synthesis, growth mechanisms, and application in lithium ion battery and water treatment. J. Phys. Chem. C 2007, 111, 10217–10225. [Google Scholar] [CrossRef]
- Zeng, S.Y.; Tang, K.B.; Li, T.W.; Liang, Z.H.; Wang, D.; Wang, Y.K.; Qi, Y.X.; Zhou, W.W. Facile route for the fabrication of porous hematite nanoflowers: Its synthesis, growth mechanism, application in the lithium ion battery, and magnetic and photocatalytic properties. J. Phys. Chem. C 2008, 112, 4836–4843. [Google Scholar] [CrossRef]
- Ma, F.-X.; Hu, H.; Wu, H.B.; Xu, C.-Y.; Xu, Z.C.; Zhen, L.; Lou, X.W. Formation of uniform Fe3O4 hollow spheres organized by ultrathin nanosheets and their excellent lithium storage properties. Adv. Mater. 2015, 27, 4097–4101. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Han, F.; Li, D.; Li, W.-C.; Sun, Q.; Zhang, X.-Q.; Lu, A.-H. Dopamine as the coating agent and carbon precursor for the fabrication of N-doped carbon coated Fe3O4 composites as superior lithium ion anodes. Nanoscale 2013, 5, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.Q.; Kan, J.; Wang, Y. Fe2O3-graphene rice-on-sheet nanocomposite for high and fast lithium ion storage. J. Phys. Chem. C 2011, 115, 20747–20753. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, H.; Zhang, Z.; Bian, Y.; Shen, A.; Xu, P.; Zhao, Y. Subunits controlled synthesis of three-dimensional hierarchical flower-like α- Fe2O3 hollow spheres as high-performance anodes for lithium ion batteries. Appl. Surf. Sci. 2018, 452, 174–180. [Google Scholar] [CrossRef]
- Mei, J.; Liao, T.; Ayoko, G.A.; Bell, J.; Sun, Z.Q. Cobalt oxide-based nanoarchitectures for electrochemical energy applications. Prog. Mater. Sci. 2019, 103, 596–677. [Google Scholar] [CrossRef]
- Liu, T.; Xue, L.; Guo, X.; Zheng, C.G. DFT study of mercury adsorption on α-Fe2O3 surface: Role of oxygen. Fuel 2014, 115, 179–185. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Camellone, M.F.; Gebauer, R. On the electronic, structural, and thermodynamic properties of Au supported on α-Fe2O3 surfaces and their interaction with CO. J. Chem. Phys. 2015, 143, 034704. [Google Scholar] [CrossRef]
- Zhang, M.L.; Luo, W.J.; Li, Z.S.; Yu, T.; Zou, Z.G. Improved photoelectrochemical responses of Si and Ti codoped α-Fe2O3 photoanode films. Appl. Phys. Lett. 2010, 97, 042105. [Google Scholar] [CrossRef]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef]
- Mei, J.; He, T.; Zhang, Q.; Liao, T.; Du, A.; Ayoko, G.; Sun, Z. Carbon-phosphorus bonds-enriched 3D graphene by self-sacrificing black phosphorus nanosheets for elevating capacitive lithium storage. ACS Appl. Mater. Interfaces 2020, 12, 21720–21729. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Zhang, Y.W.; Liao, T.; Sun, Z.Q.; Dou, S.X. Strategies for improving the lithium-storage performance of two-dimensional nanomaterials. Natl. Sci. Rev. 2018, 5, 389–416. [Google Scholar] [CrossRef]
- Pan, H.; Meng, X.; Qi, X.; Qin, G. Electronic and optical properties of MoS2/α-Fe2O3(0001) heterostructures: A first-principles investigation. CrystEngComm 2017, 19, 6333–6338. [Google Scholar] [CrossRef]
- Qu, J.; Yin, Y.-X.; Wang, Y.-Q.; Yan, Y.; Guo, Y.-G.; Song, W.G. Layer structured α-Fe2O3 nanodisk/reduced graphene oxide composites as high-performance anode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 3932–3936. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, G.M.; Weng, Z.; Shan, X.-Y.; Li, F.; Cheng, H.-M. Monolithic Fe2O3/graphene hybrid for highly efficient lithium storage and arsenic removal. Carbon 2014, 67, 500–507. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Gebauer, R. Graphene supported on hematite surface: A density functional study. J. Phys. Chem. C 2014, 118, 8455–8461. [Google Scholar] [CrossRef]
- Liu, L.; Quezada, B.R.; Stair, P.C. Adsorption, desorption, and reaction of methyl radicals on surface terminations of α-Fe2O3. J. Phys. Chem. C 2010, 114, 17105–17111. [Google Scholar] [CrossRef]
- Kiejna, A.; Pabisiak, T. Mixed termination of hematite (α-Fe2O3) (0001) surface. J. Phys. Chem. C 2013, 117, 24339–24344. [Google Scholar] [CrossRef]
- Huang, X.; Ramadugu, S.K.; Mason, S.E. Surface-specific DFT+U approach applied to α-Fe2O3 (0001). J. Phys. Chem. C 2016, 120, 4919–4930. [Google Scholar] [CrossRef]
- Wang, X.G.; Weiss, W.; Shaikhutdinov, S.K.; Ritter, M. The hematite (α-Fe2O3) (0001) surface: Evidence for domains of distinct chemistry. Phys. Rev. Lett. 1998, 81, 1038–1041. [Google Scholar] [CrossRef] [Green Version]
- Lemire, C.; Bertarione, S.; Zecchina, A.; Scarano, D.; Chaka, A.; Shaikhutdinov, S.; Freund, H.J. Ferryl (Fe=O) termination of the hematite alpha-Fe2O3(0001) surface. Phys. Rev. Lett. 2005, 94, 166101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef] [PubMed]
- Wigginton, N.S.; Rosso, K.M.; Stack, R.G.; Hochella, M.F., Jr. Long-range electron transfer across cytochrome-hematite (α-Fe2O3) interfaces. J. Phys. Chem. C 2009, 113, 2096–2103. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Govind, N.; Petersen, M.; Fitzgerald, G.; King-Smith, D.; Andzelm, J. A generalized synchronous transit method for transition state location. Comput. Mater. Sci. 2003, 28, 250–258. [Google Scholar] [CrossRef]
- Ren, J.; Guo, H.H.; Yang, J.Z.; Qin, Z.F.; Lin, J.Y.; Li, Z. Insights into the mechanisms of CO2 methanation on Ni(111) surfaces by density functional theory. Appl. Surf. Sci. 2015, 351, 504–516. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.S.; Lee, S.C.; Han, S.; Lee, K.R.; Kim, D.Y. Ab initio study of the effect of nitrogen on carbon nanotube growth. Nanotechnology 2006, 17, 909–912. [Google Scholar] [CrossRef]
- You, Y.; Yan, M.F.; Chen, H.T. Interactions of carbon-nitrogen and carbon-nitrogen-vacancy in α-Fe from first-principles calculations. Comput. Mater. Sci. 2013, 67, 222–228. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Q.; Meng, R.; Jiang, J.; Liang, Q.; Tan, C.; Sun, X. The electronic and optical properties of novel germanene and antimonene heterostructures. J. Mater. Chem. C 2016, 4, 5434–5441. [Google Scholar] [CrossRef]
- Cao, H.; Zhou, Z.; Zhou, X.; Cao, J. Tunable electronic properties and optical properties of novel stanene/ZnO heterostructure: First-principles calculation. Comp. Mater. Sci. 2017, 139, 179–184. [Google Scholar] [CrossRef]
- Chen, L.; Wu, R.; Kioussis, N.; Zhang, Q. First principles determinations of the bonding mechanism and adsorption energy for CO/MgO(001). Chem. Phys. Lett. 1998, 290, 255–260. [Google Scholar] [CrossRef]
- Shan, B.; Zhao, Y.J.; Hyun, J.; Kapur, N.; Cho, K. Coverage-dependent CO adsorption energy from first-principles calculations. J. Phys. Chem. C 2009, 113, 6088–6092. [Google Scholar] [CrossRef]
- Kirklin, S.; Meredig, B.; Wolverton, C. High-throughput computational screening of new Li-ion battery anode materials. Adv. Energy Mater. 2013, 3, 252–262. [Google Scholar] [CrossRef]
- Wang, Z.; Luan, D.; Madhavi, S.; Hu, Y.; Lou, X.W. Assembling carbon-coated α-Fe2O3 hollow nanohorns on the CNT backbone for superior lithium storage capability. Energy Environ. Sci. 2012, 5, 5252–5256. [Google Scholar] [CrossRef]
- Larcher, D.; Masquelier, C.; Bonnin, D.; Chabre, Y.; Masson, V.; Leriche, J.B.; Tarascon, J.M. Effect of particle size on lithium intercalation into alpha-Fe2O3. J. Electrochem. Soc. 2003, 150, A133–A139. [Google Scholar] [CrossRef]
- Ceder, G.; Aydinol, M.K.; Kohan, A.F. Application of first-principles calculations to the design of rechargeable Li-batteries. Comp. Mater. Sci. 1997, 8, 161–169. [Google Scholar] [CrossRef]
- Dong, Y.R.; Wei, W.; Lv, X.S.; Huang, B.B.; Dai, Y. Semimetallic Si3C as a high capacity anode material for advanced lithium ion batteries. Appl. Surf. Sci. 2019, 479, 519–524. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y. Ab initio prediction of two-dimensional Si3C enabling high specific capacity as an anode material for Li/Na/K-ion batteries. J. Mater. Chem. A 2020, 8, 4274–4282. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Liao, T.; Zhang, C.L.; Wu, X.L.; Liu, Y.S.; Qurashi, M.S.; Zheng, F.; Song, Q.S.; Han, P.D. Graphene/Cu composites: Electronic and mechanical properties by first-principles calculation. Mater. Chem. Phys. 2019, 231, 188–195. [Google Scholar] [CrossRef]
- Shan, S.Y.; Wei, T.Z. Effect of N/B doping on the electronic and field emission properties for carbon nanotubes, carbon nanocones, and graphene nanoribbons. Nanoscale 2010, 2, 1069–1082. [Google Scholar] [CrossRef]
- Yan, Q.; Huang, B.; Yu, J.; Zheng, F.W.; Zang, J.; Wu, J.; Gu, B.-L.; Liu, F.; Duan, W. Intrinsic current-voltage characteristics of graphene nanoribbon transistors and effect of edge doping. Nano Lett. 2007, 7, 1469–1473. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Han, P.; Mei, J. Interfacial Design on Graphene–Hematite Heterostructures for Enhancing Adsorption and Diffusion towards Superior Lithium Storage. Nanomaterials 2021, 11, 81. https://doi.org/10.3390/nano11010081
Zhang Q, Han P, Mei J. Interfacial Design on Graphene–Hematite Heterostructures for Enhancing Adsorption and Diffusion towards Superior Lithium Storage. Nanomaterials. 2021; 11(1):81. https://doi.org/10.3390/nano11010081
Chicago/Turabian StyleZhang, Qian, Peide Han, and Jun Mei. 2021. "Interfacial Design on Graphene–Hematite Heterostructures for Enhancing Adsorption and Diffusion towards Superior Lithium Storage" Nanomaterials 11, no. 1: 81. https://doi.org/10.3390/nano11010081




