Rapid Removal of Mercury from Water by Novel MOF/PP Hybrid Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis-Modified MOF Membrane (US-N)
2.3. Filter Removal Processes
2.4. Regeneration of US-N in Mercury Removal
3. Results and Discussion
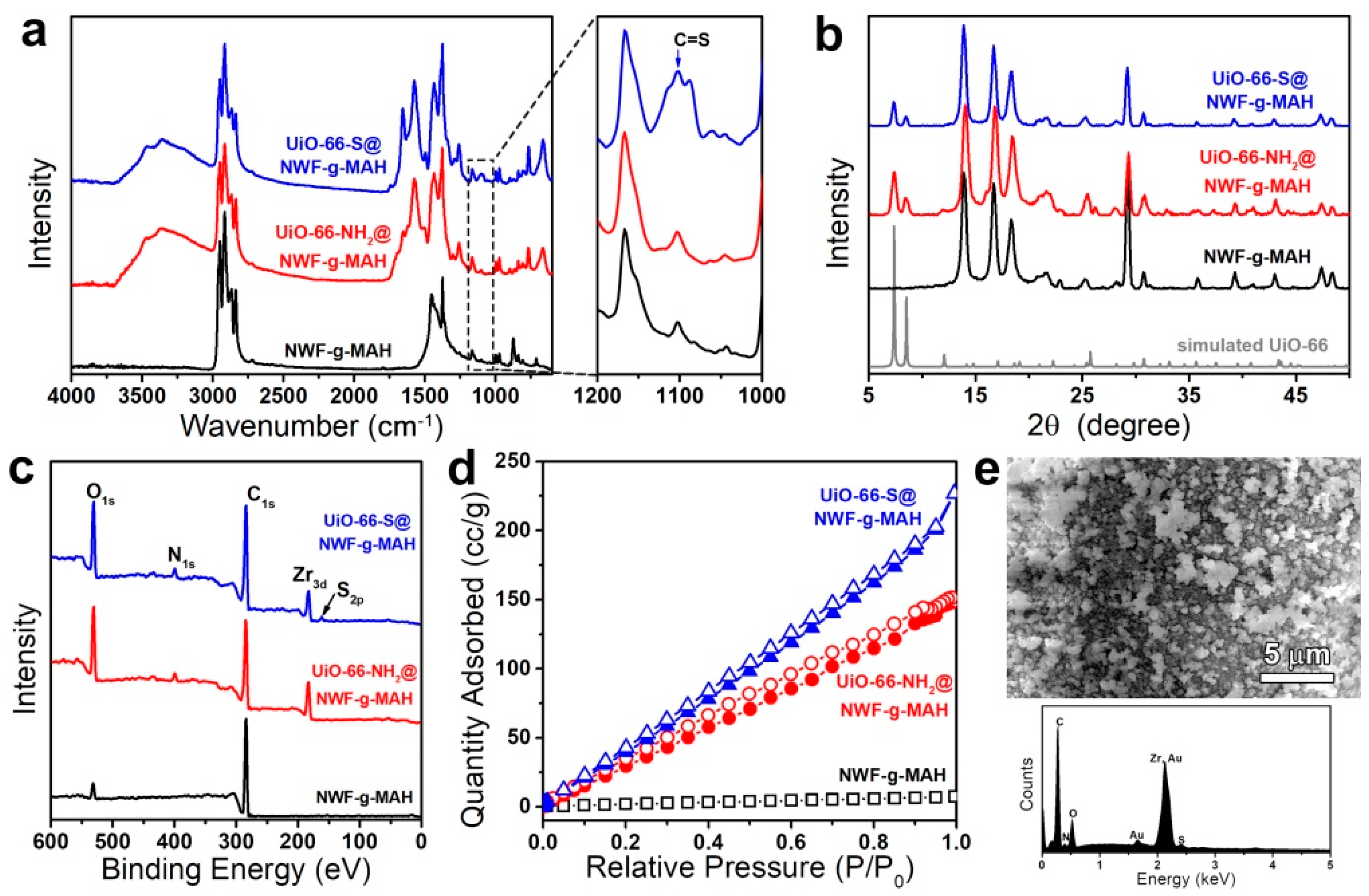
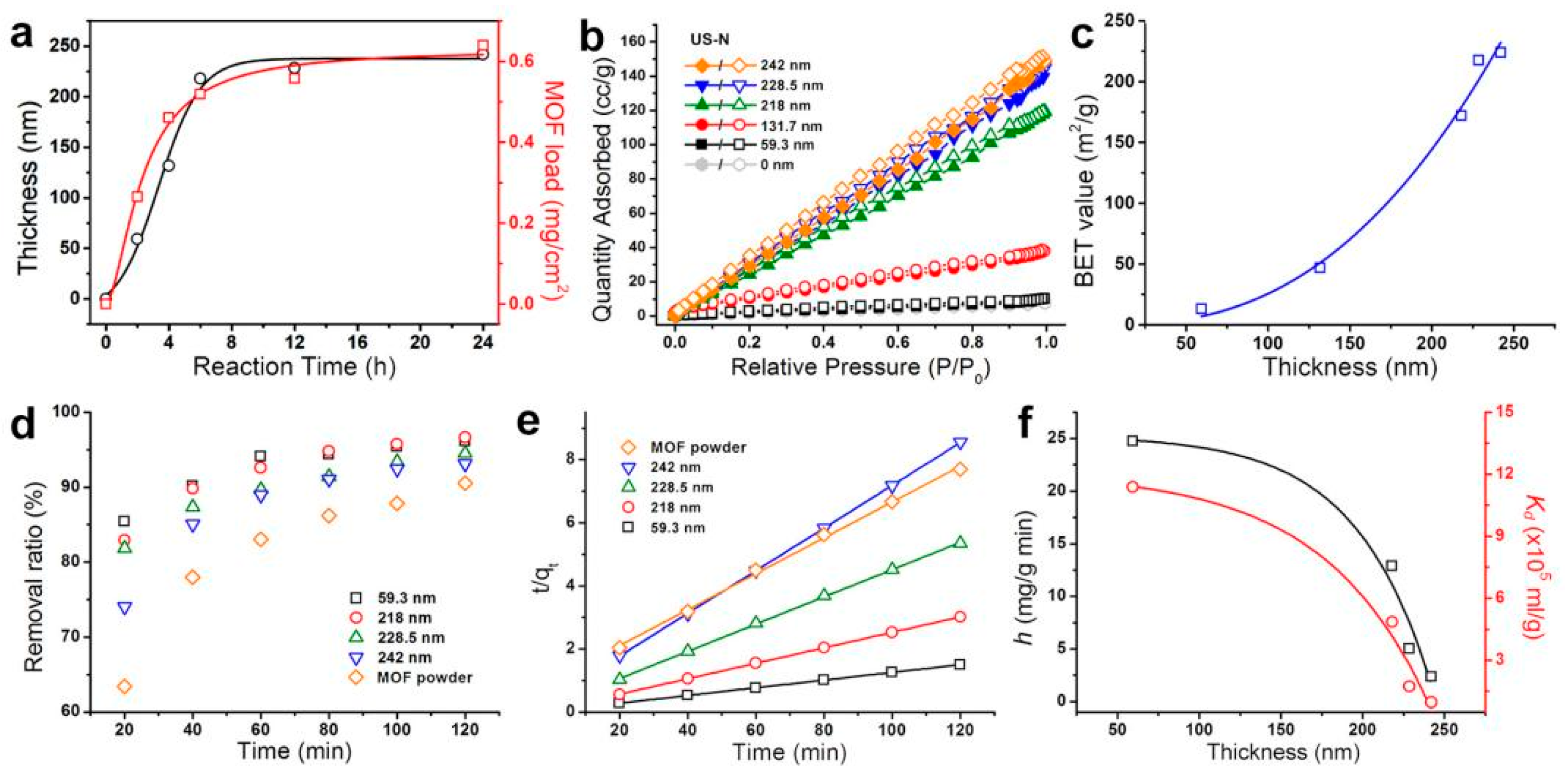
3.1. Synthesis and Characterization
3.2. Hg2+ Removal by US-N
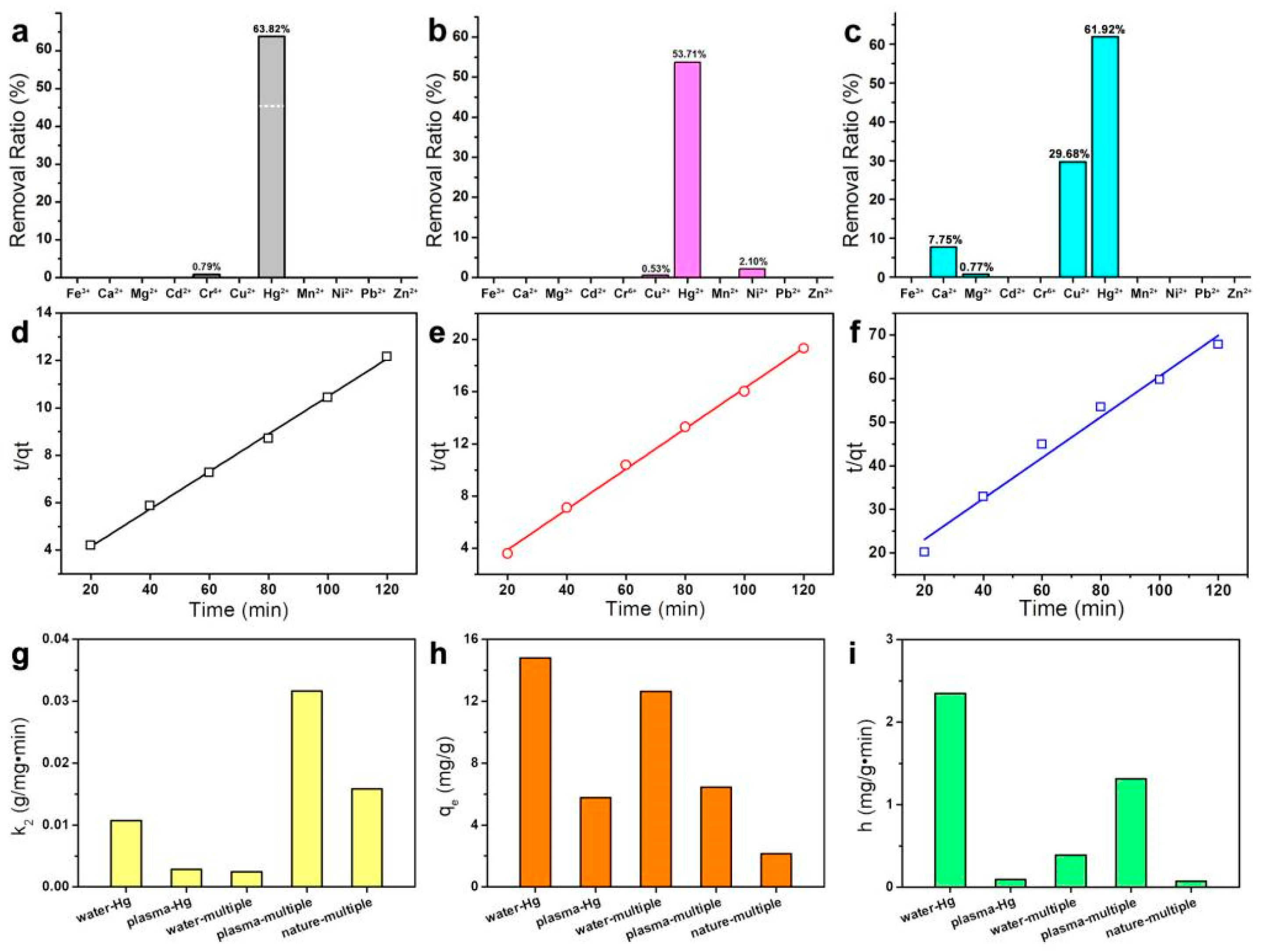
3.3. Selectivity of US-N to Hg2+
3.4. Regeneration of US-N in Mercury Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omichinski, J.G. Toward methylmercury bioremediation. Science 2007, 317, 205–206. [Google Scholar] [CrossRef]
- Mon, M.; Qu, X.; Ferrando-Soria, J.; Pellicer-Carreño, I.; Sepúlveda-Escribano, A.; Ramos-Fernandez, E.V.; Jansen, J.C.; Armentano, D.; Pardo, E. Fine-tuning of the confined space in microporous metal organic frameworks for efficient mercury removal. J. Mater. Chem. A 2017, 5, 20120–20125. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Huang, H.; Zhang, Y.; Kang, C.; Chen, S.; Song, L.; Liu, D.; Zhong, C. A versatile MOF-based trap for heavy metal ion capture and dispersion. Nat. Commun. 2018, 9, 187. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal-organic framework-based materials: Superior adsorbents for the capture of toxic and redioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Ma, D.; Shi, Z.; Ma, S. Mercury nano-trap for effective and efficient removal of mercury(II) from aqueous solution. Nat. Commun. 2014, 5, 5537. [Google Scholar] [CrossRef]
- Uzun, I.; Güzel, F. Adsorption of some heavy metal ions from aqueous solution by activated carbon and comparison of percent adsorption results of activated carbon with those of some other adsorbents. Turk. J. Chem. 2000, 24, 291–297. [Google Scholar]
- Cincotti, A.; Mameli, A.; Locci, A.M.; Orru, R.; Cao, G. Heavy metals uptake by Sardinian natural zeolites: Experiment and modeling. Ind. Eng. Chem. Res. 2006, 45, 1074–1084. [Google Scholar] [CrossRef]
- Gier, S.; Johns, W.D. Heavy metal-adsorption on micas and clay minerals studied by X-ray photoelectron spectroscopy. Appl. Clay Sci 2000, 16, 289–299. [Google Scholar] [CrossRef]
- Sun, D.T.; Peng, L.; Reeder, W.S.; Moosavi, S.M.; Tiana, D.; Oveisi, D.K.B.E.; Queen, W.L. Rapid, selective heavy metal removal from water by a metal-organic framework/polydopamine composite. ACS Cent. Sci. 2018, 4, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Panahi, P.; Nouruzi, N.; Doustkhah, E.; Mohtasham, H.; Ahadi, A.; Ghiasi-Moaser, A.; Rostamnia, S.; Mahmoudi, G.; Khataee, A. Zirconium based porous coordination polymer (PCP) bearing organocatalytic ligand: A promising dual catalytic center for ultrasonic heterocycle synthesis. Ultrason. Sonochem. 2019, 58, 104653. [Google Scholar] [CrossRef]
- Cui, W.; Hu, T.; Bu, X. Metal-organic framework materials for the separation and purification of light hydrocarbons. Adv. Mater. 2020, 32, 1806445. [Google Scholar] [CrossRef]
- Doustkhah, E.; Hassandoost, R.; Khataee, A.; Luque, R.; Assadi, M.H. Hard-templated metal-organic frameworks for advanced applications. Chem. Soc. Rev. 2021, 50, 2927–2953. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zou, L.; Pang, H.; Xu, Q. Synthesis of micro/nanoscaled metal-organic frameworks and their direct electrochemical applications. Chem. Soc. Rev. 2020, 49, 301–331. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Mondal, J.; Ortega-Castro, J.; Frontera, A.; Roy, P. A Ni-based MOF for selective detection and removal of Hg2+ in aqueous medium: A facile strategy. Dalton Trans. 2017, 46, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, S.; Lin, G.; Zhang, L.; Liu, Q.; Fang, J.; Wei, C.; Liu, G. Post-functionalization of UiO-66-NH2 by 2,5-dimercapto-1,3,4-thiadiazole for the high efficient removal of Hg(II) in water. J. Hazard. Mater. 2019, 368, 42–51. [Google Scholar] [CrossRef]
- Mon, M.; Lloret, F.; Ferrando-Soria, J.; Marti-Gastaldo, C.; Armentano, D.; Pardo, E. Selective and Efficient Removal of Mercury from Aqueous Media with the Highly Flexible Arms of a BioMOF. Angew. Chem. Int. Ed. 2016, 55, 11167–11172. [Google Scholar] [CrossRef]
- He, Y.; Hou, Y.; Wong, Y.; Xiao, R.; Li, M.Q.; Hao, Z.F.; Huang, J.; Wang, L.; Zeller, M.; He, J.; et al. Improving stability against desolvation and mercury removal performance of Zr(IV)–carboxylate frameworks by using bulky sulfur functions. J. Mater. Chem. A 2018, 6, 1648–1654. [Google Scholar] [CrossRef]
- Yang, P.; Shu, Y.; Zhuang, Q.; Li, Y.; Gu, J. A robust MOF-based trap with high-density active alkyl thiol for the super-efficient capture of mercury. Chem. Commun. 2019, 55, 12972–12975. [Google Scholar] [CrossRef]
- Mofarah, S.; Adabifiroozjaei, E.; Pardehkhorram, R.; Assadi, M.H.; Hinterstein, M.; Yao, Y.; Liu, X.; Ghasemian, M.; Kalantar-Zadeh, K.; Mehmood, R.; et al. Coordination polymer to atomically thin, holey, metal-oxide nanosheets for tuning band alignment. Adv. Mater. 2019, 31, 1905288. [Google Scholar] [CrossRef]
- Jiang, S.; He, W.; Li, S.; Su, Z.; Lan, Y. Introduction of Molecular Building Blocks to Improve the Stability of Metal–Organic Frameworks for Efficient Mercury Removal. Inorg. Chem. 2018, 57, 6118–6123. [Google Scholar] [CrossRef]
- Saleem, H.; Rafique, U.; Davies, R.P. Investigations on post-synthetically modified UiO-66-NH2 for the adsorptive removal of heavy metal ions from aqueous solution. Micropor. Mesopor. Mater. 2016, 221, 238. [Google Scholar] [CrossRef]
- Wang, X.; Yin, H.; Yin, X. MOF@COFs with Strong Multiemission for Differentiation and Ratiometric Fluorescence Detection. ACS Appl. Mater. Inter. 2020, 12, 20973–20981. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Li, Z.; Deng, Z.; Liu, M.; Wei, W.; Zheng, C.; Zhang, Y.; Chen, S.; Deng, P. Rapid Removal of Mercury from Water by Novel MOF/PP Hybrid Membrane. Nanomaterials 2021, 11, 2488. https://doi.org/10.3390/nano11102488
Gao J, Li Z, Deng Z, Liu M, Wei W, Zheng C, Zhang Y, Chen S, Deng P. Rapid Removal of Mercury from Water by Novel MOF/PP Hybrid Membrane. Nanomaterials. 2021; 11(10):2488. https://doi.org/10.3390/nano11102488
Chicago/Turabian StyleGao, Jian, Ziming Li, Ziqi Deng, Meihua Liu, Wei Wei, Chunbai Zheng, Yifan Zhang, Shusen Chen, and Pengyang Deng. 2021. "Rapid Removal of Mercury from Water by Novel MOF/PP Hybrid Membrane" Nanomaterials 11, no. 10: 2488. https://doi.org/10.3390/nano11102488




